Abstract
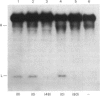
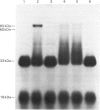
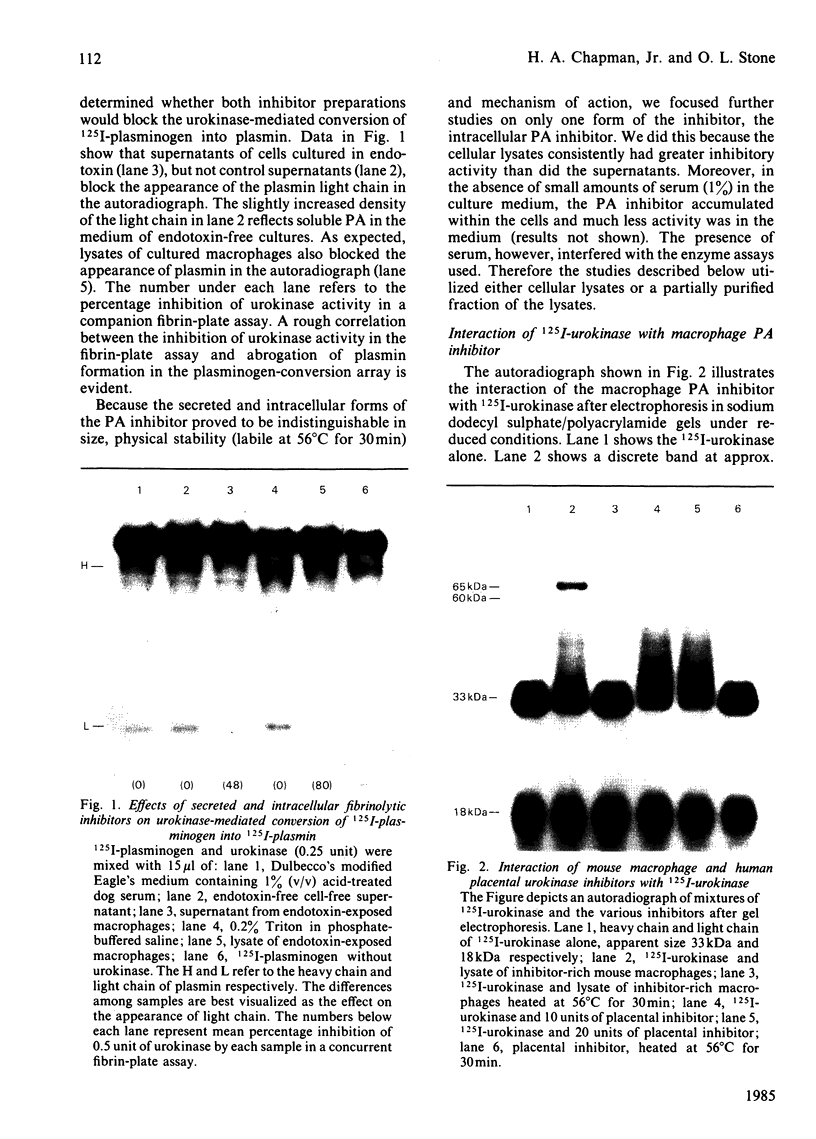
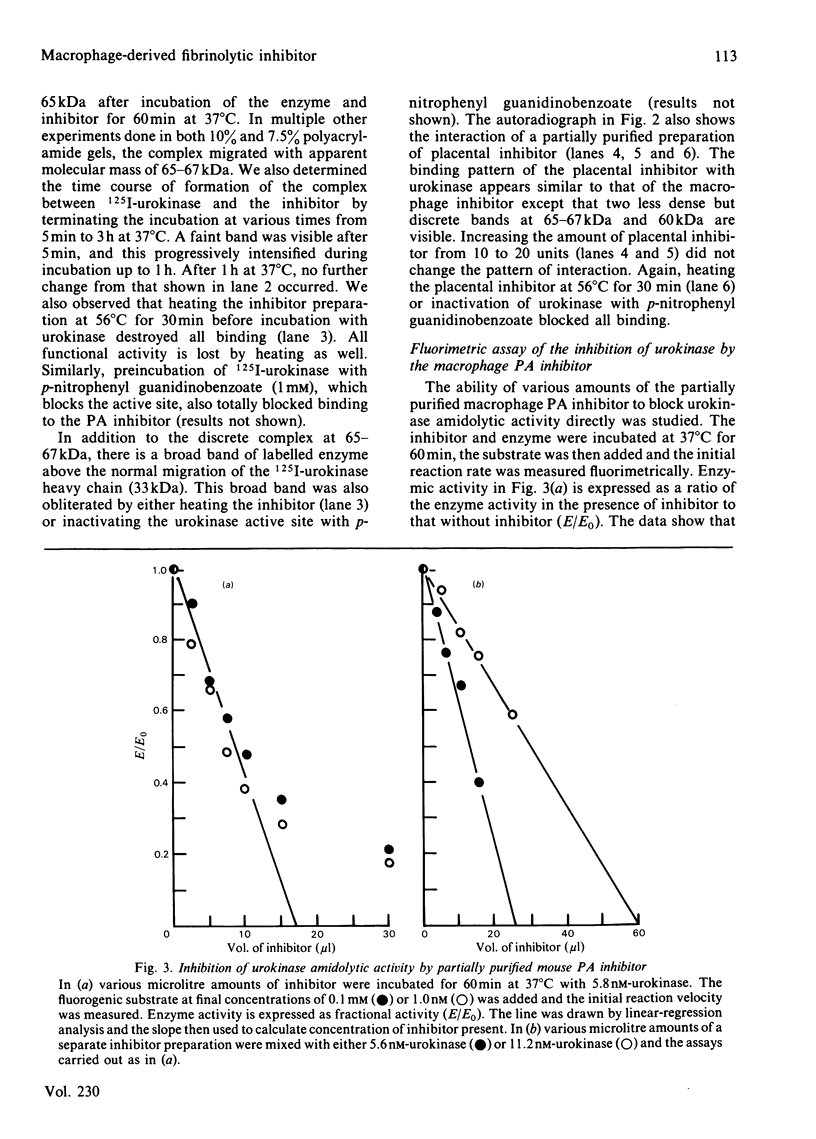
Human and mouse macrophages release a fibrinolytic inhibitor after stimulation by endotoxin in vitro. The released mouse inhibitor was indistinguishable in size by molecular-sieve chromatography from an intracellular form (approx. 50 kDa), and both inhibitors blocked urokinase directly as judged by a 125I-plasminogen conversion assay. The intracellular inhibitor was found mostly to dissociate from 125I-urokinase during sodium dodecyl sulphate/polyacrylamide-gel electrophoresis under reduced conditions, but a dodecyl sulphate-stable complex at 65-67 kDa was observed. Because of similarities in the reported size, stability and urokinase-binding properties of a placental urokinase inhibitor, the kinetic properties of the two inhibitors were compared. Under the reaction conditions employed (37 degrees C at pH7.4 in the presence of 0.2% Triton X-100), the association rate constants and equilibrium dissociation constants of the two inhibitors were indistinguishable, 3 X 10(5) M-1 X s-1 and 4 X 10(-10) M respectively. These data show that peritoneal macrophages contain a plasminogen-activator very similar to a previously recognized placental inhibitor. Although the inhibitor appears to be a trace protein in macrophages, placental macrophages may account for the accumulation of the inhibitor in placental tissue.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Low D. A., Simmer R. L., Cunningham D. D. Protease-nexin: a cellular component that links thrombin and plasminogen activator and mediates their binding to cells. Cell. 1980 Aug;21(1):37–45. doi: 10.1016/0092-8674(80)90112-9. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Adelberg S., Crystal R. G. Role of fibronectin as a growth factor for fibroblasts. J Cell Biol. 1983 Dec;97(6):1925–1932. doi: 10.1083/jcb.97.6.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Stone O. L., Vavrin Z. Degradation of fibrin and elastin by intact human alveolar macrophages in vitro. Characterization of a plasminogen activator and its role in matrix degradation. J Clin Invest. 1984 Mar;73(3):806–815. doi: 10.1172/JCI111275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Coordinate expression of macrophage procoagulant and fibrinolytic activity in vitro and in vivo. J Immunol. 1983 Jan;130(1):261–266. [PubMed] [Google Scholar]
- Chapman H. A., Jr, Vavrin Z., Hibbs J. B., Jr Macrophage fibrinolytic activity: identification of two pathways of plasmin formation by intact cells and of a plasminogen activator inhibitor. Cell. 1982 Mar;28(3):653–662. doi: 10.1016/0092-8674(82)90220-3. [DOI] [PubMed] [Google Scholar]
- Christensen U., Holmberg L., Bladh B., Astedt B. Kinetics of the reaction between urokinase and an inhibitor of fibrinolysis from placental tissue. Thromb Haemost. 1982 Aug 24;48(1):24–26. [PubMed] [Google Scholar]
- Golder J. P., Stephens R. W. Minactivin: a human monocyte product which specifically inactivates urokinase-type plasminogen activators. Eur J Biochem. 1983 Nov 15;136(3):517–522. doi: 10.1111/j.1432-1033.1983.tb07771.x. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Feld M., Minter D. Fibroblast adhesion to fibrinogen and fibrin substrata: requirement for cold-insoluble globulin (plasma fibronectin). Cell. 1980 Feb;19(2):517–525. doi: 10.1016/0092-8674(80)90526-7. [DOI] [PubMed] [Google Scholar]
- Hedelin H., Teger-Nilsson A. C., Peterson H. I., Pettersson S. Effects of tranexamic acid and local fibrin deposition of fibrinolysis and granulation tissue formation in preformed cavities. Thromb Res. 1984 Jan 1;33(1):31–38. doi: 10.1016/0049-3848(84)90152-x. [DOI] [PubMed] [Google Scholar]
- Hølund B., Clemmensen I., Wanning M. Sequential appearance of fibronectin and collagen fibres in experimental arthritis in rabbits. Histochemistry. 1984;80(1):39–44. doi: 10.1007/BF00492769. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Nielsen M. L. Analysis of macrophage surface receptors. I. Binding of alpha-macroglobulin . protease complexes to rabbit alveolar macrophages. J Biol Chem. 1979 Aug 10;254(15):7323–7328. [PubMed] [Google Scholar]
- Kawano T., Morimoto K., Uemura Y. Partial purification and properties of urokinase inhibitor from human placenta. J Biochem. 1970 Mar;67(3):333–342. doi: 10.1093/oxfordjournals.jbchem.a129257. [DOI] [PubMed] [Google Scholar]
- Kurkinen M., Vaheri A., Roberts P. J., Stenman S. Sequential appearance of fibronectin and collagen in experimental granulation tissue. Lab Invest. 1980 Jul;43(1):47–51. [PubMed] [Google Scholar]
- Kwaan H. C., Astrup T. Tissue repair in presence of locally applied inhibitors of fibrinolysis. Exp Mol Pathol. 1969 Aug;11(1):82–88. doi: 10.1016/0014-4800(69)90072-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975 Jan;78(1):71–100. [PMC free article] [PubMed] [Google Scholar]
- Lemaire G., Drapier J. C., Petit J. F. Importance, localization and functional properties of the cell-associated form of plasminogen activator in mouse peritoneal macrophages. Biochim Biophys Acta. 1983 Feb 22;755(3):332–343. doi: 10.1016/0304-4165(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Loskutoff D. J., Edgington T. S. An inhibitor of plasminogen activator in rabbit endothelial cells. J Biol Chem. 1981 May 10;256(9):4142–4145. [PubMed] [Google Scholar]
- MOSESSON M. W. The preparation of human fibrinogen free of plasminogen. Biochim Biophys Acta. 1962 Feb 26;57:204–213. doi: 10.1016/0006-3002(62)91112-5. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repesh L. A., Fitzgerald T. J., Furcht L. T. Fibronectin involvement in granulation tissue and wound healing in rabbits. J Histochem Cytochem. 1982 Apr;30(4):351–358. doi: 10.1177/30.4.6174568. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Unkeless J. C., Gordon S., Reich E. Secretion of plasminogen activator by stimulated macrophages. J Exp Med. 1974 Apr 1;139(4):834–850. doi: 10.1084/jem.139.4.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassalli J. D., Dayer J. M., Wohlwend A., Belin D. Concomitant secretion of prourokinase and of a plasminogen activator-specific inhibitor by cultured human monocytes-macrophages. J Exp Med. 1984 Jun 1;159(6):1653–1668. doi: 10.1084/jem.159.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller E. K., Schleuning W. D., Reich E. Complex-formation and inhibition of urokinase by blood plasma proteins. Biochem J. 1983 Oct 1;215(1):123–131. doi: 10.1042/bj2150123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. B., Haas J. E., Weaver W. M. Isolation, purification and characteristics of mononuclear phagocytes from human placentas. J Immunol Methods. 1983 Feb 11;56(3):305–317. doi: 10.1016/s0022-1759(83)80020-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman M., Quigley J. P., Ashe B., Dorn C., Goldfarb R., Troll W. Direct fluorescent assay of urokinase and plasminogen activators of normal and malignant cells: kinetics and inhibitor profiles. Proc Natl Acad Sci U S A. 1978 Feb;75(2):750–753. doi: 10.1073/pnas.75.2.750. [DOI] [PMC free article] [PubMed] [Google Scholar]