Abstract
BACKGROUND
Although surgery remains the primary treatment for gastric cancer (GC), the identification of effective alternative treatments for individuals for whom surgery is unsuitable holds significance. HER2 overexpression occurs in approximately 15%-20% of advanced GC cases, directly affecting treatment-related decisions. Spectral-computed tomography (sCT) enables the quantification of material compositions, and sCT iodine concentration parameters have been demonstrated to be useful for the diagnosis of GC and prediction of its invasion depth, angiogenesis, and response to systemic chemotherapy. No existing report describes the prediction of GC HER2 status through histogram analysis based on sCT iodine maps (IMs).
AIM
To investigate whether whole-volume histogram analysis of sCT IMs enables the prediction of the GC HER2 status.
METHODS
This study was performed with data from 101 patients with pathologically confirmed GC who underwent preoperative sCT examinations. Nineteen parameters were extracted via sCT IM histogram analysis: The minimum, maximum, mean, standard deviation, variance, coefficient of variation, skewness, kurtosis, entropy, percentiles (1st, 5th, 10th, 25th, 50th, 75th, 90th, 95th, and 99th), and lesion volume. Spearman correlations of the parameters with the HER2 status and clinicopathological parameters were assessed. Receiver operating characteristic curves were used to evaluate the parameters’ diagnostic performance.
RESULTS
Values for the histogram parameters of the maximum, mean, standard deviation, variance, entropy, and percentiles were significantly lower in the HER2+ group than in the HER2– group (all P < 0.05). The GC differentiation and Lauren classification correlated significantly with the HER2 status of tumor tissue (P = 0.001 and 0.023, respectively). The 99th percentile had the largest area under the curve for GC HER2 status identification (0.740), with 76.2%, sensitivity, 65.0% specificity, and 67.3% accuracy. All sCT IM histogram parameters correlated positively with the GC HER2 status (r = 0.237-0.337, P = 0.001-0.017).
CONCLUSION
Whole-lesion histogram parameters derived from sCT IM analysis, and especially the 99th percentile, can serve as imaging biomarkers of HER2 overexpression in GC.
Keywords: Gastric cancer, Spectral computed tomography, Iodine map, Histogram analysis
Core Tip: Anti-HER2 receptor is a critical biomarker in gastric cancer (GC). In this study, a total of 101 GC patients underwent preoperative spectral-computed tomography (sCT) and nineteen parameters were extracted from the iodine maps of sCT by histogram analysis. The relationship between parameters derived from whole volume histogram analysis of sCT and HER-2 status of GC were further investigated. Our present results indicated that parameters derived from sCT, particularly the 99th percentiles, could be utilized as an imaging biomarker in assessing the HER2 overexpression of GC. This founding would help Gastrointestinal Oncologists to develop an effective treatment strategy for patients with GC.
INTRODUCTION
Gastric cancer (GC) is among the most prevalent malignancies of the digestive tract and is the fourth leading cause of cancer-related death worldwide[1]. Although surgery remains the primary treatment for GC, exploration to identify effective alternative treatments for individuals for whom surgery is unsuitable holds significance. Accurate biomarker characterization is critical for the development of targeted therapeutic strategies to serve as alternatives or adjuncts to chemotherapy for GC. Upon its activation through phosphorylation, the epidermal growth factor receptor (EGFR), a highly expressed cell-surface tyrosine kinase receptor, drives cancer cell proliferation and plays significant roles in the regulation of various other tumor cell functions, such as migration, differentiation, apoptosis, and adhesion[2]. HER2, a member of the EGFR family, is a 185-kDa transmembrane tyrosine kinase receptor. It has no ligand and cannot form a ligand-dependent homodimer. To initiate downstream signaling, HER2 must form heterodimers with other EGFR proteins upon their ligand binding[3]. HER2 overexpression or amplification occurs in approximately 15%-20% of advanced GC cases[4,5]. The landmark phase-3 ToGA trial demonstrated that the addition of trastuzumab use to chemotherapy improved the median overall survival of patients with HER2+ advanced GC[6]. Consequently, this combination has become the standard first-line treatment for HER2+ GC. However, a small proportion of GC cases is HER2+, and the cost of trastuzumab treatment is notably high. Currently, HER2 status is determined primarily through immunohistochemical (IHC) or fluorescence in-situ hybridization (FISH) analysis of tissues obtained from surgery or biopsy, invasive procedures that may hinder the timely provision of treatment options. Hence, a noninvasive means of determining the HER2 status of GC cases to identify suitable candidates before treatment initiation is needed. Computed tomography (CT) is the routine imaging modality for the clinical staging of GC. Spectral-CT (sCT), which involves the acquisition of attenuation measurements from different energy spectra and the use of known attenuation changes between spectra, enables the quantification and differentiation of material compositions[7]. sCT iodine concentration (IC) parameters have been demonstrated to be useful in the diagnosis of GC and prediction of its invasion depth, lymph node metastasis, angiogenesis, and responses to systemic chemotherapy[8]. Zhao et al[9] explored correlations between these parameters and the HER2 status of GC, finding that the normalized venous-phase (VP) IC was a significant predictor of this status. To date, no report has described the prediction of GC HER2 status through histogram analysis based on sCT iodine maps (IMs). Given the potential of sCT for tumor evaluation, we investigated whether histogram and texture analysis of sCT IMs enabled the effective and noninvasive determination of the HER2 status of GC. We hypothesized that this combined analysis would aid the assessment of GC HER2 status.
MATERIALS AND METHODS
Patients
Consecutive patients with GC who underwent abdominal contrast-enhanced CT examinations at Fujian Cancer Hospital between April 2020 and September 2022 were included in this study. The hospital’s research ethics committee approved the study protocol (No. K2022-152-01). The inclusion criteria were: (1) Histopathological confirmation of gastric adenocarcinoma; (2) Performance of abdominal sCT examination before anti-tumor treatment; and (3) Availability of complete clinicopathological data. The exclusion criteria were: (1) History of chemoradiotherapy or other anti-tumor therapy; and (2) Poor image quality or inability to measure lesions on CT images. In total, 101 patients (82 males and 19 females) with a mean age of 63.15 ± 10.00 (range, 38-88) years were included in the study. The patients’ clinicopathological data are summarized in Table 1.
Table 1.
Clinical characteristics between two sub-groups of patients with gastric adenocarcinoma
|
Clinical characteristics
|
HER-2 positive (n = 21)
|
HER-2 negative (n = 80)
|
P value
|
| Age (years) | 59.33 ± 8.534 | 64.15 ± 10.152 | 0.049 |
| Sex | 0.221 | ||
| Male | 19 | 63 | |
| Female | 2 | 17 | |
| Specimen type | 0.007 | ||
| Gastrectomy | 14 | 72 | |
| Biopsy | 7 | 8 | |
| Tumor location | 0.676 | ||
| Cardia | 10 | 30 | |
| Body | 4 | 16 | |
| Antrum | 7 | 34 | |
| Differentiation degree | 0.001 | ||
| Poorly differentiated | 6 | 55 | |
| Non-poorly differentiated | 11 | 18 | |
| Lauren classification | 0.023 | ||
| Intestinal type | 8 | 22 | |
| Diffuse type | 0 | 25 | |
| Mixed type | 5 | 18 |
Pathological examination of HER2 status
The cases’ HER2 status was assessed through IHC analysis of biopsy specimens or tumor tissues obtained during resection surgery. For tissues with HER2 scores of 2+, FISH examination was performed to confirm HER2 overexpression. The patients were allocated to HER2+ (IHC 3+ or IHC 2+ with positive FISH findings) and HER2– (IHC 0, IHC 1+, or IHC 2+ with negative FISH findings) groups.
sCT protocol
All patients underwent bowel preparation to cleanse the gastrointestinal tract and consumed 800-1000 mL water to distend the stomach 30 minutes before sCT examination. The examinations were performed using a 256-channel sCT scanner (Revolution CT; GE Healthcare, Milwaukee, WI, United States). The acquisition parameters were: Tube voltage, 80,140 kV; tube current, 355 mA; pitch, 0.992; field of view, 500 mm × 500 mm; image matrix, 512 × 512; rotation speed, 0.8 seconds; slice thickness/gap, 1.25/1.25 mm; and reconstruction slice thickness, 1.25 mm. The nonionic contrast agent ioversol (320 mg I/mL, 1.5 mL/kg body weight; Hengrui Med, Jiangsu, China) was administered at a rate of 2.8-3.0 mL/s. Contrast-enhanced images were obtained 30 seconds and 65 seconds after contrast agent injection to capture arterial-phase and VP data.
Post-processing of sCT images and histogram analysis
The VP-enhanced IC images were converted to DICOM format for further analysis. Histogram analysis was performed using open-source image analysis software (FireVoxel; New York University, New York, NY, United States). In each case, two radiologists with 10 and 15 years of gastrointestinal CT diagnostics experience, respectively, manually delineated a region of interest (ROI) encompassing the entire GC lesion volume, avoiding areas of necrosis, bleeding, and gas. The software automatically generated a volume of interest for the calculation of whole-lesion histogram parameters, comprising the mean, minimum, and maximum; variance and coefficient of variation; SD and percentiles (1st, 5th, 10th, 25th, 50th, 75th, 90th, 95th, and 99th), lesion volume; and skewness, kurtosis, and entropy.
Statistical analyses
The statistical analyses were conducted using SPSS (version 26.0; IBM, Armonk, NY, United States). The Kolmogorov-Smirnov test was employed to assess the normality of the distributions of all histogram parameters. Continuous variables were compared between groups using the Mann-Whitney U test or independent-samples t test. Categorical variables were compared using the χ2 test or Fisher's exact test. Receiver operating characteristic curves were generated to evaluate the parameters’ predictive and diagnostic performance. Areas under the curve (AUCs), sensitivity, specificity, accuracy, and positive and negative predictive values were calculated. Inter-observer agreement on the sCT histogram measures was assessed using the intraclass correlation coefficient. P values < 0.05 were considered to be significant.
RESULTS
Sample characteristics and clinicopathological characteristics
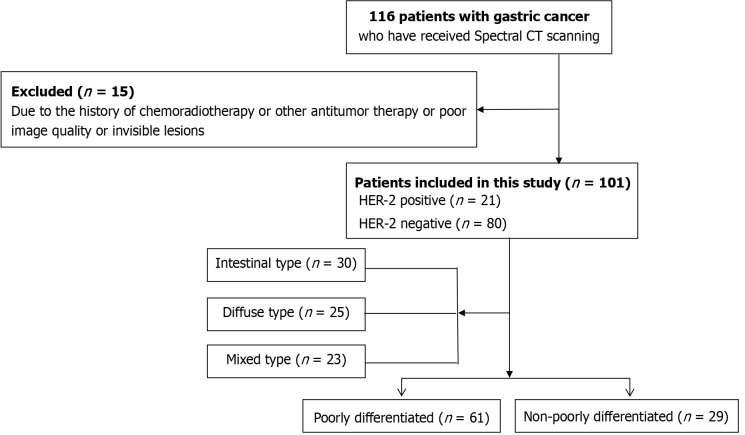
The HER2+ group consisted of 21 patients [HER2+++, n = 16 (76.2%); HER2++ with FISH+, n = 5 (23.8%)] and the HER2– group comprised 80 patients [HER2–/+, n = 68 (85.0%); HER2++ with FISH–, n = 12 (15.0%)]. Histopathological examination of surgical specimens revealed 29 cases of moderately to well-differentiated GC (11 HER2+ and 18 HER2–) and 61 cases of poorly differentiated GC (6 HER2+ and 55 HER2–). Based on the Lauren classification, 30 cases of GC were of the intestinal type (8 HER2+ and 22 HER2–), 23 cases were of the mixed type (5 HER2+ and 18 HER2–), and 25 cases were of the diffuse type (all HER2–; Figure 1). The clinicopathological features of enrolled patients are presented in Table 1.
Figure 1.
Diagram of study flow. CT: Computed tomography.
The prevalence of HER2+ GC differed significantly between patients with moderately to well-differentiated malignancies and those with poorly differentiated malignancies (37.9% vs 9.8%, P = 0.001). No significant difference was found between groups according to sex or the tumor location. However, the Lauren classification and age differed significantly between groups (P = 0.049 and 0.023, respectively). The proportion of HER2+ GC cases detected in biopsy specimens was significantly larger than that of cases detected in resection samples (46.7% vs 16.3%, P = 0.007).
Relationships of histogram parameters to the HER2 status
Nineteen parameters were generated from the histogram analysis of the sCT IMs: The minimum, maximum, mean, SD, variance, coefficient of variation, skewness, kurtosis, entropy, percentiles (1st, 5th, 10th, 25th, 50th, 75th, 90th, 95th, and 99th), and lesion volume. The maximum, mean, SD, variance, entropy, and percentile values were significantly lower in the HER2+ group than in the HER2– groups (all P < 0.05; Table 2). No significant difference was observed in the minimum, coefficient of variation, skewness, kurtosis, or lesion volume.
Table 2.
Comparison of spectral-computed tomography histogram parameters and HER-2 status in gastric cancer
|
Parameters
|
HER-2 positive (n = 21)
|
HER-2 negative (n = 80)
|
P value
|
| Min | -2.000 (-5.518, 1.000) | -0.500 (-7.750, 5.000) | 0.143 |
| Max | 37.000 (29.500, 40.622) | 41.000 (36.000, 50.750) | 0.018 |
| Mean | 17.429 (14.923, 19.341) | 21.176 (17.529, 25.899) | 0.001 |
| Std | 4.383 (3.663, 4.949) | 5.037 (4.285, 5.883) | 0.015 |
| Variance | 19.198 (13.417, 24.489) | 25.366 (18.359, 34.604) | 0.015 |
| CV | 0.269 ± 0.067 | 0.245 ± 0.071 | 0.163 |
| Skewness | -0.051 (-0.246, 0.020) | -0.064 (-0.289, 0.182) | 0.569 |
| Kurtosis | 0.282 (0.020, 0.683) | 0.280 (0.037, 0.617) | 0.861 |
| Entropy | 2.843 ± 0.256 | 3.012 ± 0.259 | 0.009 |
| 1st percentile | 6.037 (3.500, 9.000) | 9.000 (6.000, 12.75) | 0.007 |
| 5th percentile | 10.000 (7.000, 12.000) | 12.500 (10.000, 16.750) | 0.003 |
| 10th percentile | 11.000 (9.000, 13.500) | 14.000 (12.000, 18.750) | 0.003 |
| 25th percentile | 14.086 (12.000, 16.500) | 17.000 (14.000, 21.750) | 0.002 |
| 50th percentile | 18.000 (15.000, 19.500) | 21.000 (18.000, 25.750) | 0.001 |
| 75th percentile | 20.122 (18.000, 23.000) | 24.000 (21.000, 30.500) | 0.001 |
| 90th percentile | 24.000 (19.500, 26.000) | 27.000 (24.000, 35.000) | 0.001 |
| 95th percentile | 24.147 (21.000, 28.000) | 29.000 (25.000, 37.750) | 0.001 |
| 99th percentile | 28.171 (24.000, 30.500) | 32.500 (28.000, 40.750) | 0.001 |
| Lesion volume | 14.643 (4.214, 41.820) | 9.930 (4.418, 21.191) | 0.266 |
CV: Coefficient of variation.
Diagnostic performance of histogram parameters
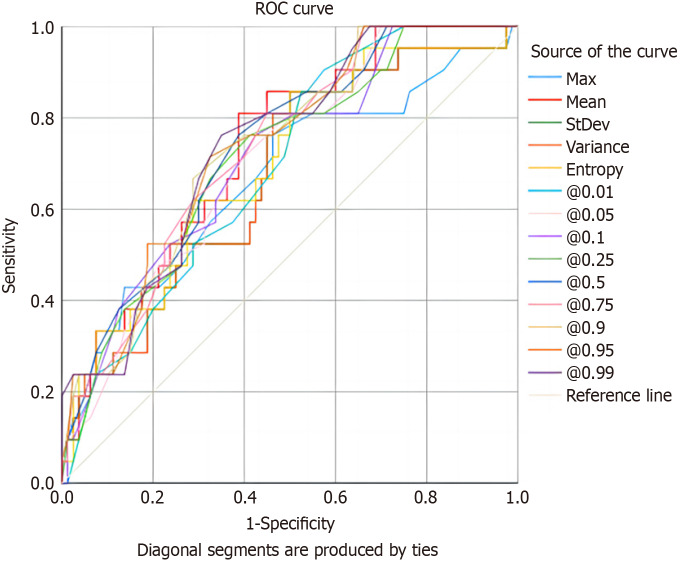
The AUC values for the IM-derived histogram parameters ranged from 0.669 to 0.740, with sensitivities and specificities ranging from 66.7% to 85.7% and 47.5% to 71.2%, respectively. The AUC for the identification of HER2 status was largest for the 99th percentile [0.740; 95% confidence interval (CI): 0.631-0.848; 76.2% sensitivity, 65.0% specificity, and 67.3% accuracy] and smallest for the maximum (0.669; 95%CI: 0.537-0.807; 76.2% sensitivity, 53.7% specificity, and 58.4% accuracy; Table 3, Figure 2). All histogram parameters derived from sCT images correlated positively with the GC HER2 status (r = 0.237-0.337, P = 0.001-0.017; Table 4, Figure 3).
Table 3.
Receiver operating characteristic curve results of the iodine map histogram parameters to identify HER-2 status
|
Parameters
|
AUC (95%CI)
|
Sensitivity (%)
|
Specificity (%)
|
Accuracy (%)
|
Cutoff value
|
P value
|
Youden index
|
| Max | 0.669 | 76.2 | 53.7 | 58.4 | 40.62 | 0.018 | 0.299 |
| Mean | 0.734 | 81.0 | 61.2 | 65.3 | 19.54 | 0.001 | 0.422 |
| SD | 0.674 | 85.7 | 50.0 | 57.4 | 5.05 | 0.015 | 0.357 |
| Variance | 0.674 | 85.7 | 50.0 | 57.4 | 25.50 | 0.015 | 0.357 |
| Entropy | 0.698 | 85.7 | 50.7 | 57.4 | 3.02 | 0.005 | 0.357 |
| 1st percentile | 0.691 | 85.7 | 47.5 | 55.4 | 9.18 | 0.007 | 0.332 |
| 5th percentile | 0.708 | 71.4 | 60.0 | 62.4 | 11.50 | 0.003 | 0.314 |
| 10th percentile | 0.713 | 76.2 | 57.5 | 61.4 | 13.50 | 0.003 | 0.337 |
| 25th percentile | 0.715 | 76.2 | 58.7 | 62.4 | 15.50 | 0.003 | 0.349 |
| 50th percentile | 0.736 | 76.2 | 61.2 | 64.4 | 19.50 | 0.001 | 0.374 |
| 75th percentile | 0.731 | 81.0 | 55.0 | 60.4 | 27.50 | 0.001 | 0.360 |
| 90th percentile | 0.733 | 66.7 | 71.2 | 70.3 | 24.57 | 0.001 | 0.379 |
| 95th percentile | 0.737 | 71.4 | 67.5 | 68.3 | 26.50 | 0.001 | 0.389 |
| 99th percentile | 0.740 | 76.2 | 65.0 | 67.3 | 30.50 | 0.001 | 0.412 |
AUC: Area under the curve.
Figure 2.
Receiver operating characteristic curves of the diagnostic performance of spectral computed tomography histogram parameters in the identification of HER2 status. The 99th percentile had the best overall area under the curve among parameters (0.740), with 76.2% sensitivity, 65.0% specificity, and 67.3% accuracy. ROC: Receiver operating characteristic.
Table 4.
Correlations between spectral-computed tomography histogram parameters and HER-2 status of gastric cancers
|
Parameters
|
Correlation coefficient
|
P value
|
| Max | -0.237 | 0.017 |
| Mean | -0.329 | 0.001 |
| SD | -0.244 | 0.014 |
| Variance | -0.244 | 0.014 |
| Entropy | -0.278 | 0.005 |
| 1st percentile | -0.270 | 0.006 |
| 5th percentile | -0.293 | 0.003 |
| 10th percentile | -0.300 | 0.002 |
| 25th percentile | -0.303 | 0.002 |
| 50th percentile | -0.332 | 0.001 |
| 75th percentile | -0.325 | 0.001 |
| 90th percentile | -0.328 | 0.001 |
| 95th percentile | -0.333 | 0.001 |
| 99th percentile | -0.337 | 0.001 |
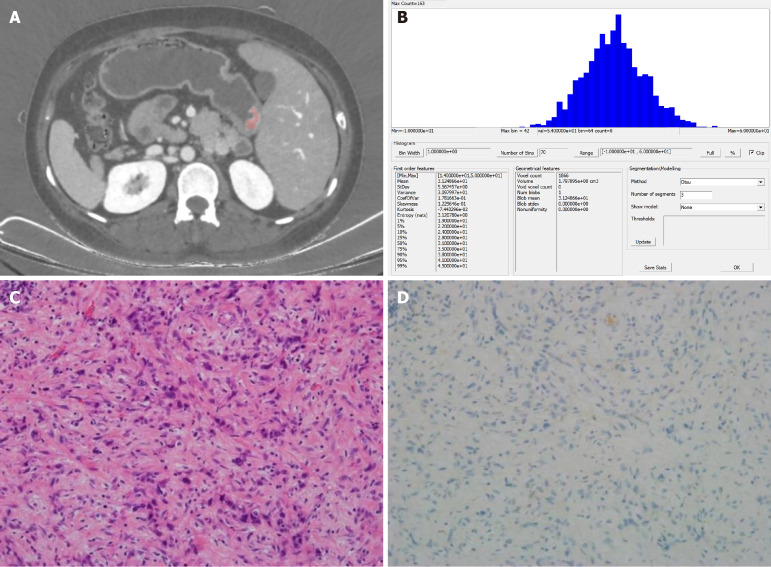
Figure 3.
Images from a 43-year-old woman with poorly differentiated gastric adenocarcinoma. A: Portal venous-phase spectral computed tomography image showing clear (pink) enhancement of the lesion, located at the gastric antrum; B: Histogram of parameter distributions for the whole tumor (minimum = 14.000, maximum = 50.000, mean = 31.249, standard deviation = 5.567, skewness = 0.123, kurtosis = 0.074, 1st-99th percentiles = 19.000, 22.000, 24.000, 28.000, 31.000, 35.000, 38.000, 41.000, and 45.000, respectively); C and D: Microscopic pathological (HE staining, 200 ×) and immunohistochemical images, respectively, showing a poorly differentiated adenocarcinoma with a Lauren classification of diffuse type, vascular and neural invasion, and negative HER2 staining.
DISCUSSION
Considering the relatively low prevalence of HER2 expression in GC cases, the identification of a noninvasive imaging biomarker discriminating individuals most likely to exhibit HER2 overexpression is essential. This study was conducted to investigate the associations of sCT-derived IM histogram parameters with the GC HER2 status and their discriminatory ability. It showed that these parameters, and especially the 99th percentile, correlated significantly with the GC HER2 status. The rate of HER2 overexpression in our GC cases was 20.8%, consistent with previous findings[5]. Similar to previous findings, a larger proportion of HER2+ cases was detected in biopsy specimens than in resection samples. This difference may be attributed to small sample sizes or the superior fixation of biopsy specimens, as proposed by Wang et al[10]. Previous studies of the correlation between HER2 overexpression and tumor location have yielded inconsistent findings[11], and these variables did not correlate in the present study. Additionally, as in the present study, previous studies have revealed consistent associations of a greater frequency of HER2 overexpression with the intestinal histological subtype of GC and moderate to high degrees of differentiation[12-14]. sCT has emerged as a valuable tool in various clinical studies of cancer[15,16], as it provides information on blood flow and quantitative lesion parameters based on IMs. sCT enables the assessment of actual iodine deposition in tissues and the indirect quantification of lesion blood-vessel density and blood volume. Several reports emphasize the diagnostic and predictive potential of sCT for GC, including the modality’s use for detailed evaluation, histological differentiation, Lauren classification, the prediction of lymph node metastasis, the assessment of angiogenesis, the determination of the Ki-67 antigen expression level, and the evaluation of response to neoadjuvant chemotherapy[16-19]. However, assessments of the use of sCT to determine the HER2 status of GC cases are limited. Zhao et al[9] reported a strong correlation of the tumor and normalized VP ICs with HER2 overexpression. Histogram analysis has become a standard tool in the diagnosis and evaluation of differentiation and treatment response of various cancer types, including GC[19-22]. The analysis of IM histogram parameters enables the estimation of the iodine distribution, reflecting the spatial distribution of gray values and providing a comprehensive view of tumor heterogeneity[23,24]. This study is the first in which HER2 overexpression in GC was identified using sCT-derived histogram features. These histogram characteristics can serve as imaging-based biomarkers that aid the selection of patients most likely to benefit from anti-HER2 targeted therapy. In this study, we focused primarily on first-order histogram parameters, which are considered to be more repeatable and stable than higher-order features. To mitigate sampling errors stemming from ROI delineation within tumors, we characterized histogram features for entire target lesion volumes. Values for the variance, skewness, kurtosis, entropy, and percentiles (1st, 5th, 10th, 25th, 50th, 75th, 90th, 95th, and 99th) were significantly lower in the HER2+ group than in the HER2– group. These findings suggest that these parameters could help clinicians devise more personalized therapeutic strategies for GC cases. This study has several limitations. First, the sample was small and the study lacked an external validation cohort. Additionally, as the study was preliminary, a limited number of sCT parameters was examined. The consideration of a more comprehensive set of sCT parameters and high-throughput radiomics features extracted from sCT images may significantly enhance the predictive power of this approach.
CONCLUSION
The results of this study suggest that quantitative parameters derived from whole-lesion histogram analysis of sCT IMs can serve as biomarkers of HER2 overexpression in GC. The use of these biomarkers could help oncologists noninvasively distinguish cases likely to be sensitive to anti-HER2 therapy and aid in clinical decision making.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Fujian Cancer Hospital Institutional Review Board (No. K2022-152-01).
Clinical trial registration statement: Not applicable for this study is not a registered clinical trial.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors declare that there is no conflict of interest regarding the publication of this paper.
CONSORT 2010 statement: The authors have read the CONSORT 2010 Statement, and the manuscript was prepared and revised according to the CONSORT 2010 Statement.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade B, Grade C
Novelty: Grade B, Grade B
Creativity or Innovation: Grade B, Grade B
Scientific Significance: Grade B, Grade C
P-Reviewer: Haque MA; Nemr MTM S-Editor: Qu XL L-Editor: A P-Editor: Wang WB
Contributor Information
Wei-Ling Zhang, Department of Radiology, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
Jing Sun, Department of Radiology, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
Rong-Fang Huang, Department of Pathology, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
Yi Zeng, Department of Gastric Surgery, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
Shu Chen, Department of Gastric Surgery, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
Xiao-Peng Wang, Department of Gastric Surgery, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
Jin-Hu Chen, Department of Gastric Surgery, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
Yun-Bin Chen, Department of Radiology, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
Chun-Su Zhu, Department of Epidemiology, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
Zai-Sheng Ye, Department of Gastric Surgery, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China.
You-Ping Xiao, Department of Radiology, Clinical Oncology School of Fujian Medical University & Fujian Cancer Hospital (Fujian Branch of Fudan University Affiliated Cancer Hospital), Fuzhou 350014, Fujian Province, China. xyp999@yeah.net.
Data sharing statement
Technical appendix, statistical code, and dataset available from the corresponding author at xyp999@yeah.net.
References
- 1.Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229–263. doi: 10.3322/caac.21834. [DOI] [PubMed] [Google Scholar]
- 2.Fadaly WAA, Nemr MTM, Kahk NM. Discovery of novel pyrazole based Urea/Thiourea derivatives as multiple targeting VEGFR-2, EGFR(WT), EGFR(T790M) tyrosine kinases and COX-2 Inhibitors, with anti-cancer and anti-inflammatory activities. Bioorg Chem. 2024;147:107403. doi: 10.1016/j.bioorg.2024.107403. [DOI] [PubMed] [Google Scholar]
- 3.Fadaly WAA, Zidan TH, Kahk NM, Mohamed FEA, Abdelhakeem MM, Khalil RG, Nemr MTM. New pyrazolyl-thiazolidinone/thiazole derivatives as celecoxib/dasatinib analogues with selective COX-2, HER-2 and EGFR inhibitory effects: design, synthesis, anti-inflammatory/anti-proliferative activities, apoptosis, molecular modelling and ADME studies. J Enzyme Inhib Med Chem. 2023;38:2281262. doi: 10.1080/14756366.2023.2281262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roviello G, Aprile G, D'Angelo A, Iannone LF, Roviello F, Polom K, Mini E, Catalano M. Human epidermal growth factor receptor 2 (HER2) in advanced gastric cancer: where do we stand? Gastric Cancer. 2021;24:765–779. doi: 10.1007/s10120-021-01182-9. [DOI] [PubMed] [Google Scholar]
- 5.Nie C, Xu W, Guo Y, Gao X, Lv H, Chen B, Wang J, Liu Y, Zhao J, Wang S, He Y, Chen X. Immune checkpoint inhibitors enhanced the antitumor efficacy of disitamab vedotin for patients with HER2-positive or HER2-low advanced or metastatic gastric cancer: a multicenter real-world study. BMC Cancer. 2023;23:1239. doi: 10.1186/s12885-023-11735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, Aprile G, Kulikov E, Hill J, Lehle M, Rüschoff J, Kang YK ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 7.Greffier J, Villani N, Defez D, Dabli D, Si-Mohamed S. Spectral CT imaging: Technical principles of dual-energy CT and multi-energy photon-counting CT. Diagn Interv Imaging. 2023;104:167–177. doi: 10.1016/j.diii.2022.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Liu YY, Zhang H, Wang L, Lin SS, Lu H, Liang HJ, Liang P, Li J, Lv PJ, Gao JB. Predicting Response to Systemic Chemotherapy for Advanced Gastric Cancer Using Pre-Treatment Dual-Energy CT Radiomics: A Pilot Study. Front Oncol. 2021;11:740732. doi: 10.3389/fonc.2021.740732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao H, Li W, Huang W, Yang Y, Shen W, Liang P, Gao J. Dual-Energy CT-Based Nomogram for Decoding HER2 Status in Patients With Gastric Cancer. AJR Am J Roentgenol. 2021;216:1539–1548. doi: 10.2214/AJR.20.23528. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Nie C, Xu W, Li J, Gou H, Lv H, Chen B, Wang J, Liu Y, He Y, Zhao J, Chen X. In era of immunotherapy: the value of trastuzumab beyond progression in patients with trastuzumab-resistant HER2-positive advanced or metastatic gastric cancer. Therap Adv Gastroenterol. 2024;17:17562848241245455. doi: 10.1177/17562848241245455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang T, Xu R, You J, Li F, Yan B, Cheng JN. Prognostic and clinical significance of HER-2 low expression in early-stage gastric cancer. BMC Cancer. 2022;22:1168. doi: 10.1186/s12885-022-10262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu Y, Zhu X, Wei X, Tang C, Zhang W. HER2-targeted therapies in gastric cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188549. doi: 10.1016/j.bbcan.2021.188549. [DOI] [PubMed] [Google Scholar]
- 13.Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–484. doi: 10.1007/s10120-014-0402-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abood RA, Alomar S, Alharoon SS. A study of human epidermal growth factor receptor 2 overexpression by immunohistochemistry in patients with gastric adenocarcinoma. J Public Health Afr. 2023;14:2721. doi: 10.4081/jphia.2023.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren T, Zhang W, Li S, Deng L, Xue C, Li Z, Liu S, Sun J, Zhou J. Combination of clinical and spectral-CT parameters for predicting lymphovascular and perineural invasion in gastric cancer. Diagn Interv Imaging. 2022;103:584–593. doi: 10.1016/j.diii.2022.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Zhu Y, Wang P, Wang B, Jiang Z, Li Y, Jiang J, Zhong Y, Xue L, Jiang L. Dual-layer spectral-detector CT for predicting microsatellite instability status and prognosis in locally advanced gastric cancer. Insights Imaging. 2023;14:151. doi: 10.1186/s13244-023-01490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Xu S, Wang Y, Fang M, Ma F, Xu C, Li H. Spectral CT-based nomogram for preoperative prediction of perineural invasion in locally advanced gastric cancer: a prospective study. Eur Radiol. 2023;33:5172–5183. doi: 10.1007/s00330-023-09464-9. [DOI] [PubMed] [Google Scholar]
- 18.Li J, Wang Y, Wang R, Gao JB, Qu JR. Spectral CT for preoperative prediction of lymphovascular invasion in resectable gastric cancer: With external prospective validation. Front Oncol. 2022;12:942425. doi: 10.3389/fonc.2022.942425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao LT, Chen WC, Lu JY, Zhang HL, Ye YS, Zhang Y, Liu B, Deng WW, Liu X. Quantitative parameters in novel spectral computed tomography: Assessment of Ki-67 expression in patients with gastric adenocarcinoma. World J Gastroenterol. 2023;29:1602–1613. doi: 10.3748/wjg.v29.i10.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xing P, Chen L, Yang Q, Song T, Ma C, Grimm R, Fu C, Wang T, Peng W, Lu J. Differentiating prostate cancer from benign prostatic hyperplasia using whole-lesion histogram and texture analysis of diffusion- and T2-weighted imaging. Cancer Imaging. 2021;21:54. doi: 10.1186/s40644-021-00423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei X, Yan XJ, Guo YY, Zhang J, Wang GR, Fayyaz A, Yu J. Machine learning-based gray-level co-occurrence matrix signature for predicting lymph node metastasis in undifferentiated-type early gastric cancer. World J Gastroenterol. 2022;28:5338–5350. doi: 10.3748/wjg.v28.i36.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.She Y, Liu X, Liu H, Yang H, Zhang W, Han Y, Zhou J. Combination of clinical and spectral-CT iodine concentration for predicting liver metastasis in gastric cancer: a preliminary study. Abdom Radiol (NY) 2024 doi: 10.1007/s00261-024-04346-0. [DOI] [PubMed] [Google Scholar]
- 23.Son JY, Lee HY, Kim JH, Han J, Jeong JY, Lee KS, Kwon OJ, Shim YM. Quantitative CT analysis of pulmonary ground-glass opacity nodules for distinguishing invasive adenocarcinoma from non-invasive or minimally invasive adenocarcinoma: the added value of using iodine mapping. Eur Radiol. 2016;26:43–54. doi: 10.1007/s00330-015-3816-y. [DOI] [PubMed] [Google Scholar]
- 24.Zeng F, Chen L, Lin L, Hu H, Li J, He P, Wang C, Xue Y. Iodine map histogram metrics in early-stage breast cancer: prediction of axillary lymph node metastasis status. Quant Imaging Med Surg. 2022;12:5358–5370. doi: 10.21037/qims-22-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Technical appendix, statistical code, and dataset available from the corresponding author at xyp999@yeah.net.