Abstract
Patient: Female, 68-year-old
Final Diagnosis: Renal infarction
Symptoms: Abdominal pain • nausea • urinary frequency • vomiting
Clinical Procedure: —
Specialty: General and Internal Medicine
Objective:
Unknown etiology
Background:
Incidental findings of renal infarct secondary to thrombosis in acutely ill patients present a unique challenge in diagnosis. We present a case of idiopathic renal infarct to highlight its workup and management and encourage further investigation of renal infarctions.
Case Report:
A 68-year-old woman with a past medical history of diet-controlled diabetes, hypertension, and hyperlipidemia presented to the Emergency Department (ED) for abdominal pain. She was found to be in diabetic ketoacidosis with pyelonephritis, so she was admitted to the Intensive Care Unit (ICU) for insulin and dextrose drip. Due to her abdominal pain, she underwent computed tomography (CT) of her abdomen and pelvis with contrast. This revealed multifocal infarcts of her right kidney with noncalcified thrombus at the proximal right renal artery. Subsequent CT angiography confirmed a right renal artery thrombus. She was started on subcutaneous enoxaparin and downgraded to basic level of care. Her history was negative for prior thrombosis, hypercoagulable state, and abdominal trauma. Echocardiogram and limited hypercoagulable workup were largely unremarkable. A multidisciplinary team evaluated the patient and recommended no surgical intervention. Following downgrade from the ICU, the patient was transitioned from enoxaparin to apixaban. She was discharged with plans for anticoagulation for 6 months, aspirin daily, and repeat CT angiogram abdomen/pelvis in 1 month.
Conclusions:
This case illustrates the difficulties in elucidating the cause of incidental renal thrombosis in an acutely ill patient. Diagnostic workup is limited in the inpatient setting, but therapeutic anticoagulation remains the standard of treatment regardless of etiology.
Key words: Diabetes Mellitus, Infarction, Ischemia, Renal Artery
Introduction
The mechanisms of renal infarction are divided into 3 major categories: embolic, thrombophilic, and idiopathic. Embolic causes can be caused by atrial fibrillation, endocarditis, or atheromatous disease, while thrombophilic causes can include antiphospholipid syndrome, sickle cell disease, cocaine use, trauma, neoplasms, or other vascular causes [1]. Previous research has estimated that approximately 45% of cases are due to embolic causes and 25% are due to thrombophilic causes [2]. However, due to the nonspecific clinical presentation of this pathology, which can include diffuse abdominal, back and/or flank pain, hematuria, or even hematochezia, a large percentage of these infarcts (23–30%) are attributed to idiopathic causes [2,3].
The prevalence of renal infarcts is typically estimated to be 1.4% [4], while some recent studies have estimated a renal infarction incidence rate of 0.004% in the ED [5]. The small sample size (<100) of other retrospective studies [6,7] continues to reflect the overall rarity of renal infarctions. No comprehensive guidelines exist for the diagnosis and treatment of idiopathic renal infarcts; however, diagnosis typically includes comprehensive bloodwork, electrocardiogram, and contrast-enhanced imaging (either CT or MRI) [8]. Management depends on time since infarction, but usually involves revascularization and/or anticoagulation [9]. Early recognition is important, as delays in diagnosis can lead to acute kidney injury (AKI) and chronic kidney disease (CKD).
Classic imaging findings will reveal “wedge-shaped focal infarcts” [10], with variations on cortical enhancements; however, in the absence of these findings, the diagnosis can be especially challenging. Furthermore, other reports proposed that elevated lactate dehydrogenase (LDH), hematuria, and leukocytosis in a urinalysis may serve as markers for renal infarction [7]. However, this clinical triad can also be observed in conditions such as autoimmune hemolytic anemia (AIHA), pyelonephritis, and renal stones. Although a marked elevation in LDH possesses higher specificity for renal infarction in the setting of elevated pretest probability, its overall specificity is low, with elevations demonstrated in many hemolytic or ischemic processes [7,11]. Thus, these laboratory studies fail to provide specific markers for diagnosis.
Current research continues to attempt to elucidate the mechanisms underscoring the “idiopathic” category, offering suggestions such as viral illnesses, genetic mutations, including vascular or connective tissue pathologies, or iatrogenic injuries [12]. However, both definitive diagnosis and conclusive explanations remain elusive. Considering these prior investigations, our case report describes a rare case of renal infarction. We do so to highlight the difficulties in the workup for idiopathic renal infarction and encourage further investigation of renal infarctions.
Case Report
A 68-year-old woman with a past medical history of diet-controlled diabetes, hypertension, and hyperlipidemia presented to the ED with a 2-day course of worsening generalized abdominal pain radiating to her back, associated with nausea and vomiting, and urinary frequency without dysuria. She denied any abdominal trauma, previous kidney infarct, prior history of thrombosis, hypercoagulable state, atrial fibrillation, or initiation of any new medications. The patient was afebrile (36.8°C) and hypertensive (151/75 mmHg), with a normal heart rate (76) in sinus rhythm, normal respiratory rate (18), and appropriate oxygen saturation on room air. Her physical exam demonstrated abdominal tenderness and right-sided costovertebral angle tenderness. Blood test results showed glucose 541, beta-hydroxybutyrate 6.041, venous pH 7.28, creatinine 1.2, eGFR 47.93, and hemoglobin A1C 13.4. A urinalysis showed >1000 mg/dL glucose, 150 mg/dL ketones, large leukocyte esterase, 270 white blood cells/hpf, 4 red blood cells/hpf, 2+ bacteria/hpf, and negative nitrite. Due her diabetic ketoacidosis and pyelonephritis, she was admitted to ICU for insulin drip, intravenous fluids, and antibiotics.
Due to her abdominal pain radiating to her back, she underwent CT abdomen and pelvis with contrast, which revealed multifocal infarcts of her right kidney with noncalcified thrombus at the proximal right renal artery (Figure 1). Further evaluation via CT angiography confirmed a thrombus in the right renal artery and ischemic changes in the right kidney (Figure 2). She was started on therapeutic enoxaparin and downgraded to basic level of care after resolution of the diabetic ketoacidosis. Echocardiography to determine the source of the embolus was unremarkable. Limited hypercoagulable workup was completed, including negative factor V Leiden mutation and normal protein C activity, cardiolipin antibody, prothrombin G20210A mutation, ANA screen, ANCA screen, myeloperoxidase antibody, beta-2 glycoprotein 1 antibodies, antithrombin III activity, homocysteine, and lupus anticoagulant. Of note, protein S activity was low, at 46% of normal. Interventional Radiology and Vascular Surgery evaluated the patient and recommended no surgical intervention.
Figure 1.
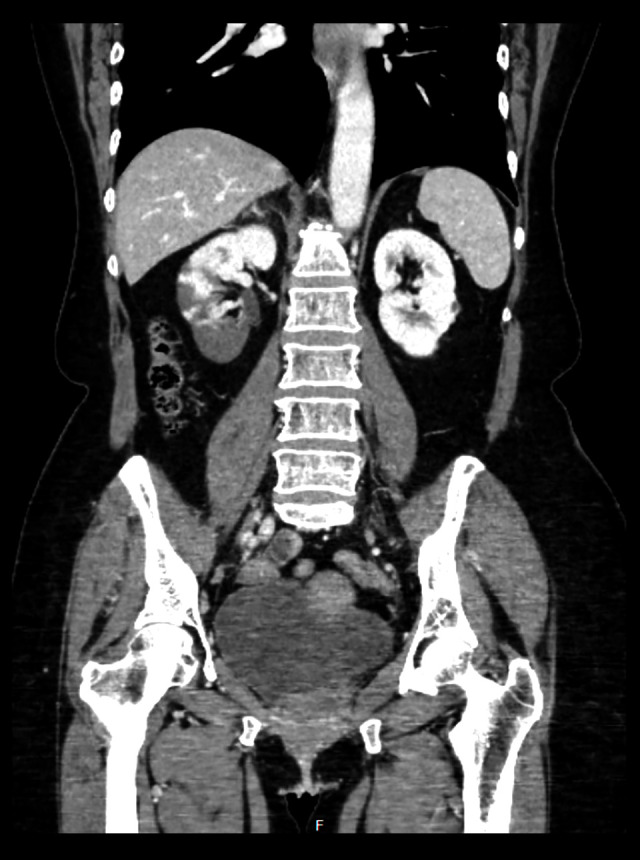
CT abdomen/pelvis showing ischemic changes in right kidney.
Figure 2.
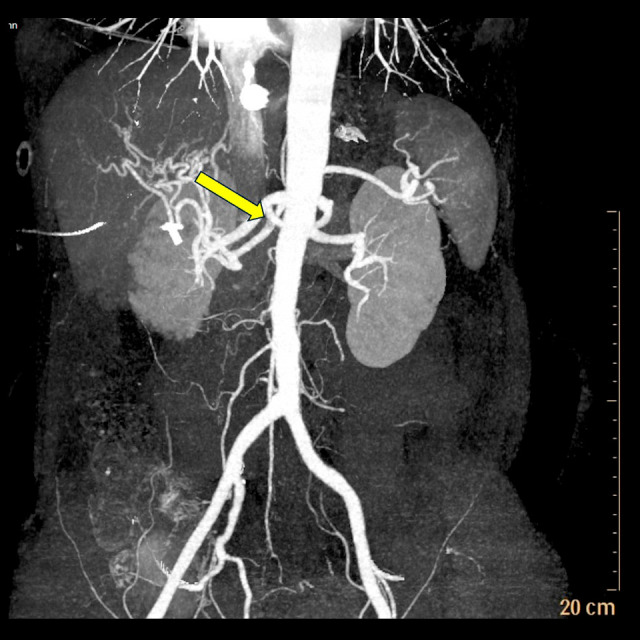
CT angiogram abdomen/pelvis showing evidence of thrombus in right renal artery.
The patient was then transitioned from enoxaparin to apixaban. Due to the idiopathic nature of her arterial thrombus and her risk factors, including diabetes mellitus, hypertension, and hyperlipidemia, she was discharged with plans for empiric anticoagulation for 6 months, aspirin daily, and repeat CT angiogram abdomen/pelvis (A/P) in 1 month. The patient was from out of state and was lost to follow-up.
Discussion
Renal infarctions remain exceedingly rare in the clinical setting, with estimated incidence rates of 0.004–0.01% in hospitalized patients [13]. The major causes of renal infarction are embolic, which are typically attributed to cardiovascular pathologies, thrombophilia due to hypercoagulability, or idiopathic, in which neither of the aforementioned causes can be attributed to the instances of renal infarction [1]. Other less common causes may include underlying arterial damage, such as fibrodysplasias or dissections [14].
Our patient had a history of hypertension, hyperlipidemia, and diet-controlled diabetes, thus putting her at greater risk for potential embolic causes; however, she had no prior history of hypercoagulability or thrombosis. Furthermore, we completed an extensive hypercoagulability examination, including assessment of factor V Leiden mutation, protein C and S activity, cardiolipin antibody, prothrombin G20210A mutation, beta-2 glycoprotein 1 antibodies, antithrombin III activity, homocysteine, and lupus anticoagulant. Despite this investigation, our findings were only notable for Protein S activity at 46% of normal and a positive repeat lupus anticoagulant test. Thus, as our patient had no clear cause for her renal infarct, but had risk factors for atherosclerosis and atherothrombosis (history of diabetes mellitus, hypertension, and hyperlipidemia), the decision was made to treat empirically with anticoagulation for 6 months and aspirin indefinitely.
The literature proposes that low total protein S levels cannot identify subjects at risk for venous thrombosis [15]. Thus, the isolated decreased Protein S activity was unlikely to contribute meaningfully to this patient’s infarction. Interestingly, the patient initially tested negative for lupus anticoagulant, but tested positive on subsequent examination and also had a positive hexagonal phase test result. However, like the Protein S activity, no significant associations can be drawn between this patient’s Hexll PE positivity and clinical complications due to the administration of anticoagulation [16]. Specifically, inflammation and the use of direct oral anticoagulants (DOAC) can induce false positives in lupus anticoagulant [17]. Furthermore, repeat testing should be done 12 weeks after initial testing to verify the diagnosis of antiphospholipid syndrome [17].
Thus, the nonspecific clinical and laboratory findings of renal infarctions make it difficult to definitively diagnose this condition early in the disease process. Although the literature cites <1% incidences of renal infarctions in clinical settings, these factors, alongside the overall rarity of the disease, may lead to an underestimation of the true number of infarctions within the population. Particularly in older patients, renal infarctions can rapidly progress to AKI or acute hypertension, leading to long-term CKD [14]. This effect is further compounded when the diagnosis is delayed [18].
Treatment methodologies vary based on the pathophysiology of the injury, but typically involve revascularization via either thrombolysis or surgery, with decreased time of occlusion associated with greater benefit from revascularization, and increased time to treatment associated with decreased ability to recover from the initial insult [9]. The literature is limited on anticoagulation in idiopathic renal infarction; however, as performed in this case, the standard of care is to treat with anticoagulation.
With difficulties elucidating clear clinical diagnosis in the setting of nonspecific laboratory findings and lack of classical imaging findings, future studies must consider further methodologies by which to conclusively diagnose renal infarctions. Furthermore, the large category of “idiopathic” classifications continues to demonstrate the need for more thorough histories and investigations in patients with renal infarcts to determine the etiology of their disease. Clinicians should maintain high suspicion for renal infarctions in patients with cardioembolic risk factors but should continue to consider this diagnosis in patients with underlying hypercoagulability or otherwise inexplicable renal insults. Furthermore, due to the many potential causes of renal infarction, a multidisciplinary approach should be emphasized in patients with this diagnosis.
Conclusions
Although renal infarctions have various etiologies, they remain exceedingly rare in the clinical setting. Failure of early recognition can rapidly progress to AKI or acute hypertension, leading to chronic CKD. Given the overall rarity of the condition and the difficulties in diagnosis, we recommend that clinicians maintain clinical suspicion for renal infarctions in those with risk factors for embolism or hypercoagulability.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Faucon AL, Bobrie G, Jannot AS, et al. Cause of renal infarction: A retrospective analysis of 186 consecutive cases. J Hypertens. 2018;36(3):634–40. doi: 10.1097/HJH.0000000000001588. [DOI] [PubMed] [Google Scholar]
- 2.Pizzarossa AC, Mérola V. [Etiology of renal infarction. A systematic review.] Rev Med Chil. 2019;147(7):891–900. doi: 10.4067/S0034-98872019000700891. [DOI] [PubMed] [Google Scholar]
- 3.Saeed K. Renal infarction. International Journal of Nephrology and Renovascular Disease. 2012;5:119–23. doi: 10.2147/IJNRD.S33768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoxie HJ, Coggin CB. Renal tnfarction: Statistical study of two hundred and five cases and detailed report of an unusual case. Arch Intern Med (Chic) 1940;65(3):587–94. [Google Scholar]
- 5.Huang C, Lo H, Huang H, et al. ED presentations of acute renal infarction. Am J Emerg Med. 2007;25(2):164–69. doi: 10.1016/j.ajem.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Bourgault M, Grimbert P, Verret C, et al. Acute renal infarction. Cl J Am Soc Nephrol. 2013;8(3):392–98. doi: 10.2215/CJN.05570612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolderman R, Oyen R, Verrijcken A, et al. Idiopathic renal infarction. Am J Med. 2006;119(4):356.e9–12. doi: 10.1016/j.amjmed.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 8.Domanovits H, Pauli M, Nikfardjam M, et al. Acute renal infarction: Clinical characteristics of 17 patients. Medicine. 1999;87(6):386–94. doi: 10.1097/00005792-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Ouriel K, Andrus CH, Ricotta JJ, et al. Acute renal artery occlusion: When is revascularization justified? J Vasc Surg. 1987;5(2):348–55. doi: 10.1067/mva.1987.avs0050348. [DOI] [PubMed] [Google Scholar]
- 10.Suzer O, Shirkhoda A, Jafri SZ, et al. CT features of renal infarction. Eur J Radiol. 2002;44(1):59–64. doi: 10.1016/s0720-048x(01)00476-4. [DOI] [PubMed] [Google Scholar]
- 11.Korzets Z, Plotkin E, Bernheim J, Zissin R. The clinical spectrum of acute renal infarction. Isr Med Assoc J. 2002;4(10):781–84. [PubMed] [Google Scholar]
- 12.Plouffe B, Van Hooren T, Barton M, et al. Infarcts – a perplexing case in the middle of the COVID-19 pandemic. Front Pediatr. 2021;9:669453. doi: 10.3389/fped.2021.669453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber E, Grangeon F, Reynaud Q, et al. Acute renal and splenic infarctions: A review. QJM. 2020;113(3):186–93. doi: 10.1093/qjmed/hcz252. [DOI] [PubMed] [Google Scholar]
- 14.Delezire A, Terrasse M, Bouet J, et al. Acute renal infarction: Long-term renal outcome and prognostic factors. J Nephrol. 2021;34:1501–9. doi: 10.1007/s40620-020-00953-4. [DOI] [PubMed] [Google Scholar]
- 15.Pintao MC, Ribeiro DD, Bezemer ID, et al. Protein S levels and the risk of venous thrombosis: results from the MEGA case-control study. Blood. 2013;122(18):3210–9. doi: 10.1182/blood-2013-04-499335. [DOI] [PubMed] [Google Scholar]
- 16.Berard M, Boffa MC, Karmochkine M, et al. Plasma reactivity to hexagonal II phase phosphatidylethanolamine is more frequently associated with lupus anticoagulant than with antiphosphatidylethanolamine antibodies. J Lab Clin Med. 1993;122(5):601–5. [PubMed] [Google Scholar]
- 17.Favaloro E, Pasalic L. Lupus anticoagulant testing during anticoagulation, including direct oral anticoagulants. Res Pract Thromb Haemost. 2022;6(2):e12676. doi: 10.1002/rth2.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bae EJ, Hwang K, Jang HN, et al. A retrospective study of short- and long-term effects on renal function after acute renal infarction. Ren Fail. 2014;36(9):1385–89. doi: 10.3109/0886022X.2014.947514. [DOI] [PubMed] [Google Scholar]


