Abstract
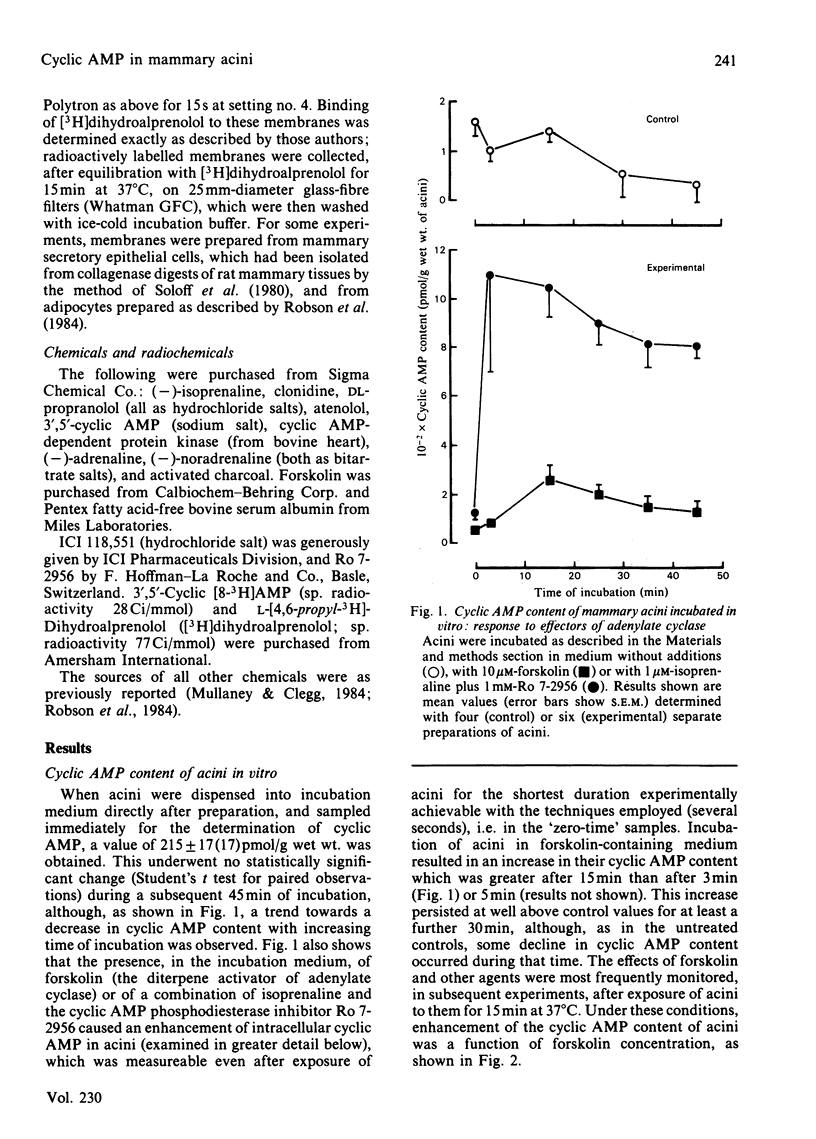
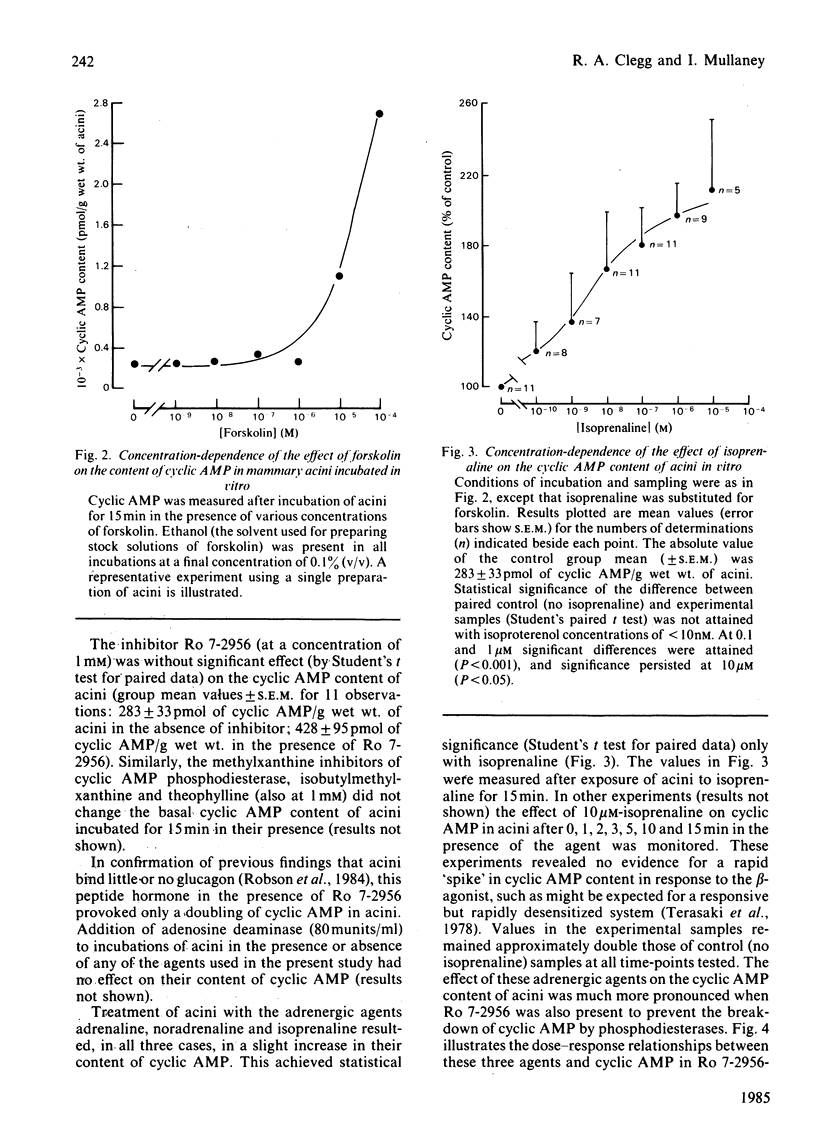
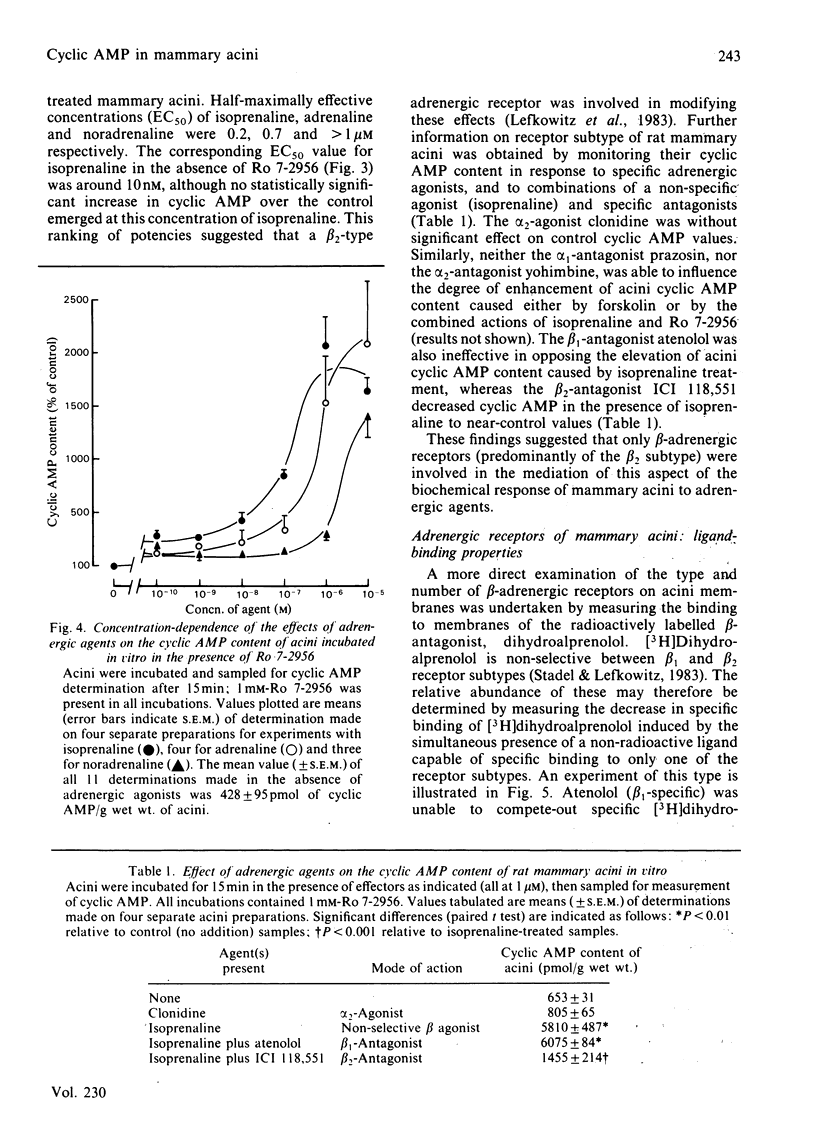
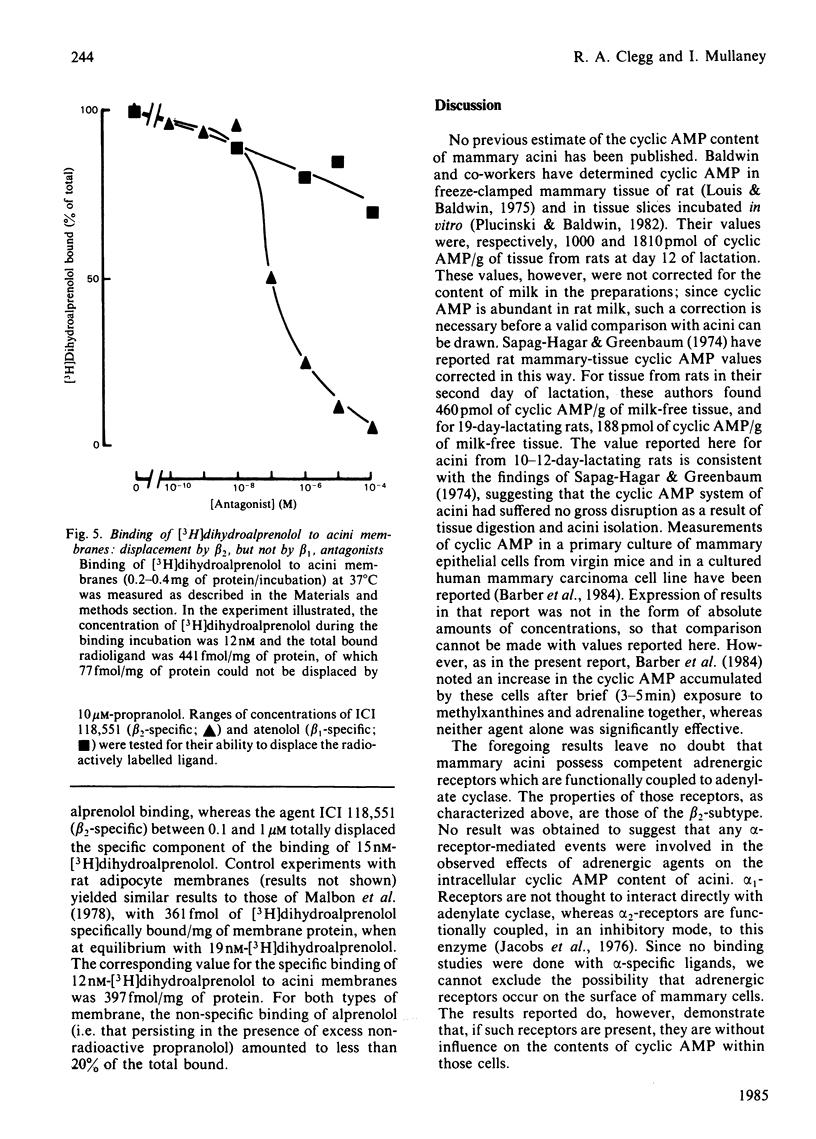
The cyclic AMP content of acini, freshly prepared from mammary tissue of lactating rats, was measured during incubation in vitro. Neither adrenergic agonists nor cyclic AMP phosphodiesterase inhibitors alone caused a change of more than 2-fold in the basal cyclic AMP content of acini. Together, however, these agents provoked increases of around 20-fold in acini cyclic AMP content. Forskolin caused similar effects. The relative potency of adrenergic agonists in increasing cyclic AMP in acini, together with the ability of selective antagonists to oppose such rises, indicated that beta 2-adrenergic receptors were involved in mediating the effects. Receptor-binding experiments using [3H]dihydroalprenolol and selective beta-antagonists confirmed the predominant presence of beta 2-adrenergic receptors on acini membranes and on membranes prepared from purified mammary secretory epithelial cells. These results elucidate some previous findings [Robson, Clegg & Zammit (1984) Biochem. J. 217, 743-749; Williamson, Munday, Jones, Roberts & Ramsey (1983) Adv. Enzyme Regul. 21, 135-145], questioning the role of cyclic AMP in the regulation of lipogenesis in mammary acini.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitchison R., West D. W., Clegg R. A. Insulin-stimulated high affinity cyclic AMP phosphodiesterase in rat mammary acini. FEBS Lett. 1984 Feb 13;167(1):25–28. doi: 10.1016/0014-5793(84)80825-x. [DOI] [PubMed] [Google Scholar]
- Barber R., Goka T. J., Butcher R. W. Hormone and methylxanthine action on breast epithelial cells. Life Sci. 1984 Jun 18;34(25):2467–2476. doi: 10.1016/0024-3205(84)90283-2. [DOI] [PubMed] [Google Scholar]
- Bussmann L. E., Ward S., Kuhn N. J. Lactose and fatty acid synthesis in lactating-rat mammary gland. Effects of starvation, re-feeding, and administration of insulin, adrenaline, streptozotocin and 2-bromo-alpha-ergocryptine. Biochem J. 1984 Apr 1;219(1):173–180. doi: 10.1042/bj2190173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E., Sutherland E. W. Effects of lipolytic and antilipolytic substances on adenosine 3',5'-monophosphate levels in isolated fat cells. J Biol Chem. 1968 Apr 25;243(8):1705–1712. [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie D. G., Guy P. S. Reversible phosphorylation and inactivation of acetyl-CoA carboxylase from lactating rat mammary gland by cyclic AMP-dependent protein kinase. Eur J Biochem. 1980 Sep;110(1):167–177. doi: 10.1111/j.1432-1033.1980.tb04852.x. [DOI] [PubMed] [Google Scholar]
- Jakobs K. H., Saur W., Schultz G. Reduction of adenylate cyclase activity in lysates of human platelets by the alpha-adrenergic component of epinephrine. J Cyclic Nucleotide Res. 1976 Nov-Dec;2(6):381–392. [PubMed] [Google Scholar]
- Kraehenbuhl J. P. Dispersed mammary gland epithelial cells. I. Isolation and separation procedures. J Cell Biol. 1977 Feb;72(2):390–405. doi: 10.1083/jcb.72.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J., Stadel J. M., Caron M. G. Adenylate cyclase-coupled beta-adrenergic receptors: structure and mechanisms of activation and desensitization. Annu Rev Biochem. 1983;52:159–186. doi: 10.1146/annurev.bi.52.070183.001111. [DOI] [PubMed] [Google Scholar]
- Loizzi R. F. Cylic AMP inhibition of mammary gland lactose synthesis: specificity and potentiation by 1-methyl-3-isobutylxanthine. Horm Metab Res. 1978 Sep;10(5):415–419. doi: 10.1055/s-0028-1093404. [DOI] [PubMed] [Google Scholar]
- Loten E. G., Assimacopoulos-Jeannet F. D., Exton J. H., Park C. R. Stimulation of a low Km phosphodiesterase from liver by insulin and glucagon. J Biol Chem. 1978 Feb 10;253(3):746–757. [PubMed] [Google Scholar]
- Louis S. L., Baldwin R. L. Changes in the cyclic 3', 5'-adenosine monophosphate system of rat mammary gland during lactation cycle. J Dairy Sci. 1975 Jun;58(6):861–869. doi: 10.3168/jds.S0022-0302(75)84650-9. [DOI] [PubMed] [Google Scholar]
- Malbon C. C., Moreno F. J., Cabelli R. J., Fain J. N. Fat cell adenylate cyclase and beta-adrenergic receptors in altered thyroid states. J Biol Chem. 1978 Feb 10;253(3):671–678. [PubMed] [Google Scholar]
- Manganiello V., Vaughan M. An effect of insulin on cyclic adenosine 3':5'-monophosphate phosphodiesterase activity in fat cells. J Biol Chem. 1973 Oct 25;248(20):7164–7170. [PubMed] [Google Scholar]
- McNeillie E. M., Clegg R. A., Zammit V. A. Regulation of acetyl-CoA carboxylase in rat mammary gland. Effects of incubation with Ca2+, Mg2+ and ATP on enzyme activity in tissue extracts. Biochem J. 1981 Dec 15;200(3):639–644. doi: 10.1042/bj2000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeillie E. M., Zammit V. A. Regulation of acetyl-CoA carboxylase in rat mammary gland. Effects of starvation and of insulin and prolactin deficiency on the fraction of the enzyme in the active form in vivo. Biochem J. 1982 Apr 15;204(1):273–280. doi: 10.1042/bj2040273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullaney I., Clegg R. A. Cyclic AMP phosphodiesterase and cyclic GMP phosphodiesterase activities of rat mammary tissue. Biochem J. 1984 May 1;219(3):801–809. doi: 10.1042/bj2190801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munday M. R., Hardie D. G. Isolation of three cyclic-AMP-independent acetyl-CoA carboxylase kinases from lactating rat mammary gland and characterization of their effects on enzyme activity. Eur J Biochem. 1984 Jun 15;141(3):617–627. doi: 10.1111/j.1432-1033.1984.tb08237.x. [DOI] [PubMed] [Google Scholar]
- Munday M. R., Williamson D. H. Role of pyruvate dehydrogenase and insulin in the regulation of lipogenesis in the lactating mammary gland of the rat during the starved-refed transition. Biochem J. 1981 Jun 15;196(3):831–837. doi: 10.1042/bj1960831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plucinski T. M., Baldwin R. L. Effects of hormones on mammary adenosine 3',5'-monophosphate levels and metabolism in normal and adrenalectomized lactating rats. Endocrinology. 1982 Dec;111(6):2062–2065. doi: 10.1210/endo-111-6-2062. [DOI] [PubMed] [Google Scholar]
- Robinson A. M., Girard J. R., Williamson D. H. Evidence for a role of insulin in the regulation of lipogenesis in lactating rat mammary gland. Measurements of lipogenesis in vivo and plasma hormone concentrations in response to starvation and refeeding. Biochem J. 1978 Oct 15;176(1):343–346. doi: 10.1042/bj1760343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson N. A., Clegg R. A., Zammit V. A. Regulation of peripheral lipogenesis by glucagon. Inability of the hormone to inhibit lipogenesis in rat mammary acini in vitro in the presence or absence of agents which alter its effects on adipocytes. Biochem J. 1984 Feb 1;217(3):743–749. doi: 10.1042/bj2170743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapag-Hagar M., Greenbaum A. L. Adenosine 3':5'-monophosphate and hormone interrelationships in the mammary gland of the rat during pregnancy and lactation. Eur J Biochem. 1974 Sep 1;47(2):303–312. doi: 10.1111/j.1432-1033.1974.tb03694.x. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Shechter Y. Differential effects of two phosphodiesterase inhibitors on fat cell metabolism. Endocrinology. 1984 Nov;115(5):1787–1791. doi: 10.1210/endo-115-5-1787. [DOI] [PubMed] [Google Scholar]
- Soloff M. S., Chakraborty J., Sadhukhan P., Senitzer D., Wieder M., Fernstrom M. A., Sweet P. Purification and characterization of mammary myoepithelial and secretory cells from the lactating rat. Endocrinology. 1980 Mar;106(3):887–897. doi: 10.1210/endo-106-3-887. [DOI] [PubMed] [Google Scholar]
- Terasaki W. L., Brooker G., de Vellis J., Inglish D., Hsu C. Y., Moylan R. D. Involvement of cyclic amp and protein synthesis in catecholamine refractoriness. Adv Cyclic Nucleotide Res. 1978;9:33–52. [PubMed] [Google Scholar]
- Tovey K. C., Oldham K. G., Whelan J. A. A simple direct assay for cyclic AMP in plasma and other biological samples using an improved competitive protein binding technique. Clin Chim Acta. 1974 Nov 8;56(3):221–234. doi: 10.1016/0009-8981(74)90133-8. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Kuhn N. J. Lactose synthesis and the utilisation of glucose by rat mammary acini. Int J Biochem. 1981;13(3):311–316. doi: 10.1016/0020-711x(81)90083-5. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Munday M. R., Jones R. G., Roberts A. F., Ramsey A. J. Short-term dietary regulation of lipogenesis in the lactating mammary gland of the rat. Adv Enzyme Regul. 1983;21:135–145. doi: 10.1016/0065-2571(83)90012-2. [DOI] [PubMed] [Google Scholar]
- Wilson C., Wilson S., Piercy V., Sennitt M. V., Arch J. R. The rat lipolytic beta-adrenoceptor: studies using novel beta-adrenoceptor agonists. Eur J Pharmacol. 1984 May 4;100(3-4):309–319. doi: 10.1016/0014-2999(84)90007-4. [DOI] [PubMed] [Google Scholar]
- de Vente J., Bast A., Van Bree L., Zaagsma J. beta-Adrenoceptor studies. 6. Further investigations on the hybrid nature of the rat adipocyte beta-adrenoceptor. Eur J Pharmacol. 1980 Apr 11;63(1):73–83. doi: 10.1016/0014-2999(80)90118-1. [DOI] [PubMed] [Google Scholar]


