Abstract
To investigate disparities in sensitisation to Staphylococcus aureus enterotoxin A/B (SEA/SEB) and olfactory function in patients with chronic rhinosinusitis (CRS) with nasal polyps (CRSwNP), those with CRS without nasal polyps (CRSsNP), and healthy controls who underwent septoplasty only. We retrospectively reviewed the medical records of 388 subjects aged ≥ 8 years, collected between January 2021 and June 2023. We analysed patient demographics, medical history, serum IgE levels against staphylococcal enterotoxins (SEs), and serum total IgE levels against inhalant allergens. We performed olfactory and taste function tests in the participants to evaluate the relationship between olfactory function and SEs. Of 388 patients enrolled, 145 were healthy controls, 111 had CRSsNP, and 133 had CRSwNP. The prevalence of SEA/SEB positivity was significantly higher among the patients with CRSwNP than among those in the CRSsNP and healthy controls. The olfactory test results showed significant differences among the groups; anosmia was observed in 9.7% of healthy controls, 22.7% of patients with CRSsNP, and 45.1% of patients with CRSwNP. Olfactory threshold deterioration was evident in patients with CRS. Distinction and identification were more impaired in patients with CRSwNP than in those of the other groups. Finally, the olfactory function scores decreased as the serum levels of SEs increased. Sinusitis patients seem to suffer from perceiving odours, and patients with CRSwNP have difficulty distinguishing odours. Olfactory function test scores decreased in patients with a history of asthma, and as serum levels of staphylococcus enterotoxin and blood eosinophil percentage increase. Furthermore, our result suggests a potential role for SE sensitisation and eosinophil percentage in deteriorating olfaction, especially in patients with CRSwNP.
Keywords: Smell (olfaction), Nasal polyps, Rhinosinusitis, Staphylococcal enterotoxins, Sensitisation
Subject terms: Risk factors, Respiratory tract diseases, Inflammation
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disease of the sinonasal mucosa, characterised by nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip), facial pain/pressure, and a reduction or loss of smell lasting for at least 12 weeks1. The prevalence of CRS in Korea ranges from 7 to 8.4%2,3 and CRS is the leading cause of olfactory dysfunction in the general population4. Olfactory dysfunction is more common in patients with CRS with nasal polyposis (CRSwNP) than in those without polyps (CRSsNP)5,6. The mechanism underlying olfactory dysfunction in CRSwNP is not fully understood; however, evidence from clinical research and animal models suggests that an obstructive component causing conductive olfactory loss and an inflammatory response in the olfactory cleft lead to sensorineural olfactory loss7.
Staphylococcus aureus (S. aureus) is a common bacterium often detected in the normal nasal microbiota of healthy individuals. Under certain conditions, this bacterium can colonise the nasal mucosa and facilitate its invasion into subepithelial regions8,9. Specific IgE against staphylococcal enterotoxins (SEs) in almost half of the nasal tissue has been reported to homogenate from nasal polyps10. Bachert et al. clustered CRS cases and demonstrated the highest concentrations of IgE and asthma prevalence in all samples expressing SE-IgE11. Moreover, biomarkers, such as IL-5, IL-5 receptor alpha, ECP, total IgE and SE-IgE, and periostin have been proposed for CRSwNP12. A study in Japan compared the level of S. aureus antibody between the CRSwNP, CRSsNP, and control groups, and found a remarkably higher level of SEA-IgE in the CRSwNP group13. However, studies examining the relationship between olfactory dysfunction in patients with CRS and sensitisation to SEs are currently lacking.
This study aimed to understand the degree of olfactory impairment in patients with CRS compared to healthy individuals, and to examine how the result of each olfactory function test differs based on the CRS phenotype. In addition, we investigated whether SE positivity, which plays a significant role in type 2 inflammation, varies according to the phenotype of rhinosinusitis and the extent to which it affects olfactory function.
Results
Demographic and clinical characteristics of patients by phenotypes of sinusitis
We included 388 patients, of whom 23 were children aged 8–18 years old and 365 were aged 18–87 years. Of them, 37.4% (145 patients) were healthy controls, 28.3% (110) had CRSsNP, and 34.3% (133) had CRSwNP. In the healthy control group, 72.4% (105) were male and 27.6% (40) were female, with a mean age of 40.7 years. In the CRSsNP group, 60.0% (66) males and 40.0% (44) females, with a mean age of 51.1 years. The CRSwNP group consisted of 75.2% (100) males, with a mean age of 51.4 years. There was no significant difference in the mean age between the CRSsNP and CRSwNP groups. Significant differences were observed in smoking rates and asthma comorbidities among the groups, with smoking rates of 17.2% in healthy controls, 24.5% in CRSsNP, and 34.6% in CRSwNP (p = 0.004), and asthma comorbidity rates of 5.5% in healthy controls, 10.0% in CRSsNP, and 18.0% in CRSwNP (p = 0.004) (Table 1).
Table 1.
Characteristic based on each group.
| Characteristic | Healthy control (n = 145, 37.4%) | CRSsNP (n = 110, 28.3%) | CRSwNP (n = 133, 34.3%) | p value |
|---|---|---|---|---|
| Age, years (range) | 40.7 (15–84) | 51.1 (14–85) | 51.4 (8–87) | |
| Sex, n (%) | ||||
| Male | 105 (72.4) | 66 (60.0) | 100 (75.2) | |
| Female | 40 (27.6) | 44 (40.0) | 33 (24.8) | |
| Current smoker, n (%) | 25 (17.2) | 27 (24.5) | 46 (34.6) | 0.004 |
| Asthma history, n (%) | 8 (5.5) | 11 (10.0) | 24 (18.0) | 0.004 |
| Serum neutrophil, % (SD) | 57.28 (10.45) | 57.38 (9.94) | 57.60 (10.45) | 0.996 |
| Serum eosinophil, % (SD) | 2.54 (1.92) | 3.08 (2.55) | 3.86 (2.91) | 0.000 |
| Total IgE, kU/L (SD) | 96.54 (65.66) | 100.55 (69.48) | 131.63 (72.82) | 0.000 |
| SEA/SEB positive, n (%) | 10 (6.9) | 11 (10.0) | 22 (16.5) | 0.035 |
| Serum levels of Staphylococcus aureus enterotoxin, kU/L (SD) | 0.09 (0.30) | 0.13 (0.49) | 0.51 (2.18) | 0.019 |
| Olfactory function test (%) | 0.000 | |||
| Normosmia | 49 (33.8) | 29 (26.4) | 16 (12.0) | |
| Hyposmia | 82 (56.5) | 56 (50.9) | 57 (42.9) | |
| Anosmia | 14 (9.7) | 25 (22.7) | 60 (45.1) | |
| Olfactory function test scores (SD) | 19.83 (4.44) | 17.79 (5.21) | 14.30 (6.11) | 0.000 |
| Threshold scores | 3.16 (2.20) | 2.45 (1.79) | 1.99 (1.64) | 0.000 |
| Discrimination scores | 6.20 (2.22) | 5.64 (2.46) | 4.56 (2.31) | 0.000 |
| Identification scores | 10.47 (2.18) | 9.70 (2.99) | 7.75 (3.75) | 0.000 |
| Taste function test (%) | 0.280 | |||
| Normogeusia | 106 (73.1) | 75 (68.2) | 83 (62.4) | |
| Hypogeusia | 33 (22.8) | 30 (27.3) | 46 (34.6) | |
| Aguesia | 6 (4.1) | 5 (4.5) | 4 (3.0) | |
| Taste function test scores (SD) | 14.05 (4.88) | 13.50 (5.08) | 12.93 (4.67) | 0.163 |
CRSsNP chronic rhinosinusitis without polyps, CRSwNP chronic rhinosinusitis with polyps, SEA Staphylococcus enterotoxin A, SEB Staphylococcus enterotoxin B.
Bold text indicates statistical significance (p < 0.05).
Impact of SE sensitisation on demographic and clinical characteristics
SE positivity rates in either group A (SEA) or B (SEB) were significantly different among the respective groups (p = 0.035), with 6.9% in healthy controls, 10.0% in CRSsNPs, and 16.5% in CRSwNPs (Table 1). Among the 43 patients who were SEA/SEB-positive, 93.0% (40) were male and only 7.0% (3) were female, with a mean age of 53.6 years. Of the 345 patients who were SEA/SEB-negative, 67.0% (231) were male and 33.0% (114) were female, with a mean age of 46.5 years (Table 2). A significant correlation was observed for sex, with 88.6% of males in the SEA/SEB-positive group compared with 66.9% in the negative group (p = 0.000). The smoking rates were also significantly higher in the SEA/SEB-positive group (44.2%) than in the SEA/SEB-negative group (22.9%; p = 0.002). However, no significant difference was found in the asthma comorbidity rates between the SEA/SEB-positive (14.0%) and -negative groups (10.7%) (p = 0.525) (Table 2).
Table 2.
Characteristic based on SEA/SEB sensitization.
| Characteristic | SEA/SEB positive (n = 43, 11.1%) | SEA/SEB negative (n = 345, 88.9%) | p value |
|---|---|---|---|
| Age, years (range) | 53.6 (17–79) | 46.5 (8–87) | 0.003 |
| Sex, n (%) | 0.000 | ||
| Male | 40 (93.0) | 231 (67.0) | |
| Female | 3 (7.0) | 114 (33.0) | |
| Current smoker, n (%) | 19 (44.2) | 79 (22.9) | 0.002 |
| Asthma history, n (%) | 6 (14.0) | 37 (10.7) | 0.525 |
| Serum neutrophil, % (SD) | 58.58 (9.86) | 57.27 (10.34) | 0.431 |
| Serum eosinophil, % (SD) | 4.13 (3.02) | 3.02 (2.44) | 0.025 |
| Total IgE, kU/L (SD) | 184.03 (58.53) | 100.44 (66.78) | 0.000 |
| Olfactory function test (%) | 0.017 | ||
| Normosmia | 4 (9.3) | 90 (26.1) | |
| Hyposmia | 22 (51.2) | 173 (50.1) | |
| Anosmia | 17 (39.5) | 82 (23.8) | |
| Olfactory function test scores (SD) | 15.33 (5.24) | 17.61 (5.79) | 0.014 |
| Threshold score | 2.00 (1.43) | 2.63 (2.01) | 0.012 |
| Discrimination score | 4.95 (2.39) | 5.54 (2.42) | 0.133 |
| Identification score | 8.37 (3.27) | 9.44 (3.21) | 0.041 |
| Taste function test (%) | 0.259 | ||
| Normogeusia | 25 (58.1) | 239 (69.3) | |
| Hypogeusia | 15 (34.9) | 94 (27.2) | |
| Aguesia | 3 (7.0) | 12 (3.5) | |
| Taste function test scores (SD) | 11.84 (5.31) | 13.72 (4.79) | 0.017 |
SEA Staphylococcus enterotoxin A, SEB Staphylococcus enterotoxin B.
Bold text indicates statistical significance (p < 0.05).
Comparison of serum total IgE and SE levels, and serum eosinophil percentage in respective groups
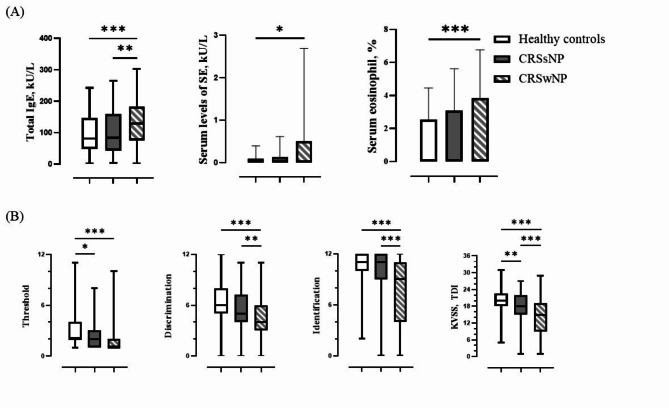
Total IgE levels were significantly different between CRSwNP and the other groups, and between the CRSsNP and the healthy controls (p = 0.003, p = 0.000) (Table 1; Fig. 1A). Among patients with SEA or SEB sensitisation, those who were positive exhibited higher IgE levels than those who were negative (p = 0.000) (Table 2; Fig. 2A). Moreover, serum SE IgE levels also had a significant difference between healthy control group and CRSwNP group (p = 0.034), 0.51 ± 2.18 in CRSwNP group, and 0.09 ± 0.30 in healthy control group (Table 1; Fig. 1A). While serum neutrophil percentage showed no significant difference between respective groups, serum eosinophil percentage showed a significant difference as follows; serum eosinophil percentage was significantly higher in CRSwNP group (3.86 ± 2.91) than in the healthy control group (2.54 ± 1.92) (p = 0.000), and higher in SE sensitisation group (4.13 ± 3.02) than in the non-SE-sensitisation group (3.02 ± 2.44) (p = 0.025) (Tables 1 and 2; Fig. 1 A).
Fig. 1.
Comparison of total IgE, staphylococcal enterotoxin, serum eosinophil percentage, and olfactory function test by each group. (A) Serum levels of total IgE, staphylococcal enterotoxin level, serum eosinophil percentage according to each group (B) Comparison of threshold, discrimination, identification score, and total score (TDI) in olfactory function test according to each group (* means statistically significant).
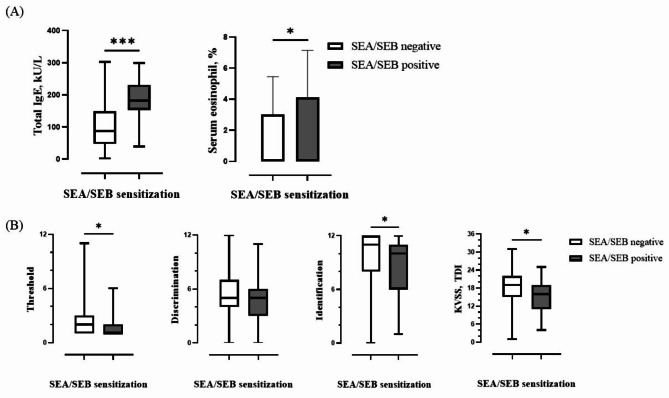
Fig. 2.
Comparison of total IgE, serum eosinophil percentage, and olfactory function test by SEA/SEB sensitization. (A) Serum levels of total IgE and serum eosinophil percentage according to SEA/SEB sensitisation (B) Comparison of threshold, discrimination, identification score, and total score (TDI) in olfactory function test according to SEA/SEB sensitisation (* means statistically significant).
Comparative analysis of olfactory dysfunction on sinusitis phenotypes
In terms of olfactory function test results, significant differences were noted among the groups: anosmia was present in 9.7% of healthy controls, 22.7% of the patients with CRSsNP, and 45.1% of the patients with CRSwNP; normosmia was observed in 33.8% of healthy controls, 26.4% of the patients with CRSsNP, and 12.0% of the patients with CRSwNP (p = 0.000). The olfactory function test is divided into three components: threshold (T), discrimination (D), and identification (I). The CRSwNP group exhibited significantly lower discrimination and identification values than the other groups. In addition, the CRS groups, including CRSsNP and CRSwNP, showed significantly lower threshold values than the healthy controls (Table 1; Fig. 1B).
Association between SE sensitisation and olfactory function test results
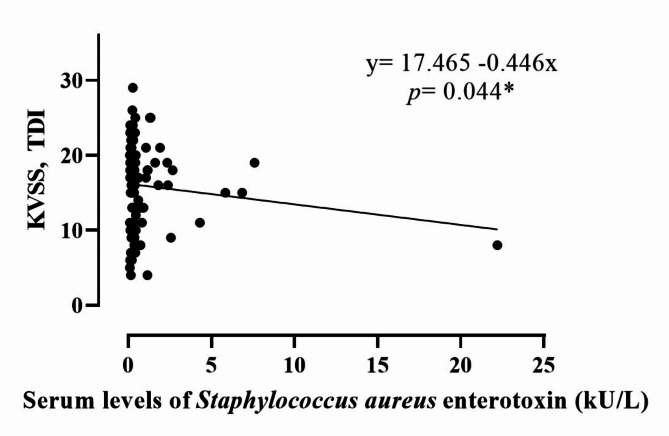
Evaluation of the impact of SE sensitisation on olfactory function revealed significant differences between the SE-sensitised- and non-SE-sensitised groups (p = 0.017) (Table 2). Unlike the SEA/SEB-negative group, the SEA/SEB-positive group showed a higher prevalence of anosmia (39.5%), hyposmia (51.2%), and normosmia (9.3%). Specifically, the olfactory threshold and identification values differed significantly (p = 0.012 and p = 0.041, respectively) between the groups, whereas the discrimination values did not show significant difference (p = 0.133) (Table 2; Fig. 2B). In the single linear regression analysis, a comparison of olfactory function scores with the maximal serum levels of SEA/SEB revealed a negative correlation (p = 0.044) (Fig. 3). This suggests that higher enterotoxin levels are associated with worsening olfactory dysfunction, highlighting the potential mechanic link between SE sensitisation and olfactory impairment.
Fig. 3.
Correlation between olfactory function test score and serum levels of Serum levels of Staphylococcus aureus enterotoxin (kU/L) by single linear regression analysis (* means statistically significant).
Taste function test results in respective groups
We also evaluated taste function in all the populations. Although the proportion of normogeusia and taste function test scores decreased in the CRSwNP group, there were no significant differences in taste function test scores and the proportions of normogeusia, hypogeusia, and ageusia among the groups (Table 1). A comparison of the taste function test between the SEA/SEB-positive group and SEA/SEB-negative group revealed no significant difference (p = 0.259) in the proportion of normogeusia, hypogeusia, and ageusia. However, taste function test scores were significantly lower in SEA/SEB-positive group (11.84 ± 5.31) than in the SEA/SEB-negative group (13.72 ± 4.79) (p = 0.017) (Table 2).
Discussion
This study was a single-centre investigation; however, its findings are representative of broader trends in South Korea. In this dataset, the prevalence of asthma in the healthy control group was 5.5%, whereas the general prevalence in South Korea ranges from 3.2 to 4.7%14. Moreover, the smoking rate in the healthy control group was 17.2%, whereas the smoking rate in South Korea in 2022 is 19.3%15. The data indicated an increase in smoking rate and comorbid asthma among patients with sinusitis, particularly those with nasal polyps, suggesting that these factors likely influence their condition. Among the group with SE sensitisation, six patients had asthma and comorbidity, all of whom were in the CRSwNP group. Previous studies have suggested that SEs play a prominent role in the pathogenesis of allergic airway disease16, and patients with asthma exhibit significantly higher SE IgE levels than non-asthmatic patients (Supplementary Table 1). Several intriguing findings from previous studies were confirmed in the present study. Smokers are known to have elevated blood neutrophil levels17, a higher prevalence of SEA/SEB positivity18, and loss of taste19, which were also observed in this dataset. Recent research indicates that smoking has a limited effect on olfactory function20, supporting our results that there was no significant difference in olfactory function between smokers and non-smokers, except for the discrimination score (p = 0.002) (Supplementary Table 2).
This study aimed to understand the degree of olfactory impairment in patients with CRS compared with healthy individuals and to examine how each olfactory function test result differs based on the CRS phenotype. Our results for each olfactory function component showed that the threshold test revealed a significant difference between the healthy controls and sinusitis groups. This threshold test evaluates the ability to detect odours and serves as a measure of olfactory sensitivity. This finding suggests potential conductive olfactory loss attributable to physical airflow obstruction in the CRS group. In addition, discrimination and identification tests, which evaluate the ability to distinguish between different smells, showed significant differences between the CRSwNP group and the other two groups. This impairment in the CRSwNP group may also be attributed to the disruption of olfactory sensory neuron signalling and processing caused by inflammation in the olfactory cleft. Thus, all patients with CRS seem to have difficulties perceiving olfaction, and patients with CRSwNP seem to have difficulties discriminating olfaction. That is, the decrease in discrimination and identification of olfactory function enables clinicians to consider CRS in patients with nasal polyps.
We investigated whether SE sensitisation varies based on CRS phenotype and the extent to which SE sensitisation affects olfactory function. Multiple linear regression analysis of the olfactory function test scores revealed significant differences associated with sinusitis phenotypes and age (p = 0.000 each) (Table 3), indicating that patients in the CRSwNP group and older individuals had lower olfactory function test scores. Djorjevic et al. evaluated olfaction in elderly patients with mild cognitive impairment and found that odour discrimination and identification performance were correlated more prominently than detection thresholds with performance on neuropsychological tests21. Some studies have also supported the correlation between CRSwNP and cognitive dysfunction in elderly22. The noticeable decline in odour discrimination and identification performance in patients with cognitively impaired CRSwNP should be studied in the future. Based on our results, a single linear regression analysis was conducted to examine the relationship between olfactory function test scores for each variable. Of all the variables assessed, serum levels of SE, blood eosinophil percentage, and asthma history were negatively correlated with olfactory function, whereas sex, smoking history, total IgE level, and neutrophil percentage were not significantly correlated (Supplementary Fig. 1). This negative correlation was not observed in multiple linear regression analysis, possibly because of the relatively small proportion of patients with a history of asthma and positive SE (both 11.1%, 43 patients). Our results align with that of a previous study23, which frequently observed olfactory dysfunction in patients with asthma, elderly, CRS, and nasal polyps to be significantly associated with olfactory dysfunction. SEs, which serve as superantigens in type 2 inflammation and eosinophils—which contribute to immune cell activation in type 2 inflammation—, may play a role in olfactory dysfunction. As in vivo analyses of SE-induced nasal polyps and CRS remain unfulfilled owing to the lack of appropriate animal models, further studies are needed to elucidate the various aspects of SE sensitisation to olfactory dysfunction in CRS.
Table 3.
Multiple and single linear regression analysis on olfactory function test score.
| Variable | Multiple linear regression analysis | Single linear regression analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| B | Standard error | Standardised coefficients beta | p value | B | Standard error | Standardised coefficients beta | p value | |
| Sinusitis phenotypes | -2.126 | 0.334 | -0.312 | 0.000 | -2.755 | 0.317 | -0.404 | 0.000 |
| Age | -0.086 | 0.015 | -0.267 | 0.000 | -0.114 | 0.015 | -0.355 | 0.000 |
| Sex | 1.105 | 0.609 | 0.088 | 0.070 | 1.082 | 0.637 | 0.086 | 0.090 |
| Asthma history | -1.049 | 0.859 | -0.057 | 0.223 | -2.702 | 0.925 | -0.147 | 0.004 |
| Smoking history (Current) | 0.275 | 0.646 | 0.021 | 0.671 | -0.926 | 0.674 | -0.070 | 0.170 |
| Serum levels of SEA/SEB | -0.086- | 0.201 | -0.020 | 0.668 | -0.446 | 0.220 | -0.102 | 0.044 |
| Serum levels of Total IgE | 0.003 | 0.004 | 0.035 | 0.477 | -0.006 | 0.004 | -0.071 | 0.160 |
| Neutrophil % | -0.062 | 0.028 | -0.110 | 0.030 | -0.035 | 0.029 | 0.063 | 0.215 |
| Eosinophil % | -0.202 | 0.119 | -0.089 | 0.089 | -0.303 | 0.115 | -0.133 | 0.009 |
SEA Staphylococcus enterotoxin A, SEB Staphylococcus enterotoxin B.
Bold text indicates statistical significance (p < 0.05). The adjusted R2 was 0.253 on multiple linear regression.
The neurotransmission mechanism of gustation differs from that of olfaction. Stinton et al. demonstrated that olfactory dysfunction did not significantly influence any measure of taste perception when the effects of sex, age, and aetiology were controlled. These data suggest that smell loss does not meaningfully influence taste function and any clinical associations observed between smell and taste dysfunction likely reflect comorbid influences24. Notably, Othieno et al. found that taste scores in patients with CRS were not related to olfactory scores or the severity of CRS25. Ahn26 classified CRS into eosinophilic CRS (ECRS) and non-eosinophilic CRS (NCRS) groups and found that taste disorders were present in 9.3% and 11.9% of patients with ECRS and NCRS, respectively, which was lower than the 28% prevalence reported by Othieno et al. In this study, the proportion of normogeusia was 58% in the SEs sensitisation group and 69% in the non-sensitisation group; however, statistical significance was not observed owing to the small number of SE-sensitisation groups. In this study, the proportion of patients with dysgeusia was 41.9% in the SEA/SEB-positive group and 30.7% in the SEA-negative group. Although there were no significant differences in the proportions of the normogeusia/hypogeusia/ageusia groups, the total taste function test scores were significantly lower in the SEA/SEB-positive group than in the SEB-negative group. Notably, the smoking rate was approximately two times higher in the SEA/SEB-positive group (44.2%) than that in the SEA/SEB-negative group, which likely influenced the taste test results. In multiple linear regression analysis of taste function test scores, significant differences were reported only for age, sex, and current smoking status (Supplementary Table 3).
Nasal inflammation induced by fungal, viral, or bacterial agents affects the morphological and physiological characteristics of the rodent olfactory bulb. Although the mechanisms by which these various microbial agents induce inflammation in the olfactory mucosa involve different receptors and signalling pathways, they have similar effects on the olfactory bulb. These effects include atrophy, characterized by thinning of superficial olfactory bulb layers, activation of glial cells, and upregulation of inflammatory cytokines. Herbert et al. investigated the profile of damaged olfactory tissues in response to bacterial challenges, tracking the infiltration of bacteria into the olfactory bulb in C57BL/6 mice27. They demonstrated that bacteria could penetrate the immunological defences of the compromised olfactory mucosa and infiltrate the olfactory bulb within 6 h27. Han et al. explored the interactions among nasal microbiota, metabolites, and the immune system and their roles in the pathogenesis of olfactory dysfunction in patients with CRS28. They found dysregulation of the nasal microbiota, differential metabolites, and elevated inflammatory mediators in patients with olfactory dysfunction28. Although SARS-CoV-2 may be capable of directly entering the central nervous system via the olfactory route or through mechanisms that degrade the blood-brain barrier integrity, whether SARS-CoV-2 can directly infiltrate the CNS through the olfactory system remains unclear29. Many variables in the deteriorating process and mechanisms of olfactory dysfunction are not well understood. Our study suggests that the infiltration of S. aureus into nasal tissue leads to cytokine upregulation, which can affect the olfactory mucosa and exacerbate olfactory disorders. However, whether the bacterial biofilm formed by S. aureus directly influences inflammation in the olfactory bulb, thereby affecting the sense of smell, remains unclear.
This study has few limitations. First, as a retrospective study; therefore, there was insufficient clinical information available, such as blood levels of essential minerals (e.g. zinc) and vitamins (e.g. vitamins A and B12), which are known to influence olfactory and taste functions. Second, we only categorised the CRS group into the CRSwNP and CRSsNP groups; therefore, the differences in olfactory dysfunction according to CRS endotyping should be further evaluated. Third, the biofilm-producing capacity of S. aureus is well-known. The potential role of SE sensitization and eosinophil percentage in deteriorating olfaction in patients with CRSwNP should be further studied to demonstrate the direct effect of SE-induced biofilm on the olfactory nerve system in vitro.
Conclusion
Sinusitis patients seem to suffer from perceiving odours, and patients with CRSwNP have difficulty distinguishing odours. Olfactory function test scores decreased in patients with a history of asthma, and as serum levels of SE and blood eosinophil percentage increase. This suggests a potential role for SE sensitisation and eosinophil percentage in deteriorating olfaction, especially in patients with CRSwNP. As the SE-sensitised CRS endotype is currently being elucidated, our findings on SE sensitisation and olfactory function in CRS will be useful to clinicians. Moreover, evaluation of SE sensitisation levels could potentially predict olfactory dysfunction in patients with CRS.
Materials and methods
Study design and patient enrolment
We retrospectively reviewed the medical records of 388 patients diagnosed with and treated for septal deviation or CRS between January 2021 and June 2023 at the Yonsei University Wonju College of Medicine, Wonju, South Korea. The baseline assessments of the study included history-taking, complete blood count test (CBC), multiple allergy simultaneous tests (MAST), and ImmunoCAP® for measuring serum total IgE levels and specific IgE levels to SE A and B (SEA/SEB). Olfactory and taste function tests were performed before surgery. Among patients who underwent endoscopic sinus surgery, those pathologically diagnosed with nasal polyps were classified as having chronic rhinosinusitis with nasal polyps (CRSwNP), while those with reports of chronic inflammation without nasal polyps were classified as having chronic rhinosinusitis without nasal polyps (CRSsNP). The exclusion criteria included pathological findings of a mucocele, retention cyst, osteoma, inverted papilloma, and fungal ball. Patients who underwent septoplasty without endoscopic sinus surgery formed the healthy control group. The final study cohort comprised 110 patients with CRSsNP (28.3%), 133 patients with CRSwNP (34.3%), and 145 patients who underwent septoplasty only, forming the healthy control group (37.4%), as shown in Table 1.
Definitions of analysis factors
Diagnosis of CRS is based on subjective symptoms and objective findings from paranasal computed tomography (CT) and nasal endoscopic findings according to the European Position Paper on Rhinosinusitis and Nasal Polyps (EPOS) 2020 guideline1. Asthma was diagnosed through a medical history review and lung function test results. Sensitisation to SEs was defined using specific immunoglobulins E (IgE) levels, with the lower cut-off level for SE sensitisation set at 0.10 kU/L and positive threshold at 0.35 kU/L, based on manufacturer recommendations and supported by finding from a previous study30.
Olfactory function test
The olfactory function test comprised of the Korean Version of Sniffin’ Stick. The olfactory threshold, odour discrimination, and odour identification tests were performed sequentially with three minutes interval between each test. The threshold was defined as the concentration at which 2-phenylethyl alcohol (highest concentration 10%, 1:2 serial dilutions in 12 steps) reached the average score of the last 4 turning points out of 7. With respect to the distinction test, triplets of odorants (two identical and one different) were presented, and the subjects were asked to choose the odd odorant. The identification test involved 12 odours familiar to Koreans. The sums of the three tests were presented as Threshold-Discrimination-Identification (TDI) score as the olfactory score. Based on a previous study, the severity of olfactory dysfunction was classified as follows: anosmia, olfactory score ≤ 14.5; hyposmia, olfactory score > 14.5 and ≤ 21.0; and nomosmia, olfactory score > 21.031.
Taste function test
Using the recently developed YSK taste function test kit (RHICO Medical Co., Seoul, Republic of Korea), we measured the detection and recognition threshold scores for five tastants (sweet, salty, bitter, sour, and umami) according to protocols established in a previous study32. We then calculated the overall gustatory function by summing the number of detected and correctly recognised taste thresholds, which were collectively expressed as the ‘taste score’, ranging from 0 to 30. Following the criteria defined by Hwang et al., patients with recognition taste scores < 12 were considered to have hypogeusia. Ageusia was defined as a recognition taste score < 5 and dysgeusia, including both hypogeusia and ageusia.
Statistical analysis
Various statistical tests were used to analyse the data, which were managed using Microsoft Excel and analysed using SPSS version 25.0. The Chi-square test was used to assess the relationship between categorical variables and the paired t-test was used to analyse continuous variables related to SE sensitisation. One-way ANOVA was used to compare means across different sinusitis phenotypes, and single and multiple linear regression analyses were conducted to examine the effects of sinusitis phenotypes, age, sex, smoking history, asthma history, and levels of serum total IgE, serum enterotoxin, neutrophils, and eosinophils on olfactory function. Continuous data are presented as mean ± standard deviation, while categorical data are presented as percentage of the group total. This comprehensive approach ensured a robust data analysis to understand the various factors affecting the study outcomes.
Ethical considerations
This study was approved by the Institutional Review Board of Wonju Severance Christian Hospital, Wonju, Republic of Korea (IRB No. CR323131), in accordance with the Declaration of Helsinki. The informed consent was obtained from all participants and/or their legal guardians.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
E.J.L. and M.K. designed the study, managed the data extraction, and interpreted the data. M.K. and E.J.L. performed statistical analysis and scientific interpretation. M.K. and E.J.L. wrote the manuscript. H.S.C. and D.H.K. formatted the manuscript. H.S.C., D.H.K., E.K.J., and Y.-H.L. interpreted the data and reviewed the manuscript. E.J.L. initiated and supervised the study. All authors read and approved the final manuscript.
Funding
This research was supported by "Regional Innovation Strategy (RIS)" through the National Research Foundation of Korea (NRF) funded by the Ministry of Education(MOE) in 2024 (2022RIS-005).
Data availability
The datasets used and analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-77459-7.
References
- 1.Fokkens, W. J. et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology58 (Suppl S29), 1–464 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Kim, Y. S. et al. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am. J. Rhinol Allergy25 (3), 117–121. 10.2500/ajra.2011.25.3630 (2011). [DOI] [PubMed] [Google Scholar]
- 3.We, J. et al. Prevalence of nasal polyps and its risk factors: Korean National Health and Nutrition Examination survey 2009–2011. Am. J. Rhinol. Allergy29 (1), e24–e28. 10.2500/ajra.2015.29.4131 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Reden, J. et al. A study on the prognostic significance of qualitative olfactory dysfunction. Eur. Arch. Otorhinolaryngol.264 (2), 139–144. 10.1007/s00405-006-0157-0 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Kohli, P. et al. The prevalence of olfactory dysfunction in chronic rhinosinusitis. Laryngoscope127 (2), 309–320. 10.1002/lary.26316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litvack, J. R., Mace, J. C. & Smith, T. L. Olfactory function and disease severity in chronic rhinosinusitis. Am. J. Rhinol Allergy23 (2), 139–144. 10.2500/ajra.2009.23.3286 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qureshi, H. A. & Lane, A. P. Olfaction now and in the future in CRSwNP. Am. J. Rhinol Allergy37 (2), 168–174. 10.1177/19458924231153485 (2023). [DOI] [PubMed] [Google Scholar]
- 8.Fraser, J. D. & Proft, T. The bacterial superantigen and superantigen-like proteins. Immunol. Rev.225, 226–243. 10.1111/j.1600-065X.2008.00681.x (2008). [DOI] [PubMed] [Google Scholar]
- 9.Huvenne, W., Hellings, P. W. & Bachert, C. Role of staphylococcal superantigens in airway disease. Int. Arch. Allergy Immunol.161 (4), 304–314. 10.1159/000350329 (2013). [DOI] [PubMed] [Google Scholar]
- 10.Rha, M. S. et al. Superantigen-related TH2 CD4 + T cells in nonasthmatic chronic rhinosinusitis with nasal polyps. J. Allergy Clin. Immunol.145 (5), 1378–1388e10. 10.1016/j.jaci.2019.12.915 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Bachert, C. & Akdis, C. A. Phenotypes and emerging endotypes of chronic rhinosinusitis. J. Allergy Clin. Immunol. Pract.4 (4), 621–628. 10.1016/j.jaip.2016.05.004 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Wang, M. et al. Association of periostin expression with eosinophilic inflammation in nasal polyps. J. Allergy Clin. Immunol.136 (6), 1700–1703e9. 10.1016/j.jaci.2015.09.005 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Kato, A. Immunopathology of chronic rhinosinusitis. Allergol. Int.64 (2), 121–130. 10.1016/j.alit.2014.12.006 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi, J. Y. et al. Current status of asthma care in South Korea: nationwide the Health Insurance Review and Assessment Service database. J. Thorac. Dis.9 (9), 3208–3214. 10.21037/jtd.2017.08.109 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korea, N. T. C. C. R. Prevalence of tobacco use. https://nosmk.khepi.or.kr/ntcc/eng/subIndex/547.do (2023).
- 16.Lee, J. H. et al. Increased levels of serum-specific immunoglobulin E to staphylococcal enterotoxin a and B in patients with allergic rhinitis and bronchial asthma. Int. Arch. Allergy Immunol.138 (4), 305–311. 10.1159/000088868 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Wiersma, V. R. et al. The decrease in serum sRAGE levels upon smoking is associated with activated neutrophils. Lung200 (6), 687–690. 10.1007/s00408-022-00585-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagasaki, T. et al. Sensitization to Staphylococcus aureus enterotoxins in smokers with asthma. Ann. Allergy Asthma Immunol.119 (5), 408–414e2. 10.1016/j.anai.2017.08.001 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Chéruel, F., Jarlier, M. & Sancho-Garnier, H. Effect of cigarette smoke on gustatory sensitivity, evaluation of the deficit and of the recovery time-course after smoking cessation. Tob. Induc. Dis.15, 15. 10.1186/s12971-017-0120-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fjaeldstad, A. W., Ovesen, T. & Hummel, T. The association between smoking on olfactory dysfunction in 3,900 patients with olfactory loss. Laryngoscope131 (1), E8–E13. 10.1002/lary.28552 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Djordjevic, J. et al. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging29 (5), 693–706. 10.1016/j.neurobiolaging.2006.11.014 (2008). [DOI] [PubMed] [Google Scholar]
- 22.Ehi, Y. & Ozlece, H. K. Electrophysiological assessment of the concentration and attention in patient with nasal polyposis. Asia Pac. Allergy8 (3), e27. 10.5415/apallergy.2018.8.e27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhyou, H. I., Bae, W. Y. & Nam, Y. H. Association between olfactory function and asthma in adults. J. Asthma Allergy14, 309–316. 10.2147/JAA.S299796 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stinton, N. et al. Influence of smell loss on taste function. Behav. Neurosci.124 (2), 256–264. 10.1037/a0018766 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Othieno, F. et al. Taste impairment in chronic rhinosinusitis. Int. Forum Allergy Rhinol.8 (7), 783–789. 10.1002/alr.22113 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn, S. H. et al. Comparison of olfactory and taste functions between eosinophilic and non-eosinophilic chronic rhinosinusitis. Auris Nasus Larynx47 (5), 820–827. 10.1016/j.anl.2020.04.006 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Herbert, R. P. et al. Cytokines and olfactory bulb microglia in response to bacterial challenge in the compromised primary olfactory pathway. J. Neuroinflamm.9, 109. 10.1186/1742-2094-9-109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han, X. et al. Disturbed microbiota-metabolites-immune interaction network is associated with olfactory dysfunction in patients with chronic rhinosinusitis. Front. Immunol.14, 1159112. 10.3389/fimmu.2023.1159112 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaFever, B. J. & Imamura, F. Effects of nasal inflammation on the olfactory bulb. J. Neuroinflammation19 (1), 294. 10.1186/s12974-022-02657-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bachert, C. et al. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J. Allergy Clin. Immunol.130 (2), 376–81e8. 10.1016/j.jaci.2012.05.012 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Ha, J. G. et al. Development of a Korean culture-friendly olfactory function test and optimization of a diagnostic cutoff value. Clin. Exp. Otorhinolaryngol.13 (3), 274–284. 10.21053/ceo.2020.00864 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang, C. S. et al. Development of a gustatory function test for clinical application in Korean subjects. Yonsei Med. J.59 (2), 325–330. 10.3349/ymj.2018.59.2.325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analysed during the current study available from the corresponding author on reasonable request.