Abstract
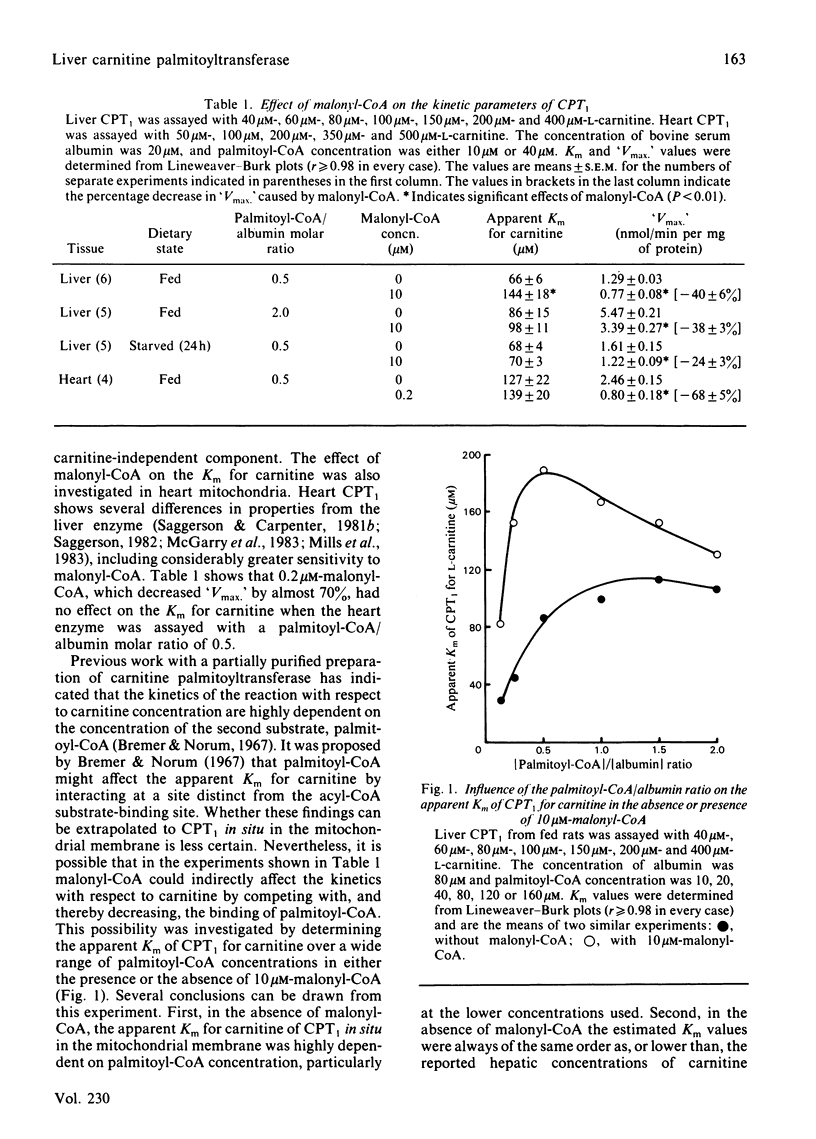
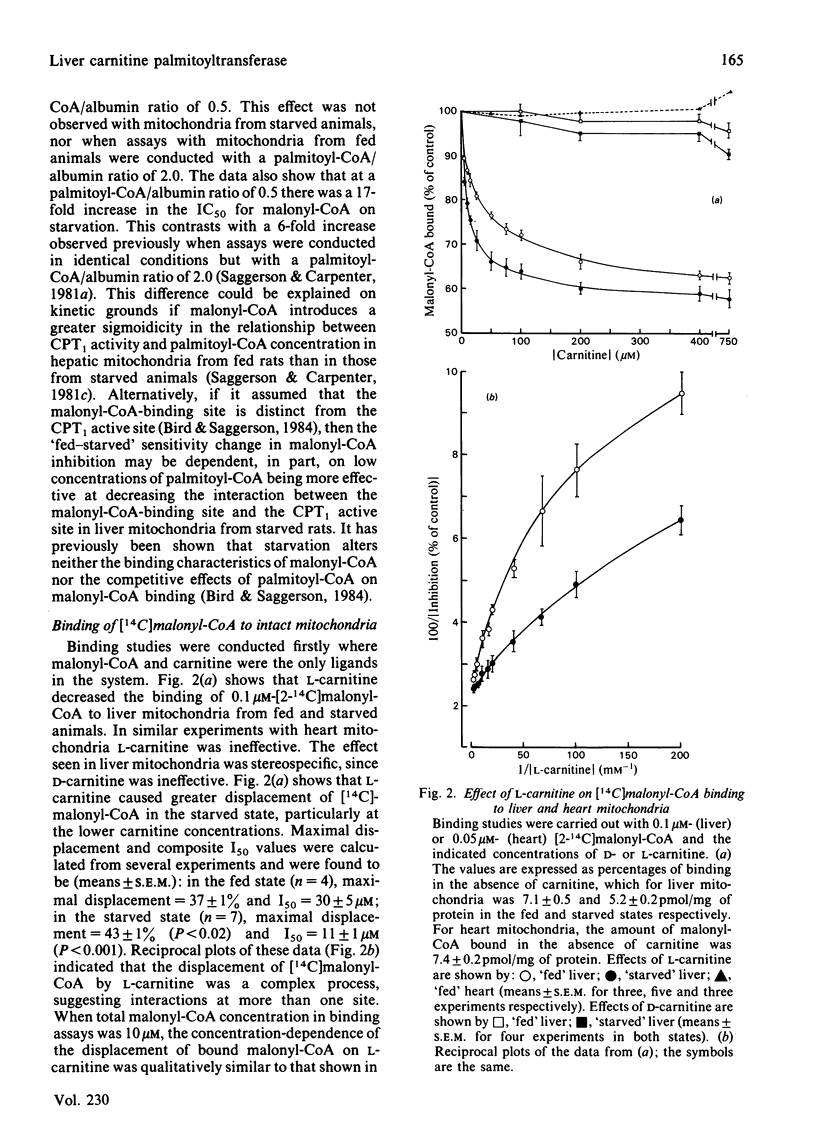
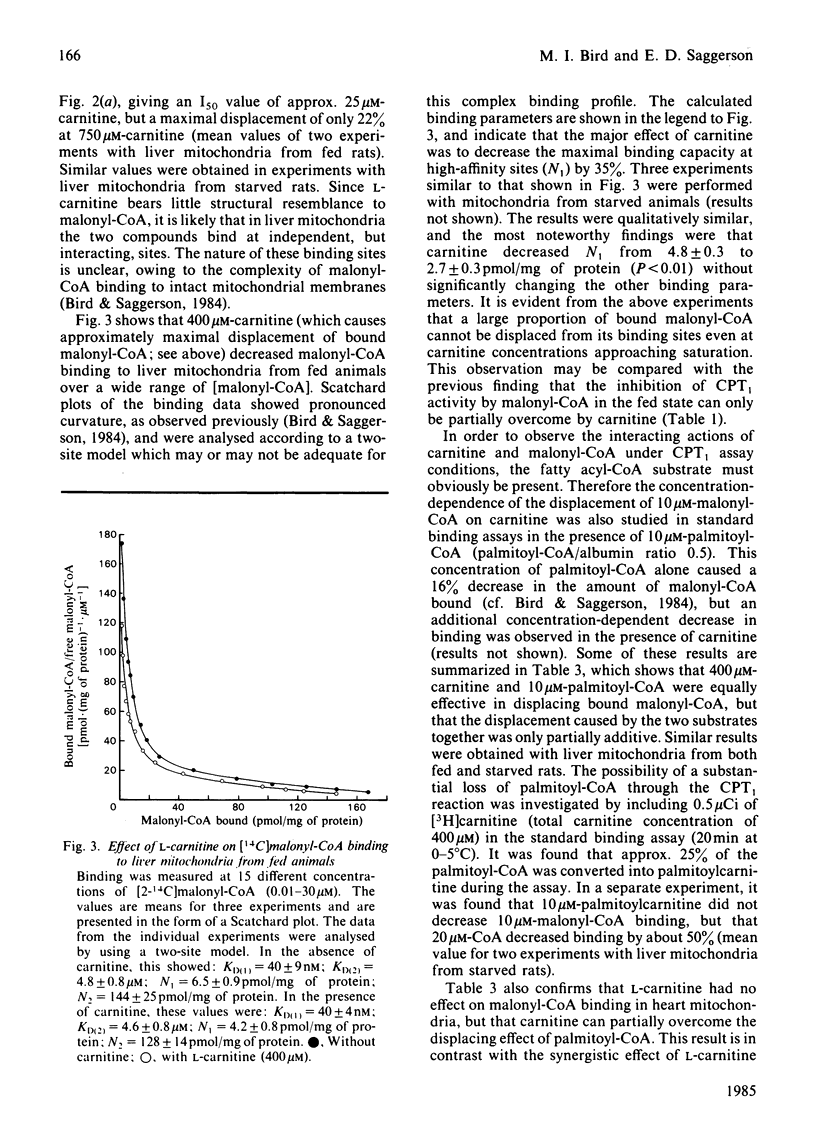
Malonyl-CoA significantly increased the Km for L-carnitine of overt carnitine palmitoyltransferase in liver mitochondria from fed rats. This effect was observed when the molar palmitoyl-CoA/albumin concentration ratio was low (0.125-1.0), but not when it was higher (2.0). In the absence of malonyl-CoA, the Km for L-carnitine increased with increasing palmitoyl-CoA/albumin ratios. Malonyl-CoA did not increase the Km for L-carnitine in liver mitochondria from 24h-starved rats or in heart mitochondria from fed animals. The Km for L-carnitine of the latent form of carnitine palmitoyltransferase was 3-4 times that for the overt form of the enzyme. At low ratios of palmitoyl-CoA/albumin (0.5), the concentration of malonyl-CoA causing a 50% inhibition of overt carnitine palmitoyltransferase activity was decreased by 30% when assays with liver mitochondria from fed rats were performed at 100 microM-instead of 400 microM-carnitine. Such a decrease was not observed with liver mitochondria from starved animals. L-Carnitine displaced [14C]malonyl-CoA from liver mitochondrial binding sites. D-Carnitine was without effect. L-Carnitine did not displace [14C]malonyl-CoA from heart mitochondria. It is concluded that, under appropriate conditions, malonyl-CoA may decrease the effectiveness of L-carnitine as a substrate for the enzyme and that L-carnitine may decrease the effectiveness of malonyl-CoA to regulate the enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bird M. I., Munday L. A., Saggerson E. D., Clark J. B. Carnitine acyltransferase activities in rat brain mitochondria. Bimodal distribution, kinetic constants, regulation by malonyl-CoA and developmental pattern. Biochem J. 1985 Feb 15;226(1):323–330. doi: 10.1042/bj2260323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Binding of malonyl-CoA to isolated mitochondria. Evidence for high- and low-affinity sites in liver and heart and relationship to inhibition of carnitine palmitoyltransferase activity. Biochem J. 1984 Sep 15;222(3):639–647. doi: 10.1042/bj2220639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J., Norum K. R. The mechanism of substrate inhibition of palmityl coenzyme A:carnitine palmityltransferase by palmityl coenzyme A. J Biol Chem. 1967 Apr 25;242(8):1744–1748. [PubMed] [Google Scholar]
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Edwards M. R., Bird M. I., Saggerson E. D. Effects of DL-2-bromopalmitoyl-CoA and bromoacetyl-CoA in rat liver and heart mitochondria. Inhibition of carnitine palmitoyltransferase and displacement of [14C]malonyl-CoA from mitochondrial binding sites. Biochem J. 1985 Aug 15;230(1):169–179. doi: 10.1042/bj2300169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Long C. S., Haller R. G., Foster D. W., McGarry J. D. Kinetics of carnitine-dependent fatty acid oxidation: implications for human carnitine deficiency. Neurology. 1982 Jun;32(6):663–666. doi: 10.1212/wnl.32.6.663. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mannaerts G. P., Foster D. W. A possible role for malonyl-CoA in the regulation of hepatic fatty acid oxidation and ketogenesis. J Clin Invest. 1977 Jul;60(1):265–270. doi: 10.1172/JCI108764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Robles-Valdes C., Foster D. W. Role of carnitine in hepatic ketogenesis. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4385–4388. doi: 10.1073/pnas.72.11.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Interaction of malonyl-CoA and related compounds with mitochondria from different rat tissues. Relationship between ligand binding and inhibition of carnitine palmitoyltransferase I. Biochem J. 1983 Jul 15;214(1):83–91. doi: 10.1042/bj2140083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvin R., Pande S. V. Enhancement of mitochondrial carnitine and carnitine acylcarnitine translocase-mediated transport of fatty acids into liver mitochondria under ketogenic conditions. J Biol Chem. 1979 Jun 25;254(12):5423–5429. [PubMed] [Google Scholar]
- Saggerson E. D. Carnitine acyltransferase activities in rat liver and heart measured with palmitoyl-CoA and octanoyl-CoA. Latency, effects of K+, bivalent metal ions and malonyl-CoA. Biochem J. 1982 Feb 15;202(2):397–405. doi: 10.1042/bj2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A. Response to starvation of hepatic carnitine palmitoyltransferase activity and its regulation by malonyl-CoA. Sex differences and effects of pregnancy. Biochem J. 1982 Dec 15;208(3):673–678. doi: 10.1042/bj2080673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson E. D., Carpenter C. A., Tselentis B. S. Effects of thyroidectomy and starvation on the activity and properties of hepatic carnitine palmitoyltransferase. Biochem J. 1982 Dec 15;208(3):667–672. doi: 10.1042/bj2080667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veerkamp J. H., Van Moerkerk H. T. The effect of malonyl-CoA on fatty acid oxidation in rat muscle and liver mitochondria. Biochim Biophys Acta. 1982 Feb 15;710(2):252–255. doi: 10.1016/0005-2760(82)90157-6. [DOI] [PubMed] [Google Scholar]


