Abstract
Rising global pediatric obesity rates, increase non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome (MetS) prevalence, with MetS being a NAFLD risk factor. NAFLD can be asymptomatic, with liver function tests insensitive to mild disease, and liver biopsy, risking complications. Thus, we investigated multiparametric MRI (mpMRI) metrics of liver fat (proton density fat fraction, PDFF) and disease activity (fibro-inflammation; iron-corrected T1, cT1), in a Hispanic pre-pubertal pediatric cohort, with increased risk of NAFLD. Pre-pubertal boys (n = 81) of varying Body-Mass Index (BMI) were recruited in Mexico City. Most children (81%) had normal liver transaminase levels, 38% had high BMI, and 14% had ≥ 3 MetS risk factors. Applying mpMRI thresholds, 12%, 7% and 4% of the cohort had NAFLD, NASH and high-risk NASH respectively. Participants with ≥ 3 MetS risk factors had higher cT1 (834 ms vs. 737 ms, p = 0.004) and PDFF (8.7% vs. 2.2%, p < 0.001) compared to those without risk factors. Those with elevated cT1 tended to have high BMI and high insulin (p = 0.005), HOMA-IR (p = 0.005) and leptin (p < 0.001). The significant association of increased risk of MetS with abnormal mpMRI, particularly cT1, proposes the potential of using mpMRI for routine pediatric NAFLD screening of high-risk (high BMI, high MetS risk score) populations.
Keywords: Obesity, BMI, Metabolic syndrome, NAFLD, Multiparametric MRI, Young children
Subject terms: Non-alcoholic fatty liver disease, Metabolic syndrome, Dyslipidaemias, Obesity, Type 2 diabetes
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of chronic liver disease worldwide1,2. NAFLD embodies a continuum of disease stages characterized by excessive lipid infiltration in hepatocytes (i.e., steatosis) that can advance via inflammatory and fibrotic pathways to more severe phenotypes such as non-alcoholic steatohepatitis (NASH) and liver cirrhosis3.
The global obesity rates have tripled in the past 50 years and are a major public health concern, with over 340 million children and adolescents being classified as overweight or obese; moreover, 5–10% of children with obesity also have NAFLD1. Although epidemiological studies typically indicate the rise in obesity to be highest in western developed countries such as the USA and UK4, industrialized developing countries such as Mexico are also showing a rapid increase in childhood obesity. The 2020 National Health Survey indicated that 19.6% of school children were overweight and 18.6% were obese in Mexico, with 26.8% of adolescents being overweight and 17% obese5,6. Crucially, obesity and NAFLD are also strongly associated with worse metabolic health and poor clinical outcomes, including increased likelihood of insulin resistance, Type 2 diabetes (T2DM), cardiovascular disease (CVD) and other chronic conditions7.
NAFLD is typically an asymptomatic disease and plasma biochemical liver health (function) markers are insensitive to mild disease3,8. It is of the utmost importance to identify individuals earlier along the disease continuum to prevent or slow progression to adverse clinical outcomes8. Liver biopsy, although recommended by clinical practice guidelines9,10 to support NAFLD/NASH diagnosis, is an imperfect reference standard with numerous well documented limitations11–13. Of particular concern in children, liver biopsy can result in pain, bleeding or infection14. Therefore, less invasive alternatives to liver biopsies are needed with similar diagnostic accuracy to support patient management and prognostication15. Currently, non-invasive technologies, such as ultrasound16 and elastography17, are being used to support patient management, however, these tests are predominantly validated only in adults. In addition, clinical guidance from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN)8, as well as that from the British Society of Paediatric Gastroenterology, Hepatology and Nutrition (BSPGHAN)18, have highlighted the need for NAFLD identification in children. More specifically, clinical guidelines recommend earlier screening in younger children with MetS risk factors as they are at higher risk of long-term adverse clinical outcomes8. Non-invasive imaging assessments such as ultrasound and computerized tomography (CT) are clinically available. However, routine ultrasound has inadequate sensitivity and specificity to screen for NAFLD in children, and CT is not recommended in children due to radiation exposure8. Hence, NASPGHAN have suggested that MRI could be a viable testing modality for chronic pediatric liver disease.
Multiparametric magnetic resonance imaging (mpMRI) is increasingly being used to assess chronic pediatric liver disease. Proton density fat fraction (PDFF) can assess liver fat whereas iron-corrected T1 (cT1), a correlate of fibro-inflammation, can characterize disease activity19. Such mpMRI metrics have been able to identify sub-clinically active disease19,20 and support longitudinal patient monitoring21, including assessment of Fontan-associated liver disease22, and presence of radiologic portal hypertension in chronic progressive pediatric chronic liver diseases23–25. In adults with NAFLD/NASH, mpMRI has been able to predict adverse outcomes including all-cause mortality26, shown to be useful for monitoring treatment response27–30, aid patient understanding of chronic liver disease31 and identify high risk disease32. In addition, mpMRI has shown clinical utility in epidemiological studies of liver disease in asymptomatic adults and promoting earlier non-invasive disease detection28,33,34.
While pediatric clinical guidelines propose the need for accurate non-invasive MRI-based assessment of NAFLD, hitherto, there has been no data on the utility of mpMRI and the association of mpMRI metrics with metabolic and liver health assessments in young children, asymptomatic for NAFLD. Thus, in this study, we evaluated the relationship between mpMRI assessment of NAFLD/NASH with Body-Mass-Index (BMI), conventional biochemical liver function tests, and metabolic risk factors and score, in a pre-pubertal Mexican male cohort, asymptomatic for NAFLD. Male Hispanic populations have been shown to have both a higher prevalence of NAFLD/NASH as well as a greater likelihood of developing cirrhosis35 due to the high prevalence of the patatin-like phospholipase domain-containing protein 3 (PNPLA3)36. As males are more likely to be affected by NASH than females, the probability of poor prognosis is higher in males compared to females. Thus, in this study, we selected boys as their gender (sex) makes them highly susceptible to adverse outcomes from NASH as they grow older—these include 3.1 odds ratio of developing type 2 diabetes37, higher premature mortality rate38, and higher cumulative incidence of overall mortality39. Thus, as NASH is primarily a silent disease40, early detection can support initiation of non-therapeutic interventions, such as lifestyle changes, which can result in disease regression.We aim to determine the clinical usefulness of mpMRI for the assessment of NAFLD in asymptomatic boys who are at risk of having or developing advanced disease.
Methods
Study cohort
Male pre-pubertal individuals were invited to have a research non-contrast MRI scan as part of a study to identify predictors of the risk of developing metabolic and cognitive dysfunction (“METCOG” study). Participants were recruited from local schools, as well as from adverts posted in local newspapers and clinics at Hospital Infantil de México Federico Gómez, Mexico City, Mexico, and had differing BMI classifications based on percentiles as defined by the Centre of Disease Control and Prevention (CDC) guidelines for children and teenagers41. All participants were asymptomatic and did not have any reported/existing liver disease.
The study cohort comprised 81 participants who all underwent mpMRI. Statistical power analysis42 showed that a sample size of N = 78 is required for statistical significance. For this calculation, the diagnostic rate of mpMRI was considered to be comparable to that reported in adults43. Employing an effect size of 5% (determined by liver PDFF which was used to define NAFLD), with an acceptable level of significance of 5% (α = 0.05), 80% power and assuming a 5% dropout rate, a minimum of N = 78 participants were required to be recruited into the study.
The inclusion criteria were that individuals had to be male, aged 7–9 years old (Tanner stage 1), with the ability to hold their breath for 10–15 s whilst lying supine (for MRI). Exclusion criteria included having Type 1 diabetes, known or suspected liver disease, chronic disease except for obesity or metabolic syndrome, acute infection, taking metabolic profile-altering medication (e.g., metformin, corticosteroids, lipid lowering agents), first degree relative with Type 1 diabetes, hypoxia, psychiatric disorder or neurological disorders affecting normal cognitive development, as well as having metallic implants/devices that are MRI contraindicated or being claustrophobic.
Participants attended two visits as part of this study (Fig. 1): the first for anthropometric measurements and blood collection for laboratory tests; and the second for an MRI scan. Randomization was not undertaken, boys were enrolled as the study progressed and their measurements defined their BMI or liver health/disease group classification.
Fig. 1.

Study design for the “METCOG” study showing patient enrolment/exclusions and study visits and clinical, laboratory and imaging data collected.
Ethical approval
This cross-sectional observational study was carried out according to the Principals of Good Clinical Practice and the Declaration of Helsinki (2013). Ethical approval was received from the King’s College London Psychiatry, Nursing and Midwifery Research Ethics Subcommittee (ethics number: HR-16/17-4156) and from the Hospital Infantil de Mexico, Federico Gómez (ethics number: HIM/2016/105 and SSA-1369). All participants gave their written informed assent and their legal guardians, also gave written informed consent for the participants to take part in the study.
Clinical and laboratory assessments
Anthropometric measurements including weight, height, waist circumference (WC) were recorded, alongside systolic and diastolic blood pressure (BP) readings. Fasted blood samples were collected, and plasma prepared for conventional liver biochemical measurements including alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP), total and direct bilirubin. Plasma markers of metabolic health including fasting insulin, lipid profile (high- and low-density lipoproteins [H/LDL], cholesterol, triglycerides) and hemoglobin A1c (HbA1c) were also measured. The Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) was calculated according to the formula: fasting insulin (uU/L) × fasting glucose (nmol/L)/22.5.
BMI was calculated and individuals categorized using CDC classifications into low (underweight/healthy) and high (overweight/obese) BMI groups. Underweight was defined as a BMI ≤ 5th percentile, while healthy was defined as a BMI within the 5th to 85th percentiles. Overweight was defined as a BMI in the 85th to 95th percentiles, and obesity as a BMI ≥ 95th percentile. Participants were classified as prediabetic if their HbA1c measurement was between 5.7 and 6.4%, as advised by the American Diabetes Association44. Liver biopsy was not performed in this study as those recruited were not patients and therefore, not clinically indicated.
Definition of metabolic syndrome (MetS) risk and NAFLD
Metabolic syndrome (MetS) is typically diagnosed in children above 10 years of age45. However, the ‘Identification and prevention of Dietary- and lifestyle-induced health Effects In Children and infantS’ (IDEFICS) study developed criteria which can be used to identify MetS in children younger than 10 years38. IDEFICS consider MetS risk factors in young children are: WC ≥ 90th percentile, high BP ≥ 90th percentile, dyslipidemia as triglycerides ≥ 90th percentile or HDL cholesterol ≤ 10th percentile and high blood glucose/insulin, with HOMA-IR ≥ 90th percentile or fasting glucose ≥ 90th percentile. In this study, the MetS risk for each participant was categorized into three groups according to the criteria developed in the IDEFICS study: no MetS risk factors, 1–2 MetS risk factors (some risk of MetS), and ≥ 3 MetS risk factors (indicative of likely MetS).
Participants were also defined and trichotomized into three groups, those with NAFLD, NASH or high-risk NASH, using mpMRI metric thresholds reported previously26–30,32,46. Those categorized as having NAFLD had PDFF of ≥ 5%; those as having NASH, PDFF was ≥ 5% and cT1 ≥ 800 ms; and those with high-risk NASH, i.e., NASH score of ≥ 4 and fibrosis stage (F) ≥ 2, PDFF was ≥ 5% and cT1 ≥ 875 ms46.
MRI acquisition and analysis
MRI was performed on a 3 T Siemen’s Skyra scanner (Siemens Healthineers, Erlangen, Germany) with mpMR images obtained using a non-contrast abdominal MRI scan following the LiverMultiScan (Perspectum Ltd, Oxford, UK) image acquisition protocol with MRI scanning sequences reported previously47. Four transverse slices obtained at the porta hepatis location in the liver were acquired for each participant using a shortened modified look-locker inversion (shMOLLI) and a multi-echo spoiled gradient-echo sequence to quantify T1, iron (T2*) and fat (PDFF)40. During image analysis, iron-corrected T1 (cT1) and PDFF maps of the liver were delineated into whole liver segmentation maps (Fig. 2) using a semi-automatic method47. Three 15-mm diameter circular regions of interest were placed on the transverse T2* maps for each slice, covering a representative sample of the liver, to calculate average T2* values for T1-correction. All images were analyzed by trained analysts blinded to the clinical data.
Fig. 2.
Typical transverse corrected T1 (cT1) and proton density fat fraction (PDFF) maps for underweight, healthy, overweight and obese participants calculated from multiparametric magnetic resonance imaging (mpMRI) data collected using LiverMultiScan (Perspectum Ltd, Oxford, UK) image acquisition protocol and MRI scanning sequences44.
Statistical analysis
Descriptive statistics were used to summarize participant characteristics of the study cohort. Continuous normally distributed variables were reported as mean ± standard deviation (SD) and categorical variables as frequency and percentage, with ranges reported where appropriate. Correlations between measurements were investigated using Spearman’s Rank correlation coefficient (rs). Differences in measurement values between low and high BMI, or low and high cT1 groups, were compared using the Kruskal–Wallis rank sum test, and differences in categorical data were compared using either the Pearson’s Chi-squared or two-sided Fisher’s exact test as appropriate.
Statistical analysis was performed using R version 4.1.2 (R Core Team, Vienna, Austria) with values of p < 0.05 considered statistically significant. For the mpMRI and MetS risk sub-analyses, where data were missing, case-wise deletion was employed.
Results
Participant demographics
The study cohort (n = 81) were aged 8.2 ± 0.8 years (range 7.0–9.9) with mean BMI of 17.4 ± 3.5 kg/m2 (range 12.9–29.4). Clinical assessment included collection of anthropometric, BP, and plasma metabolic and liver measurements, alongside a non-contrast MRI scan (Table 1). All participants were asymptomatic and did not have any reported/existing liver disease.
Table 1.
Age, anthropometric, blood pressure and laboratory measurements from the study cohort, and low and high Body-Mass-Index (BMI) subgroups.
| Measurement | Whole cohort n = 81 |
Low BMI Subgroup n = 50 |
High BMI Subgroup n = 31 |
Low vs. high BMI P-value |
|---|---|---|---|---|
| Age (years) | 8.2 (0.8) | 8.2 (0.8) | 8.2 (0.7) | 0.7 |
| Anthropometric measurements | ||||
| BMI (kg/m2) | 17.4 (3.5) | 15.3 (1.2) | 20.9 (3.1) | < 0.001 |
| Waist circumference (cm) | 63.3 (10.0) | 57.1 (4.0) | 73.2 (8.8) | < 0.001 |
| Waist/height ratio | 0.5 (0.1) | 0.5 (0.0) | 0.6 (0.1) | < 0.001 |
| Metabolic health measurements | ||||
| Systolic blood pressure (mmHg) | 99.0 (6.4) | 97.2 (5.7) | 102.0 (6.5) | < 0.001 |
| Diastolic blood pressure (mmHg) | 61.0 (5.6) | 59.4 (4.9) | 63.7 (5.7) | 0.001 |
| Glucose (mg/dL) | 85.7 (7.4) | 85.1 (7.6) | 86.6 (7.0) | 0.24 |
| Fasting insulin (pg/mL) | 213.8 (209.6) | 165.8 (87.5) | 287.4 (304.5) | 0.025 |
| HOMA-IR score | 1.3 (1.5) | 1.0 (0.5) | 1.9 (2.2) | 0.021 |
| HbA1c (%) | 5.3 (0.3) | 5.3 (0.3) | 5.3 (0.3) | 0.38 |
| Prediabetics (HbA1c 5.7–6.4%) | 9 (11%) | 3 (6.0%) | 6 (19%) | 0.079 |
| Leptin (pg/mL) | 2,446.8 (2,940.8) | 1,033.3 (964.8) | 4,617.5 (3,592.4) | < 0.001 |
| HDL (mg/dL) | 57 (26) | 63 (31) | 46 (12) | < 0.001 |
| LDL (mg/dL) | 94 (23) | 88 (18) | 103 (27) | 0.008 |
| Triglycerides (mg/dL) | 78.7 (74.1) | 57.1 (22.7) | 113.5 (108.5) | 0.003 |
| Plasma liver biochemical tests | ||||
| AST (U/L) | 29 (6) | 28 (6) | 32 (6) | 0.002 |
| ALT (U/L) | 32 (15) | 28 (9) | 38 (21) | 0.008 |
| ALP (U/L) | 288.6 (63.2) | 270.7 (58.1) | 316.8 (61.3) | 0.001 |
| Direct bilirubin (mg/dL) | 0.10 (0.03) | 0.10 (0.03) | 0.09 (0.02) | 0.041 |
| Total bilirubin (mg/dL) | 0.48 (0.16) | 0.51 (0.19) | 0.44 (0.09) | 0.15 |
Significant values are in bold.
Differences between low and high BMI groups considered significant are denoted in bold. BMI and prediabetes classification were performed according to the Centre for Disease Control classification and American Diabetes Association, respectively. HDL high-density lipoprotein, LDL low-density lipoprotein, AST aspartate aminotransferase, ALT alanine aminotransaminase, ALP alkaline phosphatase, HOMA-IR Homeostatic Model Assessment for Insulin Resistance.
Metabolic and liver health
BMI, cT1 and PDFF had significant correlations with anthropometric parameters, measures of metabolic and liver health (Fig. 3). BMI correlated with all measures except for HbA1c, glucose, direct and total bilirubin. Whilst both cT1 and PDFF correlated with BMI, and with each other, they also correlated with waist/height index, insulin, HOMA-IR, HDL and leptin. Additionally, PDFF (liver fat) also correlated with AST and ALT, and cT1 with triglycerides.
Fig. 3.
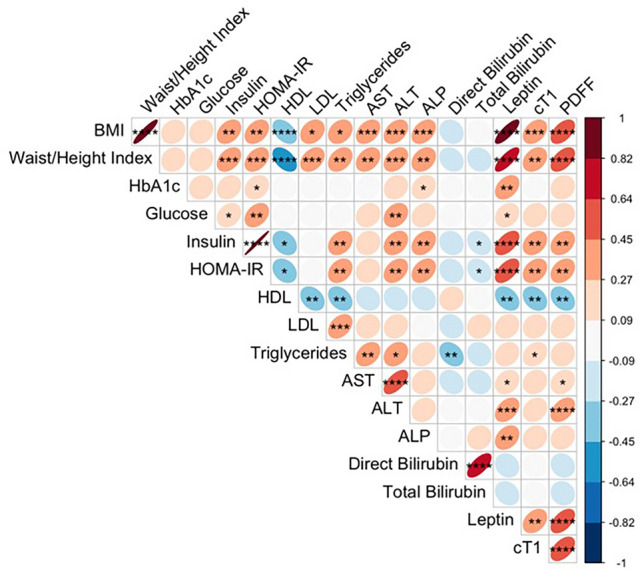
A correlation plot showing the associations between anthropometric, plasma liver and metabolic, and multiparametric magnetic resonance imaging measurements. Ellipse area reflect the absolute value of the corresponding Spearman’s correlation coefficient, and their eccentricity is parametrically scaled to the correlation value. All significant correlations are denoted in bold with the following levels of significance: *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001. BMI Body-Mass-Index, HbA1c hemoglobin A1c, HOMA-IR Homeostatic Model Assessment for Insulin Resistance, HDL high-density lipoprotein, LDL low-density lipoprotein, AST aspartate aminotransferase, ALT alanine aminotransaminase, ALP alkaline phosphatase, cT1 corrected T1, PDFF proton density fat fraction.
Of the study cohort, 38% had high BMI (overweight/obese). Whilst most plasma biochemical markers were in reported normal ranges48, 11% had HbA1c in the prediabetic range (Table 2). The high BMI group had higher fasting insulin (p = 0.001) and triglyceride levels (p < 0.001), alongside lower serum HDL (p < 0.001), than the low BMI group (Table 1). Similarly, biochemical markers of liver health were also in the normal range, but AST (p = 0.023), ALT (p < 0.001), ALP (p = 0.008) and direct bilirubin (p = 0.015), were higher in the high BMI group compared to the low BMI group. In addition, both diastolic (p = 0.006) and systolic (p = 0.013) BP were also higher in the high BMI group (Table 1). Inspection of a Manhattan plot where individual datapoints are shown for the liver transaminases and selected measurements that contribute to the IDEFCS MetS risk score, many of the abnormal values are from subjects with MetS risk score of 1–2 or ≥ 3, but some are also from subjects that have no MetS risk factors (Fig. 4A).
Table 2.
Anthropometric and liver measurements, and their comparisons between low and high Body-Mass-Index (BMI), and between low and high cT1, subgroups.
| Variable | Whole cohort n = 81 |
Low BMI subgroup n = 50 |
High BMI subgroup n = 31 |
Low vs High BMI P-value |
cT1 < 800 ms subgroup n = 72 |
cT1 800 ms subgroup n = 9 |
Low vs. high cT1 P-value |
|---|---|---|---|---|---|---|---|
| Age (years) | 8.2 (0.8) | 8.2 (0.8) | 8.2 (0.7) | 0.7 | 8.2 (0.8) | 8.6 (0.7) | 0.12 |
| Anthropometric measurements | |||||||
| BMI (kg/m2) | 17.4 (3.5) | 15.3 (1.2) | 20.9 (3.1) | < 0.001 | 16.7 (2.5) | 23.4 (4.5) | < 0.001 |
| High BMI (%) | 31 (38%) | 0 (0%) | 31 (100%) | < 0.001 | 23 (32%) | 8 (89%) | 0.002 |
| Waist/height ratio | 0.5 (0.1) | 0.5 (0.0) | 0.6 (0.1) | < 0.001 | 0.5 (0.1) | 0.6 (0.1) | < 0.001 |
| Plasma liver biochemical tests | |||||||
| AST (U/L) | 29 (6) | 28 (6) | 32 (6) | 0.002 | 28 (5) | 35 (8) | 0.033 |
| ALT (U/L) | 32 (15) | 28 (9) | 38 (21) | 0.008 | 29 (9) | 53 (32) | 0.01 |
| ALP (U/L) | 288.6 (63.2) | 270.7 (58.1) | 316.8 (61.3) | 0.001 | 284.9 (64.5) | 316.7 (45.7) | 0.076 |
| Direct bilirubin (mg/dL) | 0.10 (0.03) | 0.10 (0.03) | 0.09 (0.02) | 0.041 | 0.10 (0.03) | 0.09 (0.02) | 0.39 |
| Total bilirubin (mg/dL) | 0.48 (0.16) | 0.51 (0.19) | 0.44 (0.09) | 0.15 | 0.49 (0.16) | 0.41 (0.09) | 0.11 |
| Multiparametric magnetic resonance imaging metrics and liver health/disease assessment | |||||||
| cT1 (ms) | 758 (62) | 738 (31) | 790 (83) | < 0.001 | 742 (30) | 887 (95) | < 0.001 |
| PDFF (%) | 3.5 (3.5) | 2.2 (0.8) | 5.5 (4.9) | < 0.001 | 2.6 (1.3) | 10.0 (7.2) | < 0.001 |
| NAFLD (PDFF ≥ 5%) | 10 (12%) | 0 (0%) | 10 (32%) | < 0.001 | 4 (5.6%) | 6 (67%) | < 0.001 |
| NASH (PDFF ≥ 5% and cT1 ≥ 800 ms) | 6 (7.4%) | 0 (0%) | 6 (19%) | 0.002 | 0 (0%) | 6 (67%) | < 0.001 |
| High risk NASH (PDFF ≥ 5% and cT1 ≥ 875 ms) | 3 (3.7%) | 0 (0%) | 3 (9.7%) | 0.053 | 0 (0%) | 3 (33%) | < 0.001 |
Significant values are in bold.
BMI category was determined according to the Centre for Disease Control classification. AST aspartate aminotransferase, ALT alanine aminotransaminase, ALP alkaline phosphatase, cT1 corrected T1, PDFF proton density fat fraction.
Fig. 4.
Radar plots showing multivariate observations for group-wise comparison between those with low (< 800 ms) and elevated cT1 (≥ 800 ms) (A), and with individual children with elevated cT1 (B). (C) A Manhattan plot showing individual z-scores for selected markers of metabolic and liver health (dashed lines represent the mean and standard deviation of the z-scores), and their relationship to the metabolic syndrome (MetS) risk score (Key: black, orange and red symbols indicate no MetS; some risk of MetS, 1–2 risk factors; and likely MetS, ≥ 3 risk factors, respectively); and a circle or a triangle indicate cT1 < 800 ms or ≥ 800 ms, respectively. BMI Body-Mass-Index, HbA1c hemoglobin A1c, HOMA-IR Homeostatic Model Assessment for Insulin Resistance, HDL high-density lipoprotein, LDL low-density lipoprotein, AST aspartate aminotransferase, ALT alanine aminotransaminase, ALP alkaline phosphatase, cT1 corrected T1, PDFF proton density fat fraction.
Liver mpMRI, BMI, metabolic syndrome factors and risk
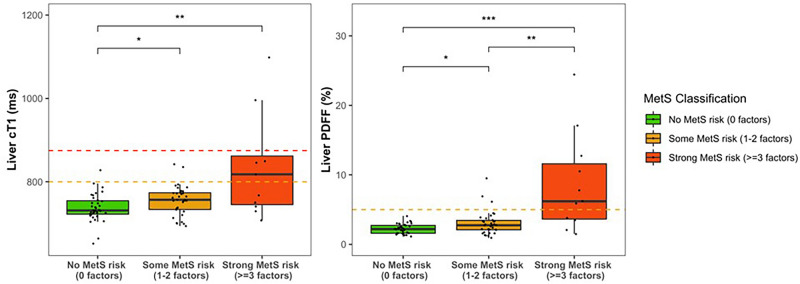
In this cohort, employing thresholds defined previously in literature26–30,32,39, 12% of the cohort had NAFLD (PDFF ≥ 5%), 7% had NASH (both PDFF ≥ 5% and cT1 ≥ 800 ms) and 4% high-risk NASH (both PDFF ≥ 5% and cT1 ≥ 875 ms) (Table 2). Both PDFF and cT1 were higher in the high BMI group (p < 0.001) (Table 2). When considering the individual components of MetS risk, individuals with elevated cT1 (≥ 800 ms) had significantly greater hyperinsulinemia/glycaemia, hyperlipidemia and low HDL when compared to those with cT1 < 800 ms (Table 3). In addition, children with higher cT1 also had significantly elevated fasting insulin, triglycerides and HOMA-IR (Table 3). Fasting leptin was also significantly higher in those with high BMI as well as those with elevated cT1 (Table 3). Figure 5 shows the relationship between cT1 and PDFF with MetS risk. Participants with ≥ 3 MetS risk factors (likely MetS) had significantly higher cT1 compared to those without any MetS risk factors (834 ± 121 ms vs 737 ± 33 ms, p = 0.047) (Table 3). Indeed, the odds ratio of having cT1 ≥ 800 ms were 7.4, 12.8, 24.5 and 49.8 for MetS risk scores, 0, 1, 2, and ≥ 3, respectively. This is also consistent with the radar plot, where those that have cT1 < 800 ms shows relatively less perturbation of plasma liver and selected metabolic measures compared to those that have high cT1 on average (Fig. 4B), and individually (Fig. 4C). Inspection of the Manhattan plot of individual measurement values for each subject individual, show that not all individuals with high cT1 had high BMI, increased MetS risk, abnormal liver or metabolic measures (Fig. 4A).
Table 3.
Metabolic health measurements, and Identification and prevention of dietary- and lifestyle-induced health effects in children and infants (IDEFICS) metabolic syndrome (MetS) risk factors categories and MetS risk criteria; and their comparisons between low and high Body-Mass-Index (BMI), and between low and high cT1, subgroups.
| Variable | Whole cohort n = 81 |
Low BMI subgroup n = 50 |
High BMI subgroup n = 31 |
Low vs High BMI P-value |
cT1 < 800 ms subgroup n = 72 |
cT1 800 ms subgroup n = 9 |
Low vs. High cT1 P-value |
|---|---|---|---|---|---|---|---|
| Metabolic health measurements | |||||||
| Systolic blood pressure (mmHg) | 99.0 (6.4) | 97.2 (5.7) | 102.0 (6.5) | < 0.001 | 98.7 (6.3) | 101.7 (6.8) | 0.18 |
| Diastolic blood pressure (mmHg) | 61.0 (5.6) | 59.4 (4.9) | 63.7 (5.7) | 0.001 | 60.6 (5.4) | 64.6 (6.1) | 0.078 |
| Fasting glucose (mg/dL) | 85.7 (7.4) | 85.1 (7.6) | 86.6 (7.0) | 0.24 | 85.4 (7.4) | 87.9 (7.4) | 0.35 |
| Fasting insulin (pg/mL) | 213.8 (209.6) | 165.8 (87.5) | 287.4 (304.5) | 0.025 | 179.3 (99.7) | 586.6 (552.9) | 0.005 |
| HOMA-IR score | 1.3 (1.5) | 1.0 (0.5) | 1.9 (2.2) | 0.021 | 1.1 (0.6) | 4.0 (4.1) | 0.005 |
| HbA1c (%) | 5.3 (0.3) | 5.3 (0.3) | 5.3 (0.3) | 0.38 | 5.3 (0.3) | 5.4 (0.4) | 0.054 |
| Prediabetics | 9 (11%) | 3 (6.0%) | 6 (19%) | 0.079 | 6 (8.3%) | 3 (33%) | 0.058 |
| Leptin (pg/mL) | 2,446.8 (2,940.8) | 1,033.3 (964.8) | 4,617.5 (3,592.4) | < 0.001 | 1,918.9 (2,228.6) | 8,165.7 (3,825.4) | < 0.001 |
| Total cholesterol (mg/dL) | 162.7 (24.5) | 159.5 (24.6) | 167.9 (23.9) | 0.15 | 161.2 (23.0) | 174.6 (33.9) | 0.38 |
| Triglycerides (mg/dL) | 78.7 (74.1) | 57.1 (22.7) | 113.5 (108.5) | 0.003 | 67.8 (51.8) | 165.6 (146.8) | 0.022 |
| HDL (mg/dL) | 57 (26) | 63 (31) | 46 (12) | < 0.001 | 59 (27) | 37 (7) | < 0.001 |
| LDL (mg/dL) | 94 (23) | 88 (18) | 103 (27) | 0.008 | 91 (20) | 114 (34) | 0.055 |
| IDEFICS MetS risk factors and mets risk assessment | |||||||
| Obesity: ≥ 90th percentile as assessed by WC | 37 (46%) | 6 (12%) | 31 (100%) | < 0.001 | 29 (40%) | 8 (89%) | 0.01 |
| Blood pressure: systolic or diastolic ≥ 90th percentile | 4 (4.9%) | 0 (0%) | 4 (13%) | 0.019 | 2 (2.8%) | 2 (22%) | 0.059 |
| Glucose: Insulin or fasting glucose ≥ 90th percentile | 17 (21%) | 7 (14%) | 10 (32%) | 0.05 | 12 (17%) | 5 (56%) | 0.017 |
| Triglycerides: ≥ 90th percentile | 17 (21%) | 5 (10%) | 12 (39%) | 0.002 | 11 (15%) | 6 (67%) | 0.002 |
| HDL Cholesterol: ≤ 10th percentile | 9 (11%) | 0 (0%) | 9 (29%) | < 0.001 | 4 (5.6%) | 5 (56%) | < 0.001 |
| No MetS risk | 36 (44%) | 36 (72%) | 0 (0%) | < 0.001 | 35 (49%) | 1 (11%) | 0.039 |
| Some MetS risk | 34 (42%) | 13 (26%) | 21 (68%) | < 0.001 | 32 (44%) | 2 (22%) | 0.29 |
| MetS 3 risk factors | 11 (14%) | 1 (2.0%) | 10 (32%) | < 0.001 | 5 (6.9%) | 6 (67%) | < 0.001 |
Significant values are in bold.
BMI and prediabetes classification were performed according to the Centre for Disease Control classification and American Diabetes Association, respectively. HbA1c hemoglobin A1c, HOMA-IR Homeostatic Model Assessment for Insulin Resistance, HDL high-density lipoprotein, LDL low-density lipoprotein.
Fig. 5.
The relationship between corrected T1 (cT1), proton density fat fraction (PDFF) and increasing metabolic syndrome (MetS) risk groups. (A) Graphs showing the cT1 in groups with different numbers of metabolic syndrome (MetS) risk factors; and (B), A radar plot illustrating the levels of liver (alanine and aspartate) transaminases and selected metabolic parameters contributing to MetS risk factor scores with cT1 < 800 ms and those with cT1 ≥ 800 ms, irrespective of MetS risk factor score. All significant Spearman’s correlations are denoted in bold with the following levels of significance: *p < 0.05; **p < 0.01; ***p < 0.001.
Similar to that for cT1, PDFF (liver fat) was found to increase with increasing risk of MetS such that individuals with ≥ 3 MetS risk factors had significantly higher PDFF compared to those without any risk factors (8.7 ± 7.1% vs 2.2 ± 0.7%, p < 0.001). In addition to having elevated liver health markers (biochemical and imaging), participants with high BMI had significantly higher diastolic and systolic (p = 0.001) BP compared to their counterparts with low BMI (Tables 1, 3).
Discussion
In this study, we showed high BMI, increased MetS factors and risk scores, and liver transaminases, with elevated mpMRI metrics, in a Hispanic pre-pubertal children cohort. Notably, liver transaminases were within normal ranges and consistent with their insensitivity to early NAFLD. To the best of our knowledge, this is the first study investigating the relationship between BMI (obesity), biochemical measurements of liver health, metabolic disease risk and mpMR metrics in a young asymptomatic Hispanic pediatric population, predisposed to developing NAFLD.
The pathogenesis of NAFLD and progression to NASH is associated with the dysregulation of metabolic parameters3. Adiposity, insulin resistance and hyperlipidemia, all characteristics of MetS have been shown to increase individual risk of NAFLD1,2. BMI has traditionally been used as part of the criteria to identify children with highest risk of long-term adverse outcomes, thus, guiding the intensity of intervention9,10. Findings from this study corroborated with these insights as children with high BMI (overweight/obese) and elevated metabolic risk markers showed increased PDFF and cT1; and notably, with plasma liver health measurements generally in the normal range. However, although BMI is closely associated with NAFLD, those with low BMI can still develop the disease, and as NAFLD is typically asymptomatic in its early stages, with liver health measurements such as ALT and AST being within the normal range10, mpMRI may play a critical role in identifying such individuals.
Large patient registries have recently shown that patients with NASH, regardless of their fibrosis stage, are at high risk of poor clinical outcomes48,49. Furthermore, the burden of NASH on healthcare systems and the economy has been acknowledged to be significant on a global scale50,51, and that early diagnosis and treatment of NASH patients could potentially reduce future healthcare costs. Therefore, early identification of individuals with NASH, with or without fibrosis, is important to provide more effective prevention, surveillance, and intervention strategies. Non-invasive technologies are currently being used to support adult management and triaging in NAFLD/NASH52,53, and to enrich and assess treatment response in adult pharmaceutical clinical trials27. This is also needed in the pediatric arena as systematic reviews on the global prevalence of NAFLD have indicated rates of 7.6% in the general pediatric population and 34.2% in the obese population54. Furthermore, epidemiological studies have shown NASH prevalence range from 20 to 50% in these NAFLD populations48.
In adults with NAFLD, mpMRI markers (cT1 and PDFF) have been reported to show good diagnostic utility to stratify NASH with fibrosis (with an AUC ranging 0.74–0.89)32,46,55,56; outperform other commonly used non-invasive markers, including biochemical markers, vibration-controlled transient elastography and magnetic resonance elastography32; perform similarly in different ethnic and racial cohorts (including black, white, Asian and Hispanic)32,46,55,56; and have the same performance as liver biopsy in predicting adverse clinical outcomes26. In our study, 12% had elevated cT1 indicative of NAFLD, with 7% suggested to have NASH, and 4%, high-risk NASH. High-risk NASH, i.e., NASH with fibrosis, has been linked to severe adverse clinical events including poor cardiovascular outcomes57. In this asymptomatic cohort, children with high cT1 also had significantly higher (but within the normal range) transaminase levels as well as increased MetS risk compared to those with low cT1 (< 800 ms). As these MRI markers seem to perform similarly in children as in adults, we suggest that cT1 can be used for early disease diagnosis and stratify children for appropriate intervention. Interventions include more robust weight-loss intervention, or treatment with drug therapies licensed for diabetes or weight-loss that have beneficial effects in the liver as proposed in adults52,58.
Elevated cT1 and PDFF are especially important clinical findings in this cohort as male Hispanic populations have higher prevalence of NAFLD/NASH and greater risk of developing cirrhosis35. These findings are further strengthened by the significant associations between cT1 and PDFF with leptin, an independent predictor of the presence and development of NAFLD, and strongly associated with insulin resistance and body adiposity59. It is worth noting that both pediatric and adult clinical guidelines are demanding studies that evaluate the clinical utility of non-invasive technologies that can be used to identify early NAFLD/NASH. Regulatory bodies, such as the FDA, are also calling for non-invasive technologies as only 3% of FDA-approved artificial imaging solutions are indicated in pediatrics60. Our study demonstrates the potential added benefit of mpMRI by characterizing the relationship between metabolic and liver health, suggesting mpMRI can be used to provide early liver disease detection and support clinical management. This is especially relevant as recent literature authored by members of the European Reference Network for Hepatological Diseases have described mpMRI as a “virtual biopsy” that not only provides a panoramic view of the liver but also facilitates risk stratification of patients61.
The term “non-alcoholic” to describe metabolic-associated liver disease related to overweight/obesity and metabolic syndrome in children has been the subject of much debate, especially as the possibility of alcohol-related liver disease is unlikely62. In recognition of this, some experts have proposed the use of the term “metabolic (dysfunction)-associated fatty liver disease” (MAFLD). However, the characterization of MAFLD in pediatrics is still poorly understood62, and the manifestation of MAFLD in children differs from that in adults62, increasing the complexity of disease screening. Nevertheless, while avoiding this ongoing controversy, our findings presented here contribute to these current discussions and support the development of clinical algorithms combining traditional biomarkers with mpMRI to improve diagnostic accuracy and obviate the need for invasive diagnostic liver biopsies62. These algorithms for non-invasive early detection of disease, have the potential to reduce the health consequences in the long-term associated with chronic childhood liver disease63.
This study has several strengths. Our findings highlight the potential relationships between mpMRI-assessed NAFLD/NASH with BMI, metabolic health and conventional liver assessments in an unusual cohort. Not only are findings from pre-pubertal children scarce, findings from a cohort living in a low- to middle-income countries such as Mexico is also uncommon. Furthermore, this study is important as amongst Hispanic persons, NAFLD is the most prevalent chronic liver disease, resulting in high incidences of hepatocellular carcinoma with worse prognosis compared to other ethnicities64.
The study does have a limitation, in that as it was a real-world study, concurrent liver biopsy was unethical in asymptomatic children. We acknowledge we are unable to assess investigated relationships with histological findings of NAFLD/NASH. Nevertheless, previous studies have shown significant correlations between cT1 and histology in both pediatrics19–21 and adults26,27,32.
In conclusion, the increasing prevalence of pediatric obesity has been strongly linked with rising global rates of NAFLD. This prospective study has demonstrated that elevated cT1 and PDFF, indicating liver dysfunction, are correlated with higher BMI and a greater risk of MetS. We propose non-invasive cT1 has clinical utility in routine pediatric NAFLD screening programs for NAFLD and its severity, alongside fibrosis markers for those with advanced disease. This would support stratification of young asymptomatic children at increased risk of developing NAFLD, e.g. those with high BMI and increased metabolic disease risk, and has the potential to enable early disease detection and reduce adverse clinical outcomes in young children.
Acknowledgements
The investigators gratefully thank the children and their guardians for their participation.
Author contributions
Conception and design of the study: P.-W.S. Data collection and curation: B.dC.A., S.H.T., B.L.M., P.D.S., A.L.M., M.K.K. & E.B.P. Data analysis: C.B., E.S. Writing original draft: C.B., E.S., H.T.B., R.B. Writing—review and editing: S.H.T., P.-W.S., B.dC.A., E.L.T., B.L.M., P.D.S., M.K.K., A.L.M.L., E.B.P., J.D.B. All authors reviewed, discussed, and agreed with manuscript.
Funding
This paper presents independent research funded by the Newton Fund programme and the grant was awarded by the Medical Research Council (MR/N029194/1) and CONACyT México (FONCICIT/37/2016) on behalf of the UK Research and Innovation to P-WS, BdCA, JDB, EBP and RR. For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) license to any Author Accepted Manuscript version arising.
Data availability
The data and analytic methods used in this study remain the property of the individual study sponsors. All deidentified participant data are not openly available presently to allow privileged use of the data to the funded researchers and may be made available to other researchers upon request to P-WS, following permission, investigator support and a signed data access agreement. Study metadata will be made available through the MRC Research Data Gateway to allow potential users to make contact and request access to the data.
Competing interests
ES, CB, HTB, RB are employees of Perspectum. Perspectum Ltd is a privately funded commercial enterprise that develops medical devices to address unmet clinical needs, including LiverMultiScan®. All remaining authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi, Z. M. et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: A systematic review and meta-analysis. J. Hepatol.71, 793–801. 10.1016/j.jhep.2019.06.021 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Saklayen, M. G. The global epidemic of the metabolic syndrome. Curr. Hypertens. Rep.20, 12. 10.1007/s11906-018-0812-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parthasarathy, G., Revelo, X. & Malhi, H. Pathogenesis of nonalcoholic steatohepatitis: an overview. Hepatol. Commun.4, 478–492. 10.1002/hep4.1479 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fox, A., Feng, W. & Asal, V. What is driving global obesity trends? Globalization or “modernization”?. Glob. Health.15, 32. 10.1186/s12992-019-0457-y (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aceves-Martins, M., Llauradó, E., Tarro, L., Solà, R. & Giralt, M. Obesity-promoting factors in Mexican children and adolescents: challenges and opportunities. Glob. Health Action.9, 29625. 10.3402/gha.v9.29625 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shamah-Levy, T. et al. Nutrition status of children, teenagers, and adults from National Health and Nutrition Surveys in Mexico From 2006 to 2020. Front. Nutr.25, 777246. 10.3389/fnut.2021.777246 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kopp, W. How western diet and lifestyle drive the pandemic of obesity and civilization diseases. Diabetes Metab. Syndr. Obes. Targets Ther.12, 2221–2236. 10.2147/DMSO.S216791 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vos, M. B. et al. NASPGHAN Clinical Practice Guideline for the Diagnosis and Treatment of Nonalcoholic Fatty Liver Disease in Children: Recommendations from the Expert Committee on NAFLD (ECON) and the North American Society of Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN). J. Pediatr. Gastroenterol. Nutr.64, 319–334. 10.1097/MPG.0000000000001482 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EASL–EASD–EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease, 2016. J. Hepatol.64, 1388–1402. 10.1016/j.jhep.2015.11.004 (2016). [DOI] [PubMed]
- 10.Hunter, A. K. & Lin, H. C. Review of clinical guidelines in the diagnosis of pediatric nonalcoholic fatty liver disease. Clin. Liver Dis.18, 40–44. 10.1002/cld.1094 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bejarano, P. A., Koehler, A. & Sherman, K. E. Second opinion pathology in liver biopsy interpretation. Am. J. Gastroenterol.96, 3158–3164. 10.1111/j.1572-0241.2001.05273.x (2001). [DOI] [PubMed] [Google Scholar]
- 12.Goldin, R. D. et al. Intra-observer and inter-observer variation in the histopathological assessment of chronic viral hepatitis. J. Hepatol.25, 649–654. 10.1016/s0168-8278(96)80234-0 (1996). [DOI] [PubMed] [Google Scholar]
- 13.Ratziu, V. et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology.128, 1898–1906. 10.1053/j.gastro.2005.03.084 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Thomaides-Brears, H. B. et al. Incidence of complications from percutaneous biopsy in chronic liver disease: a systematic review and meta-analysis. Dig. Dis. Sci.67, 3366–3394. 10.1007/s10620-021-07089-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuberger, J. & Cain, O. The need for alternatives to liver biopsies: non-invasive analytics and diagnostics. Hepatic Med. Evid. Res.13, 59–69. 10.2147/HMER.S278076 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwok, R. et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease—the role of transient elastography and plasma cytokeratin-18 fragments. Aliment. Pharmacol. Ther.39, 254–269. 10.1111/apt.12569 (2014). [DOI] [PubMed] [Google Scholar]
- 17.da Silva, L. et al. Ultrasound elastography in patients with fatty liver disease. Radiol. Bras.53, 47–55. 10.1590/0100-3984.2019.0028 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.British Society of Pediatric Gastroenterlogy Hepatology and Nutrition (BSPGHAN) Liver Steering Group. UK Fatty Liver Guideline August 2020. https://bspghan.org.uk/wp-content/uploads/2020/08/LSG_UK-Fatty-Liver-Guideline-August-2020.pdf (2020).
- 19.Janowski, K. et al. Quantitative multiparametric MRI as a non-invasive stratification tool in children and adolescents with autoimmune liver disease. Sci. Rep.11, 15261. 10.1038/s41598-021-94754-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janowski, K. et al. Multiparametric MRI as a noninvasive monitoring tool for children with autoimmune hepatitis. J. Pediatr. Gastroenterol. Nutr.72, 108–114. 10.1097/MPG.0000000000002930 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Mahalingam, N. et al. Associations between MRI T1 mapping, liver stiffness, quantitative MRCP, and laboratory biomarkers in children and young adults with autoimmune liver disease. Abdom. Radiol.47, 672–683. 10.1007/s00261-021-03378-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rijnberg, F. M. et al. 4D flow cardiovascular magnetic resonance derived energetics in the Fontan circulation correlate with exercise capacity and CMR-derived liver fibrosis/congestion. J. Cardiovasc. Magn. Reson.24, 21. 10.1186/s12968-022-00854-4 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dillman, J. R. et al. Comparison of liver T1 relaxation times without and with iron correction in pediatric autoimmune liver disease. Pediatr. Radiol.50, 935–942. 10.1007/s00247-020-04663-8 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Dillman, J. R. et al. Diagnostic performance of quantitative magnetic resonance imaging biomarkers for predicting portal hypertension in children and young adults with autoimmune liver disease. Pediatr. Radiol.49, 332–341. 10.1007/s00247-018-4319-1 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levick, C. et al. Non-invasive assessment of portal hypertension by multi-parametric magnetic resonance imaging of the spleen: A proof of concept study. PLoS One.14, e0221066. 10.1371/journal.pone.0221066 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jayaswal, A. N. A. et al. Prognostic value of multiparametric magnetic resonance imaging, transient elastography and blood-based fibrosis markers in patients with chronic liver disease. Liver Int. Off. J. Int. Assoc. Study Liver.40, 3071–3082. 10.1111/liv.14625 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Harrison, S. A. et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Lond. Engl.394, 2012–2024. 10.1016/S0140-6736(19)32517-6 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Harrison, S. A. et al. Safety, tolerability, and biologic activity of AXA1125 and AXA1957 in subjects with nonalcoholic fatty liver disease. Am. J. Gastroenterol.116, 2399–2409. 10.14309/ajg.0000000000001375 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrison, S. A. et al. Utility and variability of three non-invasive liver fibrosis imaging modalities to evaluate efficacy of GR-MD-02 in subjects with NASH and bridging fibrosis during a phase-2 randomized clinical trial. PLoS One.13, e0203054. 10.1371/journal.pone.0203054 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison, S. A. et al. NGM282 improves liver fibrosis and histology in 12 weeks in patients with nonalcoholic steatohepatitis. Hepatol.71, 1198–1212. 10.1002/hep.30590 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKay, A. et al. Patient understanding and experience of non-invasive imaging diagnostic techniques and the liver patient pathway. J. Patient-Rep. Outcomes.5, 89. 10.1186/s41687-021-00363-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imajo, K. et al. Quantitative multiparametric magnetic resonance imaging can aid non-alcoholic steatohepatitis diagnosis in a Japanese cohort. World J. Gastroenterol.27, 609–623. 10.3748/wjg.v27.i7.609 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mojtahed, A. et al. Repeatability and reproducibility of deep-learning-based liver volume and Couinaud segment volume measurement tool. Abdom. Radiol. N. Y.47, 143–151. 10.1007/s00261-021-03262-x (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilman, H. R. et al. Characterisation of liver fat in the UK Biobank cohort. PLoS One.12, e0172921. 10.1371/journal.pone.0172921 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan, J.-J. & Fallon, M. B. Gender and racial differences in nonalcoholic fatty liver disease. World J. Hepatol.6, 274–283. 10.4254/wjh.v6.i5.274 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villavicencio, E. A. Communicating PNPLA3 genetic risk status for NAFLD among Mexican-origin men. Front. Public Health.10, 1090101. 10.3389/fpubh.2022.1090101 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newton, K. P. et al. Nonalcoholic steatohepatitis clinical research network. Prevalence of prediabetes and type 2 diabetes in children with nonalcoholic fatty liver disease. JAMA Pediatr.170, e161971. 10.1001/jamapediatrics.2016.1971 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cioffi, C. E. et al. Natural history of NAFLD diagnosed in childhood: a single-center study. Children4, Article 34. 10.3390/children4050034 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon, T. G., Roelstraete, B., Khalili, H., Hagström, H. & Ludvigsson, J. F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut70, 1375–1382. 10.1136/gutjnl-2020-322786 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO); European Association for the Study of the Liver (EASL). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 81, 492–542. 10.1016/j.jhep.2024.04.031 (2024). [DOI] [PubMed]
- 41.Kuczmarski, R.J. et al. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat.11, 1–190. https://stacks.cdc.gov/view/cdc/6451 (2002). [PubMed]
- 42.Kadam, P. & Bhalerao, S. Sample size calculation. Int. J. Ayurveda Res.1, 55–57. 10.4103/0974-7788.59946 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison, S. A. et al. Prospective evaluation of the prevalence of non-alcoholic fatty liver disease and steatohepatitis in a large middle-aged US cohort. J. Hepatol.75, 284–291. 10.1016/j.jhep.2021.02.034 (2021). [DOI] [PubMed] [Google Scholar]
- 44.American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care.45, S17–S38. 10.2337/dc22-S002 (2022). [DOI] [PubMed]
- 45.Ahrens, W. et al. Metabolic syndrome in young children: definitions and results of the IDEFICS study. Int. J. Obes.38(Suppl 2), S4-14. 10.1038/ijo.2014.130 (2014). [DOI] [PubMed] [Google Scholar]
- 46.Andersson, A. et al. Clinical utility of MRI biomarkers for identifying NASH patients’ high risk of progression: A multi-center pooled data and meta-analysis. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 20, 2451–2461. 10.1016/j.cgh.2021.09.041 (2021). [DOI] [PubMed]
- 47.Bachtiar, V. et al. Repeatability and reproducibility of multiparametric magnetic resonance imaging of the liver. PLoS One.14, e0214921. 10.1371/journal.pone.0214921 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, E. L. & Schwimmer, J. B. Epidemiology of pediatric nonalcoholic fatty liver disease. Clin. Liver. Dis.17, 196–199. 10.1002/cld.1027 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon, T. G. et al. Non-alcoholic fatty liver disease in children and young adults is associated with increased long-term mortality. J. Hepatol.75, 1034–1041. 10.1016/j.jhep.2021.06.034 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fuji, H. et al. Clinical outcomes in biopsy-proven nonalcoholic fatty liver disease patients: a multicenter registry-based cohort study. Clin. Gastroenterol. Hepatol.21, 370–379. 10.1016/j.cgh.2022.01.002 (2022). [DOI] [PubMed] [Google Scholar]
- 51.O’Hara, J. et al. Cost of non-alcoholic steatohepatitis in Europe and the USA: The GAIN study. JHEP Rep.2, 100142. 10.1016/j.jhepr.2020.100142 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cusi, K. et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol.28, 528–562. 10.1016/j.eprac.2022.03.010 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Long, M. T., Noureddin, M. & Lim, J. K. AGA clinical practice update: diagnosis and management of nonalcoholic fatty liver disease in lean individuals: expert review. Gastroenterology.163, 764–774. 10.1053/j.gastro.2022.06.023 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anderson, E. L. et al. The prevalence of non-alcoholic fatty liver disease in children and adolescents: a systematic review and meta-analysis. PloS One.10, e0140908. 10.1371/journal.pone.0140908 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pavlides, M. et al. Multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease severity. Liver. Int.37, 1065–1073. 10.1111/liv.13284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eddowes, P. J. et al. Utility and cost evaluation of multiparametric magnetic resonance imaging for the assessment of non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther.47, 631–644. 10.1111/apt.14469 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Baratta, F. et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin. Gastroenterol. Hepatol.18, 2324–2331. 10.1007/s11739-012-0819-4 (2020). [DOI] [PubMed] [Google Scholar]
- 58.Newsome, P. N. et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N. Engl. J. Med.384, 1113–1124. 10.1056/NEJMoa2028395 (2021). [DOI] [PubMed] [Google Scholar]
- 59.Jiménez-Cortegana, C. et al. Role of leptin in non-alcoholic fatty liver disease. Biomedicines.9, 762. 10.3390/biomedicines9070762 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sammer, M. B. K. et al. Use of artificial intelligence in radiology: impact on pediatric patients, a white paper from the ACR pediatric AI workgroup. J. Am. Coll. Radiol.20, 730–737. 10.1016/j.jacr.2023.06.003 (2023). [DOI] [PubMed] [Google Scholar]
- 61.Muratori, L., Lohse, A. W. & Lenzi, M. Diagnosis and management of autoimmune hepatitis. BMJ.380, e070201. 10.1136/bmj-2022-070201 (2023). [DOI] [PubMed] [Google Scholar]
- 62.Carrion, A. F., Ghanta, R., Carrasquillo, O. & Martin, P. Chronic liver disease in the hispanic population of the United States. Clin. Gastroenterol. Hepatol.9, 834-e110. 10.1016/j.cgh.2011.04.027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Le Garf, S., Negre, V., Anty, R. & Gual, P. Metabolic fatty liver disease in children: A growing public health problem. Biomedicines.9, 1–23. 10.3390/biomedicines9121915 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shingina, A. et al. Future trends in demand for liver transplant: birth cohort effects among patients with NASH and HCC. Transplantation.103, 140–148. 10.1097/TP.0000000000002497 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and analytic methods used in this study remain the property of the individual study sponsors. All deidentified participant data are not openly available presently to allow privileged use of the data to the funded researchers and may be made available to other researchers upon request to P-WS, following permission, investigator support and a signed data access agreement. Study metadata will be made available through the MRC Research Data Gateway to allow potential users to make contact and request access to the data.