Abstract
BACKGROUND
Frailty is a complex aging-related syndrome characterized by a cumulative loss of physiological reserve and increased vulnerability to adverse clinical outcomes, including falls, disability, incapacity and death. While an increasing number of studies suggest that the gut microbiota may play a key role in the pathophysiology of frailty, direct evaluation of the association between gut microbiome alterations and frailty in older adults remains limited.
AIM
To gain insight into gut dysbiosis in frail older adults.
METHODS
Seven electronic databases (China National Knowledge Infrastructure, VIP, SinoMed, Wanfang, PubMed, Web of Science and EMBASE) were searched for articles published before October 31, 2023 to identify observational studies that compared the microbiomes of older adults with and without frailty. The diversity and composition of the gut microbiota were the main outcomes used to analyze the associations of changes in the gut microbiota with frailty in older adults. The quality of the included studies was assessed via the Newcastle-Ottawa Scale and the Agency for Healthcare Research and Quality.
RESULTS
Eleven observational studies with 912 older adults were included in this review. Consistent results revealed a significant difference in the gut microbiota composition between frail and non-frail older adults, with a significant decrease in α diversity and a significant increase in β diversity in frail older adults. The pooled results revealed that at the phylum level, four microbiota (Actinobacteria, Proteobacteria, Verrucomicrobia and Synergistetes) were significantly enriched, and two microbiota (Firmicutes and Fusobacteria) were significantly depleted in frail older adults. At the family level, the results consistently revealed that the abundances of 6 families, most of which belong to the Actinobacteria or Proteobacteria phylum, were greater in frail than in non-frail older adults. At the genus or species level, consistent results from more than two studies revealed that the abundances of the genera Prevotella, Faecalibacterium, and Roseburia were significantly lower in frail older adults; individual studies revealed that the abundances of some genera or species (e.g., Megamonas, Blautia, and Megasphaera) were significantly lower, whereas those of other genera or species (e.g., Bifidobacterium, Oscillospira, Ruminococcus and Pyramidobacter) were significantly greater in frail older adults.
CONCLUSION
This systematic review suggests that changes in the gut microbiota are associated with frailty in older adults, which is commonly reflected by a reduction in beneficial species and an increase in pathogenic species. However, further studies are needed to confirm these findings.
Keywords: Frailty, Gut microbiota, Observational study, Older adults, Systematic review
Core Tip: A growing number of studies have reported changes in the composition and diversity of the gut microbiota between frail and healthy older adults, suggesting that alterations in the gut microbiota may play a key role in the pathophysiology of frailty; however, direct assessment of the associations between changes in the gut microbiome and frailty in older adults remains limited. This review revealed a significant decrease in α diversity and a significant increase in β diversity in frail older adults compared with non-frail older adults, which was commonly reflected by a reduction in beneficial species and an increase in pathogenic species. This study provides a comprehensive overview of the relationship between changes in the gut microbiota and frailty in older adults and suggests a possible role for the gut microbiota in the pathogenesis of frailty.
INTRODUCTION
Frailty is a complex age-related geriatric syndrome characterized by decreased physiological reserves in the body with decreased anti-stress ability and vulnerability in the face of external stimuli, leading to increased risks of multiple adverse health outcomes, including falls, hospitalization, and even mortality[1,2]. Frailty is common among community-dwelling older adults, with a prevalence ranging from 4% to 59% among those aged 65 years and older and 25% among older adults over 85 years[3]. Current studies have shown that the risk factors associated with frailty in older adults are mainly related to increasing age, lower weight, female sex, living alone, low levels of physical activity, polypharmacy, unhealthy lifestyle, smoking, alcohol consumption, and poor diet. These factors interact and form a cycle to cause chronic malnutrition, inflammation, and disruption of hormone regulation[4-7]. Recently, intestinal dysbacteriosis has been newly identified as a risk factor for frailty in older adults[6,8]. For example, a study of 728 female twins revealed a negative association between gut microbiota α diversity and frailty, with increases in Eubacterium dolichum and Eggerthella lenta in the frail group[9].
The gut microbiota is a relatively stable community consisting of a large number of bacteria, fungi, and viruses that colonize the human gut. Gut dysbiosis has been shown to contribute to human diseases, including metabolic diseases, neurodegenerative disorders, and chronic inflammatory diseases[10-11]. The pathogenesis of frailty syndrome may involve chronic inflammation, immune activation, and the musculoskeletal system[12]. Many studies have shown that the diversity and composition of the gut microbiota are significantly altered in community-dwelling older adults with frailty, which in turn may play an important role in the development of frailty in community-dwelling older adults. For example, one study suggested that an imbalance in the gut microbiota triggers an inflammatory response, leading to an increase in intestinal permeability and the entry of pathogen-associated antibodies into the circulation[6,13]. However, most of the previous studies on frailty and the gut microbiota have only examined changes in the composition of bacterial species, but the characteristics of the gut microbiota of frail older people are still unclear, although interest in this topic is increasing. Therefore, there is a lack of adequate evidence on changes in the gut microbiota and frailty in older adults. This systematic literature review mainly summarizes the associations between changes in the gut microbiota and frailty in people over 60 years of age.
MATERIALS AND METHODS
Search strategy
Seven databases (PubMed, EMBASE, Web of Science, SinoMed, China National Knowledge Infrastructure, VIP, and Wanfang) were used to search for Chinese and English articles, respectively, using Medical Subject Headings terms or free words (e.g., “gastrointestinal microbiome” or “gut microbiota” or “intestinal microbiomes” or “gastric microbiome” or “enteric bacteria” or “gut microbiome”) and (“frailty” or “frailty syndrome” or “frailties” or “frail elder”). The final search began in October 2023, with no publication date restrictions. A summary of the search strategy for the different databases is described in Supplementary Table 1.
Inclusion criteria
Eligible studies were identified according to the following inclusion criteria: (1) Participants were adults over 60 years of age; (2) The profile of the gut microbiota was compared between frail and non-frail older adults; and (3) The primary outcome was the abundance of bacterial phyla, families, genera and species of the human gut microbiota. Studies for which the required data could not be retrieved were excluded.
Study screening, data extraction, and assessment of risk bias
The retrieved records were imported into reference management software (Note Express 3.1) for repeated screening. Two reviewers independently identified the eligibility of the retrieved articles according to the inclusion criteria after the duplicate records were removed. Disagreements were discussed and resolved in consultation with a third reviewer. Data from the eligible studies were extracted by one reviewer via prepared data extraction tables and checked by another reviewer. The information extracted included study design, participants, methodological characteristics, sample size, outcomes, and measurement methods. The risk of bias for the eligible studies was assessed via the Newcastle-Ottawa Scale (NOS)[14] and the Agency for Healthcare Research and Quality (AHRQ)[15]. Disagreements were resolved by discussion with a third reviewer.
RESULTS
Literature search
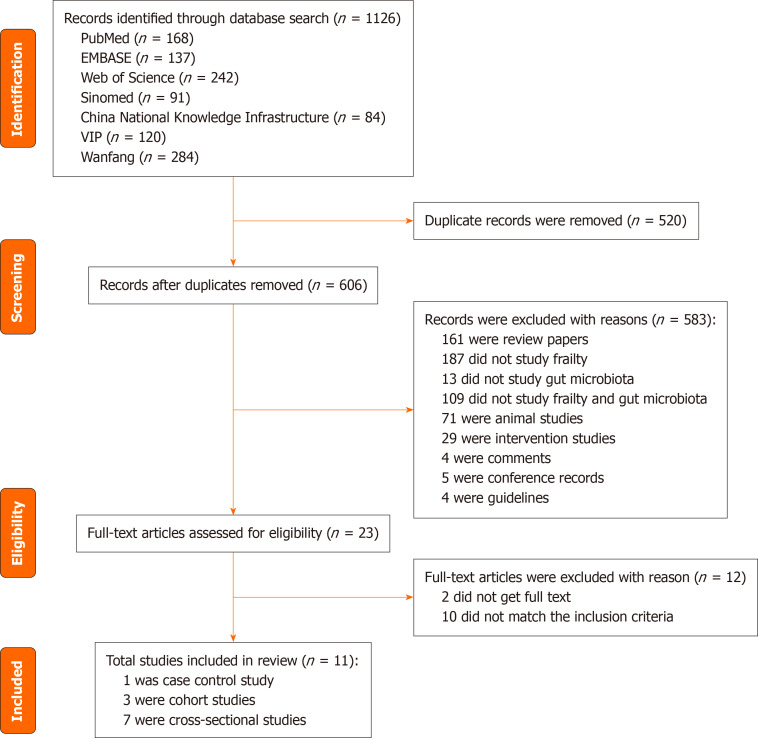
A total of 1126 records were found by searching seven electronic databases, and 520 records were deleted because of duplication. A total of 583 studies were excluded based on the title and abstract. The remaining 23 studies were further assessed by reading the full texts. As a result, 12 studies were excluded for various reasons (10 did not match the inclusion criteria, and 2 did not have the full text available). Eleven studies were ultimately included in this review. A detailed flow chart of the literature screening process is shown in Figure 1.
Figure 1.
Preferred Items for Systematic Reviews and Meta-Analysis flow diagram of literature search.
Characteristics of the included studies
Table 1 summarizes the characteristics of the 11 studies included in the systematic review[16-26]. All included studies consisted of 7 cross-sectional studies[16-22], 3 cohort studies[23-25], and 1 case-control study[26], including 912 older adults ranging in age from 65 years to 100 years. Seven studies analyzed the α diversity of the gut microbiota[17-20,22-23,26], whereas seven studies analyzed the β diversity of the gut microbiota[17,19,21-24,26]. For the outcome measures, two studies reported changes in the gut microbiota at the phylum level[17,22], two at the family level[19,22], eight at the genus level[16-22,25], and five at the species level[16,20,23-25]. Among these included studies, seven studies performed genetic analysis of the gut microbiota via the 16S rRNA method[17-22,26], three studies used the metagenomic sequencing method[23-25], and one study used the fluorescence in situ hybridization method[16]. The frailty measures used in the included studies varied widely, with the rockwood frailty index[22,25,26], FI[18], Clinical Frailty Scale[23,24], short physical performance battery[19], Groningen Frailty Indicator[16], Fried's definition[17], Fried’s Frailty Phenotype[20] and Frailty Phenotype[21] being used. The non-frail controls were mainly healthy older adults.
Table 1.
Main characteristics of the included studies in this review
|
Ref.
|
Picca et al[19], 2020
|
Van Tongeren et al[16], 2005
|
Xu et al[17], 2021
|
Lim et al[18], 2021
|
Zhang et al[22], 2020
|
Margiotta et al[20], 2020
|
Ticinesi et al[26], 2017
|
Haran et al[24], 2018
|
Haran et al[23], 2021
|
Larson et al[25], 2020
|
Zhang et al[21], 2022
|
|
| Study design | CSS | CSS | CSS | CSS | CSS | CSS | CCS | CHS | CHS | CHS | CSS | |
| Participants | Samples | 35 |
23 |
94 |
176 |
27 |
64 |
76 |
23 |
166 |
47 |
181 |
| Age (years) | > 70 | 70-100 | 80.7 ± 5.7 | 74.7 | 81.63 ± 7.90 | ≥ 65 | 83.3 ± 7.5 | ≥ 65 | 86.2 ± 9.1 | > 65 | ≥ 65 | |
| Male/female | 20/15 | 4/19 | 44/50 | 54/122 | 17/10 | 43/21 | 39/37 | 23 | 30/136 | 47 | 72/109 | |
| Genetic analysis | 16S rRNA V3-V4 | Fluorescence in situ hybri dization |
16sRNA V3-V4 | 16S rRNA | 16S rRNA | 16sRNA V3-V4 | 16S rRNA | metagenomic sequencing | metagenomic sequencing | metagenomic sequencing | 16sRNA V3-V4 | |
| Frailty diagnosis | Short physical performance battery | Groningen Frailty Indicator | Fried's definition | Frailty index | Rockwood Frailty Index | Fried’s Frailty Phenotype | Rockwood Index | CFS | CFS | Rockwood Index | Frailty Phenotype | |
| Outcome measurement |
α-diversity, β-diversity, family level, genus level | Genus level, species level | α-diversity, β-diversity, phylum level, genus level | α-diversity, genus level, dpecies level | α-diversity, β-diversity, phylum level, family level, genus level | α-diversity, phylum level, genus level | α-diversity, β-diversity |
β-diversity, species level |
α-diversity, β-diversity, species level | Genus level, species level | β-diversity, phylum level, genus level | |
CSS: Cross-sectional study: CCS: Case-control study; CHS: Cohort study; CFS: Clinical Frailty Scale.
Quality assessment
Table 2, 3 and 4 summarizes the study quality of the included studies, as assessed by the AHRQ for 7 cross-sectional studies and by the NOS tool for 3 cohort studies and one case-control study[16-21,23-25]. Of the seven cross-sectional studies, six were of moderate quality[16-21], and one was of high quality[22]. All three cohort studies were of moderate quality[23-25], and one case-control study was of high quality [26].
Table 2.
The Agency for Healthcare Research and Quality assessment for the cross-sectional study
|
Ref.
|
Item 1
|
Item 2
|
Item 3
|
Item 4
|
Item 5
|
Item 6
|
Item 7
|
Item 8
|
Item 9
|
Item 10
|
Item 11
|
Scores
|
| Picca et al[19], 2020 | Yes | Yes | Yes | Yes | Yes | Yes | 6 | |||||
| Van Tongeren et al[16], 2005 | Yes | Yes | Yes | Yes | Yes | Yes | 6 | |||||
| Xu et al[17], 2021 | Yes | Yes | Yes | Yes | Yes | Yes | 6 | |||||
| Lim et al[18], 2021 | Yes | Yes | Yes | Yes | Yes | Yes | 6 | |||||
| Zhang et al[22], 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | |||
| Margiotta et al[20], 2020 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | ||||
| Zhang et al[21], 2022 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 |
Yes is one point, the total number of stars represents a number of points. Item 1: Define the source of information (survey, record review)? Item 2: Inclusion and exclusion criteria for exposed and unexposed subjects (cases and controls) or refer to previous publications? Item 3: Indicate time period used for identifying patients? Item 4: Indicate whether or not subjects were consecutive if not population-based? Item 5: Indicate if evaluators of subjective components of study were masked to other aspects of the status of the participant? Item 6: Describe any assessments undertaken for quality assurance purposes (e.g., testretest of primary out come measuroments)? Item 7: Explain any patient exclusions from analysis? Item 8: Describe how confounding was assessed and/or controlled? Item 9: If applicable, explain how missing data were handled in the analysis? Item 10: Summarize patient response rates and completeness of data collection? Item 11: Clarify what follow-up, if any, was expected and the percentage of patients for which incomplete data or follow-up was obtained?
Table 3.
The Newcastle-Ottawa Scale assessment for the cohort studies
| Ref. |
Selection
|
Comparability
|
Exposure
|
Scores | ||||||
|
Representativeness of selection of the non- exposed cohort
|
Selection of the non-exposed cohort
|
Ascertainment of exposure
|
Demonstration that outcome of interest was not present at start of study
|
Comparability of cohort on the basis of the design or analysis
|
Ascertainment of outcome
|
Ascertainment of outcome
|
Was follow-up long enough for outcomes to occur
|
Adequacy of follow-up of cohorts
|
||
| Haran et al[24], 2018 | Yes | Yes | Yes | Yes, Yes | 5 | |||||
| Haran et al[23], 2021 | Yes | Yes | Yes, Yes | Yes | Yes | 6 | ||||
| Larson et al[25], 2020 | Yes | Yes | Yes | Yes, Yes | 5 | |||||
Yes is one point, the total number of stars represents a number of points.
Table 4.
The Newcastle-Ottawa Scale assessment for the case-control study
| Ref. |
Selection
|
Comparability
|
Exposure
|
Scores | |||||
|
Adequate definition of case
|
Representativeness of the case
|
Selection of controls
|
Definition of controls
|
Control
for important factor |
Ascertainment of exposure
|
Same method of ascertain for cases and controls
|
Non-responsese rate
|
||
| Ticinesi et al[26], 2017 | Yes | Yes | Yes | Yes | Yes | Yes, Yes | Yes | 7 | |
Yes is one point, the total number of stars represents a number of points.
Outcome assessment
Changes in the diversity of the gut microbiota: A total of seven studies analyzed the difference in α diversity of the gut microbiota measured by the Chao index, Simpson index, and Shannon index between frail older adults and non-frail controls[17-20,22,23,26], and two studies reported a significant decrease in frail older adults[18,23]. Seven studies[17,19,21,23,24,26,27] compared the β diversity measured by principal coordinate analysis in frail older adults with that in controls, and two studies reported significantly greater β diversity in frail than non-frail older adults[21,22].
Changes in the gut microbiota composition: Figure 2 summarizes the changes in the gut microbiota composition at each level in frail older adults compared with non-frail older adults. Two studies reported results at the phylum level[17,22], and all of them reported that frail older adults had a significantly greater relative abundance of the Actinobacteria phylum[17,22]. An individual study reported that frail older adults had significantly greater relative abundances of the Proteobacteria, Verrucomicrobia and Synergistetes phyla[17] and significantly lower abundances of the Firmicutes[17] and Fusobacteria phyla[22].
Figure 2.
Changes in the gut microbiota composition in older adults with frailty compared to the controls. Red arrows indicate increasing relative abundance; blue arrows indicate decreasing relative abundance in older adults with frailty compared to the controls; One arrow represents one analyzed study.
Two studies reported differences in the gut microbial composition at the family level between frail and non-frail older adults[19,22], with significantly greater abundances of the Peptostreptococcaceae[19], Mogibacteriaceae, Bifidobacteriaceae[19], Coriobacteriaceae, Enterobacteriaceae, and Moraxellaceae families[22] in frail older adults.
Eight studies reported changes in gut microbiota composition at the genus level. Three studies reported a significantly lower abundance of the genus Prevotella in frail older adults[16-18]; two studies reported that frail older adults presented significantly lower relative abundances of the genera Faecalibacterium and Roseburia[17,21]. Several individual studies reported that the relative abundances of Megamonas, Blautia, Megasphaera, Haemophilus[17], Adlercreutzia, Clostridium, Coprococcus, Phascolarctobacterium, Turicibacter[21], Eubacterium[19], Gemella, Lachnoanaerobaculum, [Eubacterium]_ruminantium_group, Tyzzerella, Azospira, Cloacibacterium and EU455341_g[22] genera, most of which belong to the Firmicutes phylum, were significantly lower in frail older adults. Moreover, individual studies reported that frail older adults presented increased relative abundances of Bifidobacterium[17], Eggerthella, Olsenella[16], Atopobium[22], Parabacteroides[17], Alistipes[17], Bacteroides[18], Oscillospira, Ruminococcus, Pyramidobacter and Dialister[19], Akkermansia and Klebsiella[17], KF843164_g, Pseudoxanthomonas, EF434341_g and Prevotella_9[22], and Oscillospira and Coprobacillus[20]. In addition, the genera of Lactobacillus in the included studies were heterogeneous, and three studies[17,20-21] reported a significantly higher relative abundance, but one study[16] reported a significantly lower relative abundance in frail older adults.
At the species level, two studies[16,23] reported that the relative abundance of Faecalibacterium prausnitzii was significantly lower in frail than in non-frail older adults. Furthermore, individual studies reported that the abundances of the Prevotella copri[18], Coprococcus eutactus[18], Bacteroides vulgatus[23] and Anaerostipes hadrus species[23] were significantly lower in frail older adults. However, the abundances of Bacteroides fragilis[18], Clostridium hathewayi[18], Eggerthella lenta[20], Flavonifractor plautii[23] and Ruminococcus gnavus[24] were significantly increased in frail older adults.
DISCUSSION
This qualitative systematic review, which included 11 eligible studies with 912 older adults over 65 years of age, investigated the relationships between changes in gut microbiota diversity and composition and frailty in older adults. For gut microbiota diversity, the results, which are based on consistent findings reported by more than two eligible studies, revealed a significant decrease in α diversity and a significant increase in β diversity in frail older adults. In terms of the gut microbiota composition, although there was wide variation in the gut microbiota composition reported in the included studies, the consistent results revealed significant differences in the relative abundance of some gut microbiota compositions at different levels, including phylum, family, genus and species, between frail and non-frail older adults. These findings suggest that changes in the gut microbiota may be associated with frailty in older adults.
An increasing number of studies have reported that altered gut microbiota play an important role as a risk factor in the development of many chronic diseases[28-30]. The gut microbiota in healthy individuals maintains a symbiotic relationship with the host but also triggers some pathological processes and causes the evolution of some diseases if potentially pathogenic bacteria overgrow and alter the diversity and abundance of the gut microbiota[31]. The mechanism is related to a deficiency or excess of metabolites resulting from an imbalance in the gut microbiota, which fundamentally affects the physiological status of the host cells and has direct or indirect toxic effects on hormones and the host organism[32]. The gut microbiota is a highly complex and diverse ecosystem of microorganisms living in the digestive tract, and the balance of beneficial and pathogenic bacteria in the gut microbiome is helpful for maintaining host health and homeostasis[33]. However, both environmental factors and host genetics can affect the homeostatic balance of the gut microbiota and lead to a dysbiotic microbiome configuration by altering the diversity and richness of the gut microbiota[34]. Seven studies included in this review compared the differences in gut microbiota diversity between frail and non-frail older adults. Two of the seven studies reported significantly greater β diversity and significantly lower α diversity in frail than non-frail older adults. These findings suggest a possible separation in gut microbiota diversity in older adults with frailty.
The composition of the gut microbiota in older adults can be altered by the constant influence of external environmental factors, such as diet, medication, physical activity, and social environment. Altered gut microbiota composition has also been shown to play an important role in the development of age-related chronic diseases[35]. The gut microbiome can influence host physical function by regulating nutrient absorption, inflammation, oxidative stress, immune function, and anabolic balance and is associated with the progression of aged-physical frailty[35,36]. Among the relative abundances at the phylum level between frail and non-frail older adults, the current review revealed a significant increase in the Actinobacteria, Proteobacteria, Verrucomicrobia and Synergistetes phyla and a significant decrease in the Firmicutes and Fusobacteria phyla in frail older adults. These phyla are dominant in healthy humans and are pivotal in the maintenance of gut homeostasis[37,38]. There is evidence of positive associations between increased Actinobacteria, Proteobacteria, Verrucomicrobia, and Synergistetes phyla and inflammation-related diseases[39,40]. Conversely, the abundance of the Firmicutes phylum was negatively associated with inflammatory responses[41].
With respect to the relative abundance of families between frail and non-frail older adults, the current review revealed that the abundances of Peptostreptococcaceae, Bifidobacteriaceae, Mogibacteriaceae, and Coriobacteriaceae as well as Enterobacteriaceae and Moraxellaceae families were greater in frail than in non-frail older adults. Most of them (Bifidobacteriaceae, Mogibacteriaceae, Coriobacteriaceae, Enterobacteriaceae X and Moraxellaceae) belong to the Actinobacteria or Proteobacteria phylum and have previously been implicated in accelerating the aging process through telomere attrition, cellular senescence, inflammasome activation and impaired mitochondrial function, which have been described as correlates of biological aging or are abundant in elderly individuals[42,43]; furthermore, some of them also seem to be positively correlated with various nutritional and physical features[44,45].
With respect to the genera and species levels, more than one study in the current review reported that the abundances of genera (Roseburia, Faecalibacterium, and Prevotella) and species (Faecalibacterium prausnitzii and Prevotella copri) were significantly lower in frail older adults. The Roseburia and Faecalibacterium genera have anti-inflammatory properties, which are likely mediated by the short-chain fatty acid butyrate[46,47]. Roseburia is a anaerobic bacteria that produces butyrate that metabolizes indigestible carbohydrates to produce short-chain fatty acids (particularly high levels of butyric acid), which maintain intestinal function, immune function, and anti-inflammatory properties[47,48]. Furthermore, a lower Roseburia level was also found to be associated with inflammation-related diseases such as diabetes, obesity, atherosclerosis, and nonalcoholic liver steatohepatitis[47]. Faecalibacterium prausnitzii, which is the only species of the Faecalibacterium genus, is a genus of bacteria that produces butyrate and has anti-inflammatory effects[46]. Moreover, the abundance of Faecalibacterium prausnitzii is obviously lower in patients with gastrointestinal inflammation and ulcerative colitis (UC)[49]. Hedin et al[50] also reported that the abundances of Faecalibacterium prausnitzii and Roseburia are decreased in patients with the inflammatory Crohn's disease. Prevotella and Prevotella copri, which belong to the Prevotellaceae in this review, were reported to be decreased in frail older adults. Prevotella species significantly colonize the human intestine, especially Prevotella copri, which is prevalent in populations fed high-fiber diets and is associated with beneficial outcomes, including reduced visceral fat and improved glucose tolerance[51,52]. Studies have shown that Prevotella copri transplantation may attenuate oxidative stress and blood-brain barrier damage and alleviate motor and cognitive deficits[53]. In addition, some single studies included in this review reported that the abundances of some genera or species, such as Eubacterium, Gemella, Lachnoanaerobaculum, Bacteroides vulgatus, Megasphaera, Haemophilus, Adlercreutzia, Clostridium, Coprococcus, and Blautia, were significantly lower in frail than in non-frail older adults. Most of these microbiomes have been found to be beneficial. For example, several members of the genus Eubacterium can produce butyrate, which plays important roles in the immunomodulation and inhibition of inflammation in the gut microbiome[54]. Eubacterium, Gemella, Lachnoanaerobaculum and Tyzzerella belong to Firmicutes, and an increase in Firmicutes is associated with a reduction in inflammatory responses[41]. Moreover, the current review revealed that some genera or species, including Oscillospira, Ruminococcus, Alistipes, Bacteroides, Bacteroides fragilis, Pyramidobacter, Eggerthella, Olsenella, Atopobium, Parabacteroides, etc., were more enriched in the frail than the non-frail older adults in the individual included studies; some of these genera or species may be related to the pathological mechanisms of frailty. It has been reported that Oscillospira abundance is positively correlated with inflammation in type II diabetes mellitus patients[55] and is associated with a lower body mass index[56]. Ruminococcus gnavus, which is a type of Ruminococcus, is enriched in inflammatory diseases, such as inflammatory bowel disease[57]. Treatment of UC patients with fecal microbiome transplants revealed that disease progression was more likely to recrudesce in those who received high concentrations of Ruminococcus donors[58]. Ruminococcus also aggravated amyotrophic lateral sclerosis in mice, leading to further frailty[59]. Alistipes, Bacteroides and Bacteroides vulgatus are commonly associated with chronic intestinal inflammation[41,60]. In this review, the results of Lactobacillus in the included studies were not consistent, and its relative abundance was greater in the frail older adults in three studies but was lower in the frail older adults in one study. The reason may be related to the different diets of the participants in those studies.
This systematic review provides a comprehensive summary and overview of the current research on the gut microbiota and frailty in adults over 60 years of age. By focusing on older adults over 60 years of age, the frailty states assessed by comprehensive tools, and the non-frail control group consisting mainly of community-dwelling healthy older adults or those with comorbidities, the findings of this review may therefore provide informative guidance for the prevention or rehabilitation of frailty in community-dwelling older adults. However, the following limitations should be acknowledged, as they may affect the interpretation of these findings. First, most of the included studies were cross-sectional in design, which limits the interpretation of the results regarding causality between changes in the gut microbiota and frailty in older adults. Second, the small sample size and insufficient number of studies may hinder the generalization of the findings of this review. The human gut microbiota is complex and may be influenced by internal and external factors. The small number of included studies may limit the ability to observe the influence of confounding factors such as diet, physical activity, comorbid conditions, and medications. In addition, the variation in the measurement of frailty and the gut microbiota across the included studies also makes accurate assessment or analysis of the associations between the gut microbiota outcomes and frailty difficult.
CONCLUSION
The current review revealed significant changes in the α-diversity and β-diversity and composition of the gut microbiota at the phylum, family, genus, or species level in frail and non-frail older adults aged over 60 years. These changes are commonly reflected by a decrease in the beneficial microbiota (e.g., Faecalibacterium prausnitzii at the species level; Roseburia, Eubacterium, and Faecalibacterium at the genus level) and an increase in the pathogenic microbiota (e.g., Oscillospira, Ruminococcus, Alistipes, Eggerthella, and Bacteroides at the genus level). Future research with large samples and a prospective design is needed to further investigate the impact of specific gut microbiota on frailty in adults over 60 years of age.
Footnotes
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Duan SL S-Editor: Luo ML L-Editor: A P-Editor: Xu ZH
Contributor Information
Na-Na Wen, College of Nursing and Health Management, Shanghai University of Medicine and Health Sciences, Shanghai 201318, China; Graduate School, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China.
Li-Wei Sun, College of Nursing and Health Management, Shanghai University of Medicine and Health Sciences, Shanghai 201318, China.
Qian Geng, College of Nursing and Health Management, Shanghai University of Medicine and Health Sciences, Shanghai 201318, China.
Guo-Hua Zheng, College of Nursing and Health Management, Shanghai University of Medicine and Health Sciences, Shanghai 201318, China. zhenggh@sumhs.edu.cn.
References
- 1.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394:1365–1375. doi: 10.1016/S0140-6736(19)31786-6. [DOI] [PubMed] [Google Scholar]
- 3.Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60:1487–1492. doi: 10.1111/j.1532-5415.2012.04054.x. [DOI] [PubMed] [Google Scholar]
- 4.Rensa R, Setiati S, Laksmi PW, Rinaldi I. Factors Associated with Physical Frailty in Elderly Women with Low Socioeconomic Status in Urban Communities: A Cross-Sectional Study. Acta Med Indones. 2019;51:220–229. [PubMed] [Google Scholar]
- 5.Xie B, Ma C, Chen Y, Wang J. Prevalence and risk factors of the co-occurrence of physical frailty and cognitive impairment in Chinese community-dwelling older adults. Health Soc Care Community. 2021;29:294–303. doi: 10.1111/hsc.13092. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Wu M. Research progress of gut microbiota and frailty syndrome. Open Med (Wars) 2021;16:1525–1536. doi: 10.1515/med-2021-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizka A, Indrarespati A, Dwimartutie N, Muhadi M. Frailty among Older Adults Living in Nursing Homes in Indonesia: Prevalence and Associated Factors. Ann Geriatr Med Res. 2021;25:93–97. doi: 10.4235/agmr.21.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen SY, Wang TY, Zhao C, Wang HJ. Oxidative stress bridges the gut microbiota and the occurrence of frailty syndrome. World J Gastroenterol. 2022;28:5547–5556. doi: 10.3748/wjg.v28.i38.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson MA, Jeffery IB, Beaumont M, Bell JT, Clark AG, Ley RE, O'Toole PW, Spector TD, Steves CJ. Erratum to: signatures of early frailty in the gut microbiota. Genome Med. 2016;8:21. doi: 10.1186/s13073-016-0262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng X, Zhang G, Cao H, Yu D, Fang X, de Vos WM, Wu H. Gut dysbacteriosis and intestinal disease: mechanism and treatment. J Appl Microbiol. 2020;129:787–805. doi: 10.1111/jam.14661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schirmer M, Garner A, Vlamakis H, Xavier RJ. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 2019;17:497–511. doi: 10.1038/s41579-019-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan DB, Maxwell CJ, Afilalo J, Arora RC, Bagshaw SM, Basran J, Bergman H, Bronskill SE, Carter CA, Dixon E, Hemmelgarn B, Madden K, Mitnitski A, Rolfson D, Stelfox HT, Tam-Tham H, Wunsch H. A Scoping Review of Frailty and Acute Care in Middle-Aged and Older Individuals with Recommendations for Future Research. Can Geriatr J. 2017;20:22–37. doi: 10.5770/cgj.20.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ticinesi A, Nouvenne A, Cerundolo N, Catania P, Prati B, Tana C, Meschi T. Gut Microbiota, Muscle Mass and Function in Aging: A Focus on Physical Frailty and Sarcopenia. Nutrients. 2019;11:1633. doi: 10.3390/nu11071633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 15.Chou R, Baker WL, Bañez LL, Iyer S, Myers ER, Newberry S, Pincock L, Robinson KA, Sardenga L, Sathe N, Springs S, Wilt TJ. Agency for Healthcare Research and Quality Evidence-based Practice Center methods provide guidance on prioritization and selection of harms in systematic reviews. J Clin Epidemiol. 2018;98:98–104. doi: 10.1016/j.jclinepi.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 16.van Tongeren SP, Slaets JP, Harmsen HJ, Welling GW. Fecal microbiota composition and frailty. Appl Environ Microbiol. 2005;71:6438–6442. doi: 10.1128/AEM.71.10.6438-6442.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Wang Y, Li H, Dai Y, Chen D, Wang M, Jiang X, Huang Z, Yu H, Huang J, Xiong Z. Altered Fecal Microbiota Composition in Older Adults With Frailty. Front Cell Infect Microbiol. 2021;11:696186. doi: 10.3389/fcimb.2021.696186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim MY, Hong S, Kim JH, Nam YD. Association Between Gut Microbiome and Frailty in the Older Adult Population in Korea. J Gerontol A Biol Sci Med Sci. 2021;76:1362–1368. doi: 10.1093/gerona/glaa319. [DOI] [PubMed] [Google Scholar]
- 19.Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho-Junior HJ, Gervasoni J, Primiano A, Putignani L, Del Chierico F, Reddel S, Gasbarrini A, Landi F, Bernabei R, Marzetti E. Gut Microbial, Inflammatory and Metabolic Signatures in Older People with Physical Frailty and Sarcopenia: Results from the BIOSPHERE Study. Nutrients. 2019;12:65. doi: 10.3390/nu12010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Margiotta E, Miragoli F, Callegari ML, Vettoretti S, Caldiroli L, Meneghini M, Zanoni F, Messa P. Gut microbiota composition and frailty in elderly patients with Chronic Kidney Disease. PLoS One. 2020;15:e0228530. doi: 10.1371/journal.pone.0228530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Yu J, Yang Y, Wang Z, Lu S, Chen R, Lu W, Yu Z, Hong K. Relationship between frailty degree and intestinal microbiome in elderly patients with hypertension complicated with type 2 diabetes mellitus in community. Shiyong Laonian Yixue. 2022;36:663–669. [Google Scholar]
- 22.Zhang L, Liao J, Chen Q, Chen M, Kuang Y, Chen L, He W. Characterization of the gut microbiota in frail elderly patients. Aging Clin Exp Res. 2020;32:2001–2011. doi: 10.1007/s40520-019-01385-2. [DOI] [PubMed] [Google Scholar]
- 23.Haran JP, Zeamer A, Ward DV, Dutta P, Bucci V, McCormick BA. The Nursing Home Older Adult Gut Microbiome Composition Shows Time-dependent Dysbiosis and Is Influenced by Medication Exposures, Age, Environment, and Frailty. J Gerontol A Biol Sci Med Sci. 2021;76:1930–1938. doi: 10.1093/gerona/glab167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haran JP, Bucci V, Dutta P, Ward D, McCormick B. The nursing home elder microbiome stability and associations with age, frailty, nutrition and physical location. J Med Microbiol. 2018;67:40–51. doi: 10.1099/jmm.0.000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larson PJ, Oh J, Robison J, Grady J, Kuchel G. 1206. Association of Aging, Frailty and Place of Residence with Skin, Oral and Gut Microbiome Characteristics and Pathogenicity Reservoirs. Open Forum Infectious Diseases. 2020;7:S625–S625. [Google Scholar]
- 26.Ticinesi A, Milani C, Lauretani F, Nouvenne A, Mancabelli L, Lugli GA, Turroni F, Duranti S, Mangifesta M, Viappiani A, Ferrario C, Maggio M, Ventura M, Meschi T. Gut microbiota composition is associated with polypharmacy in elderly hospitalized patients. Sci Rep. 2017;7:11102. doi: 10.1038/s41598-017-10734-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang L, Li P, Wang D, Wang T, Hao D, Qu X. Alterations in intestinal microbiota diversity, composition, and function in patients with sarcopenia. Sci Rep. 2021;11:4628. doi: 10.1038/s41598-021-84031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei W, Wang S, Xu C, Zhou X, Lian X, He L, Li K. Gut microbiota, pathogenic proteins and neurodegenerative diseases. Front Microbiol. 2022;13:959856. doi: 10.3389/fmicb.2022.959856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiwari P, Dwivedi R, Bansal M, Tripathi M, Dada R. Role of Gut Microbiota in Neurological Disorders and Its Therapeutic Significance. J Clin Med. 2023;12:1650. doi: 10.3390/jcm12041650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katsimichas T, Theofilis P, Tsioufis K, Tousoulis D. Gut Microbiota and Coronary Artery Disease: Current Therapeutic Perspectives. Metabolites. 2023;13:256. doi: 10.3390/metabo13020256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olvera-Rosales LB, Cruz-Guerrero AE, Ramírez-Moreno E, Quintero-Lira A, Contreras-López E, Jaimez-Ordaz J, Castañeda-Ovando A, Añorve-Morga J, Calderón-Ramos ZG, Arias-Rico J, González-Olivares LG. Impact of the Gut Microbiota Balance on the Health-Disease Relationship: The Importance of Consuming Probiotics and Prebiotics. Foods. 2021;10:1261. doi: 10.3390/foods10061261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu J, Tan Y, Cheng H, Zhang D, Feng W, Peng C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022;13:1106–1126. doi: 10.14336/AD.2022.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DAS B, Nair GB. Homeostasis and dysbiosis of the gut microbiome in health and disease. J Biosci. 2019;44:117. [PubMed] [Google Scholar]
- 34.Sommer F, Anderson JM, Bharti R, Raes J, Rosenstiel P. The resilience of the intestinal microbiota influences health and disease. Nat Rev Microbiol. 2017;15:630–638. doi: 10.1038/nrmicro.2017.58. [DOI] [PubMed] [Google Scholar]
- 35.Coman V, Vodnar DC. Gut microbiota and old age: Modulating factors and interventions for healthy longevity. Exp Gerontol. 2020;141:111095. doi: 10.1016/j.exger.2020.111095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni Lochlainn M, Bowyer RCE, Steves CJ. Dietary Protein and Muscle in Aging People: The Potential Role of the Gut Microbiome. Nutrients. 2018;10:929. doi: 10.3390/nu10070929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Binda C, Lopetuso LR, Rizzatti G, Gibiino G, Cennamo V, Gasbarrini A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig Liver Dis. 2018;50:421–428. doi: 10.1016/j.dld.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, Bertalan M, Borruel N, Casellas F, Fernandez L, Gautier L, Hansen T, Hattori M, Hayashi T, Kleerebezem M, Kurokawa K, Leclerc M, Levenez F, Manichanh C, Nielsen HB, Nielsen T, Pons N, Poulain J, Qin J, Sicheritz-Ponten T, Tims S, Torrents D, Ugarte E, Zoetendal EG, Wang J, Guarner F, Pedersen O, de Vos WM, Brunak S, Doré J MetaHIT Consortium, Antolín M, Artiguenave F, Blottiere HM, Almeida M, Brechot C, Cara C, Chervaux C, Cultrone A, Delorme C, Denariaz G, Dervyn R, Foerstner KU, Friss C, van de Guchte M, Guedon E, Haimet F, Huber W, van Hylckama-Vlieg J, Jamet A, Juste C, Kaci G, Knol J, Lakhdari O, Layec S, Le Roux K, Maguin E, Mérieux A, Melo Minardi R, M'rini C, Muller J, Oozeer R, Parkhill J, Renault P, Rescigno M, Sanchez N, Sunagawa S, Torrejon A, Turner K, Vandemeulebrouck G, Varela E, Winogradsky Y, Zeller G, Weissenbach J, Ehrlich SD, Bork P. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Z, Lu G, Li Z, Wu B, Luo E, Qiu X, Guo J, Xia Z, Zheng C, Su Q, Zeng Y, Chan WY, Su X, Cai Q, Xu Y, Chen Y, Wang M, Poon WS, Luo X. Altered Actinobacteria and Firmicutes Phylum Associated Epitopes in Patients With Parkinson's Disease. Front Immunol. 2021;12:632482. doi: 10.3389/fimmu.2021.632482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rodriguez-Palacios A, Cominelli F. Myeloperoxidases and Proteobacteria: Reliable Interspecies Biomarkers to Identify and Monitor Pro-inflammatory Diets in Humans. Inflamm Bowel Dis. 2019;25:e1–e2. doi: 10.1093/ibd/izy197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Samaddar A, van Nispen J, Armstrong A, Song E, Voigt M, Murali V, Krebs J, Manithody C, Denton C, Ericsson AC, Jain AK. Lower systemic inflammation is associated with gut firmicutes dominance and reduced liver injury in a novel ambulatory model of parenteral nutrition. Ann Med. 2022;54:1701–1713. doi: 10.1080/07853890.2022.2081871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boopathi S, Kumar RMS, Priya PS, Haridevamuthu B, Nayak SPRR, Chulenbayeva L, Almagul K, Arockiaraj J. Gut Enterobacteriaceae and uraemic toxins - Perpetrators for ageing. Exp Gerontol. 2023;173:112088. doi: 10.1016/j.exger.2023.112088. [DOI] [PubMed] [Google Scholar]
- 43.Clavel T, Desmarchelier C, Haller D, Gérard P, Rohn S, Lepage P, Daniel H. Intestinal microbiota in metabolic diseases: from bacterial community structure and functions to species of pathophysiological relevance. Gut Microbes. 2014;5:544–551. doi: 10.4161/gmic.29331. [DOI] [PubMed] [Google Scholar]
- 44.Aleman FDD, Valenzano DR. Microbiome evolution during host aging. PLoS Pathog. 2019;15:e1007727. doi: 10.1371/journal.ppat.1007727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rautio M, Eerola E, Väisänen-Tunkelrott ML, Molitoris D, Lawson P, Collins MD, Jousimies-Somer H. Reclassification of Bacteroides putredinis (Weinberg et al., 1937) in a new genus Alistipes gen. nov., as Alistipes putredinis comb. nov., and description of Alistipes finegoldii sp. nov., from human sources. Syst Appl Microbiol. 2003;26:182–188. doi: 10.1078/072320203322346029. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira-Halder CV, Faria AVS, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol. 2017;31:643–648. doi: 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Tamanai-Shacoori Z, Smida I, Bousarghin L, Loreal O, Meuric V, Fong SB, Bonnaure-Mallet M, Jolivet-Gougeon A. Roseburia spp.: a marker of health? Future Microbiol. 2017;12:157–170. doi: 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]
- 48.Kasahara K, Krautkramer KA, Org E, Romano KA, Kerby RL, Vivas EI, Mehrabian M, Denu JM, Bäckhed F, Lusis AJ, Rey FE. Interactions between Roseburia intestinalis and diet modulate atherogenesis in a murine model. Nat Microbiol. 2018;3:1461–1471. doi: 10.1038/s41564-018-0272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laursen MF, Laursen RP, Larnkjær A, Mølgaard C, Michaelsen KF, Frøkiær H, Bahl MI, Licht TR. Faecalibacterium Gut Colonization Is Accelerated by Presence of Older Siblings. mSphere. 2017;2:e00448–17. doi: 10.1128/mSphere.00448-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hedin CR, McCarthy NE, Louis P, Farquharson FM, McCartney S, Taylor K, Prescott NJ, Murrells T, Stagg AJ, Whelan K, Lindsay JO. Altered intestinal microbiota and blood T cell phenotype are shared by patients with Crohn's disease and their unaffected siblings. Gut. 2014;63:1578–1586. doi: 10.1136/gutjnl-2013-306226. [DOI] [PubMed] [Google Scholar]
- 51.Ley RE. Gut microbiota in 2015: Prevotella in the gut: choose carefully. Nat Rev Gastroenterol Hepatol. 2016;13:69–70. doi: 10.1038/nrgastro.2016.4. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed HS. The Impact of Prevotella on Neurobiology in Aging: Deciphering Dendritic Cell Activity and Inflammatory Dynamics. Mol Neurobiol. 2024;61:9240–9251. doi: 10.1007/s12035-024-04156-x. [DOI] [PubMed] [Google Scholar]
- 53.Gu N, Yan J, Tang W, Zhang Z, Wang L, Li Z, Wang Y, Zhu Y, Tang S, Zhong J, Cheng C, Sun X, Huang Z. Prevotella copri transplantation promotes neurorehabilitation in a mouse model of traumatic brain injury. J Neuroinflammation. 2024;21:147. doi: 10.1186/s12974-024-03116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mukherjee A, Lordan C, Ross RP, Cotter PD. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes. 2020;12:1802866. doi: 10.1080/19490976.2020.1802866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu Y, Dong L, Huang L, Shi Z, Dong J, Yao Y, Shen R. Effects of oat β-glucan, oat resistant starch, and the whole oat flour on insulin resistance, inflammation, and gut microbiota in high-fat-diet-induced type 2 diabetic rats. JFF. 2020;69:103939. [Google Scholar]
- 56.Tims S, Derom C, Jonkers DM, Vlietinck R, Saris WH, Kleerebezem M, de Vos WM, Zoetendal EG. Microbiota conservation and BMI signatures in adult monozygotic twins. ISME J. 2013;7:707–717. doi: 10.1038/ismej.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hall AB, Yassour M, Sauk J, Garner A, Jiang X, Arthur T, Lagoudas GK, Vatanen T, Fornelos N, Wilson R, Bertha M, Cohen M, Garber J, Khalili H, Gevers D, Ananthakrishnan AN, Kugathasan S, Lander ES, Blainey P, Vlamakis H, Xavier RJ, Huttenhower C. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017;9:103. doi: 10.1186/s13073-017-0490-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fuentes S, Rossen NG, van der Spek MJ, Hartman JH, Huuskonen L, Korpela K, Salojärvi J, Aalvink S, de Vos WM, D'Haens GR, Zoetendal EG, Ponsioen CY. Microbial shifts and signatures of long-term remission in ulcerative colitis after faecal microbiota transplantation. ISME J. 2017;11:1877–1889. doi: 10.1038/ismej.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blacher E, Bashiardes S, Shapiro H, Rothschild D, Mor U, Dori-Bachash M, Kleimeyer C, Moresi C, Harnik Y, Zur M, Zabari M, Brik RB, Kviatcovsky D, Zmora N, Cohen Y, Bar N, Levi I, Amar N, Mehlman T, Brandis A, Biton I, Kuperman Y, Tsoory M, Alfahel L, Harmelin A, Schwartz M, Israelson A, Arike L, Johansson MEV, Hansson GC, Gotkine M, Segal E, Elinav E. Potential roles of gut microbiome and metabolites in modulating ALS in mice. Nature. 2019;572:474–480. doi: 10.1038/s41586-019-1443-5. [DOI] [PubMed] [Google Scholar]
- 60.Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front Immunol. 2020;11:906. doi: 10.3389/fimmu.2020.00906. [DOI] [PMC free article] [PubMed] [Google Scholar]