Abstract
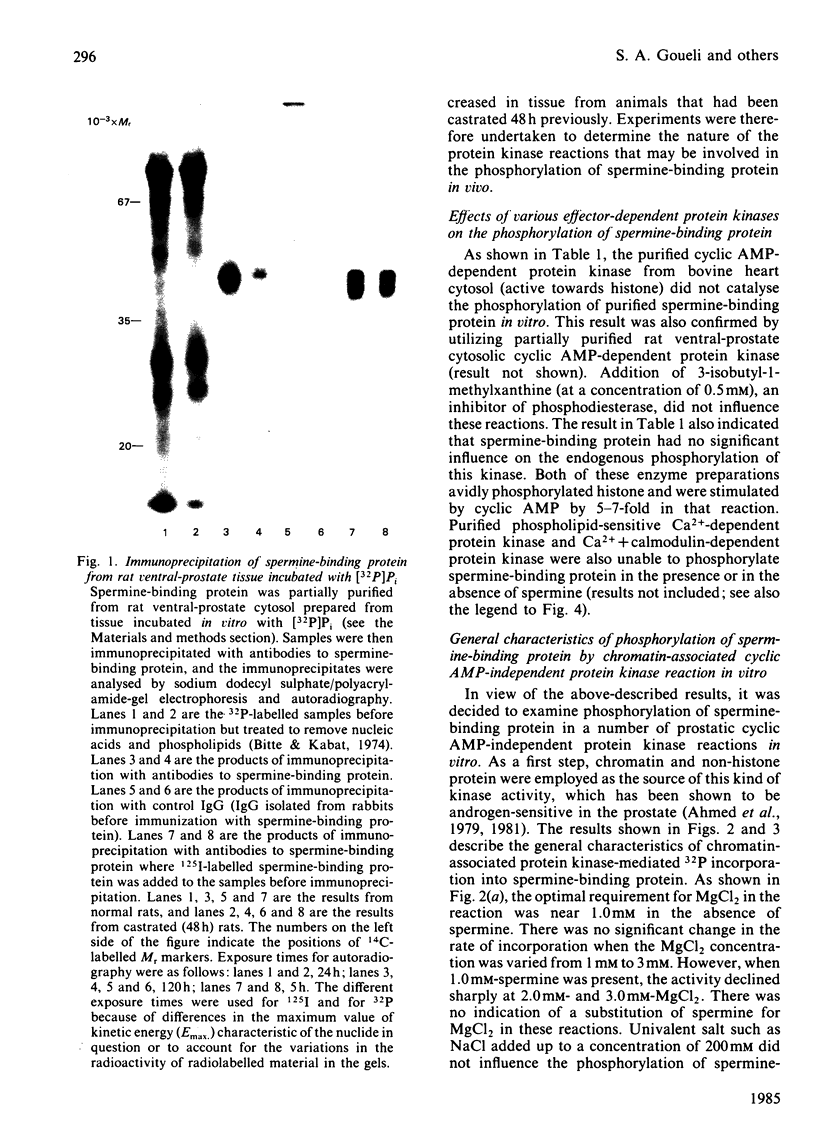
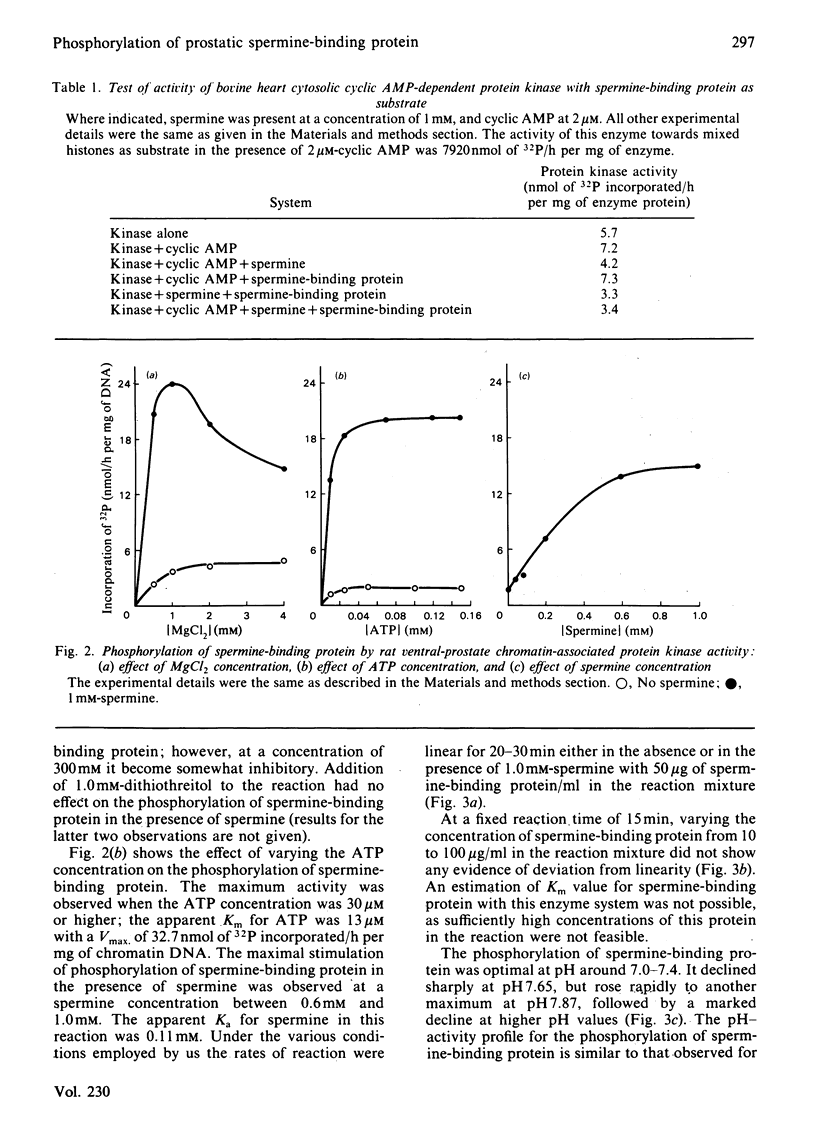
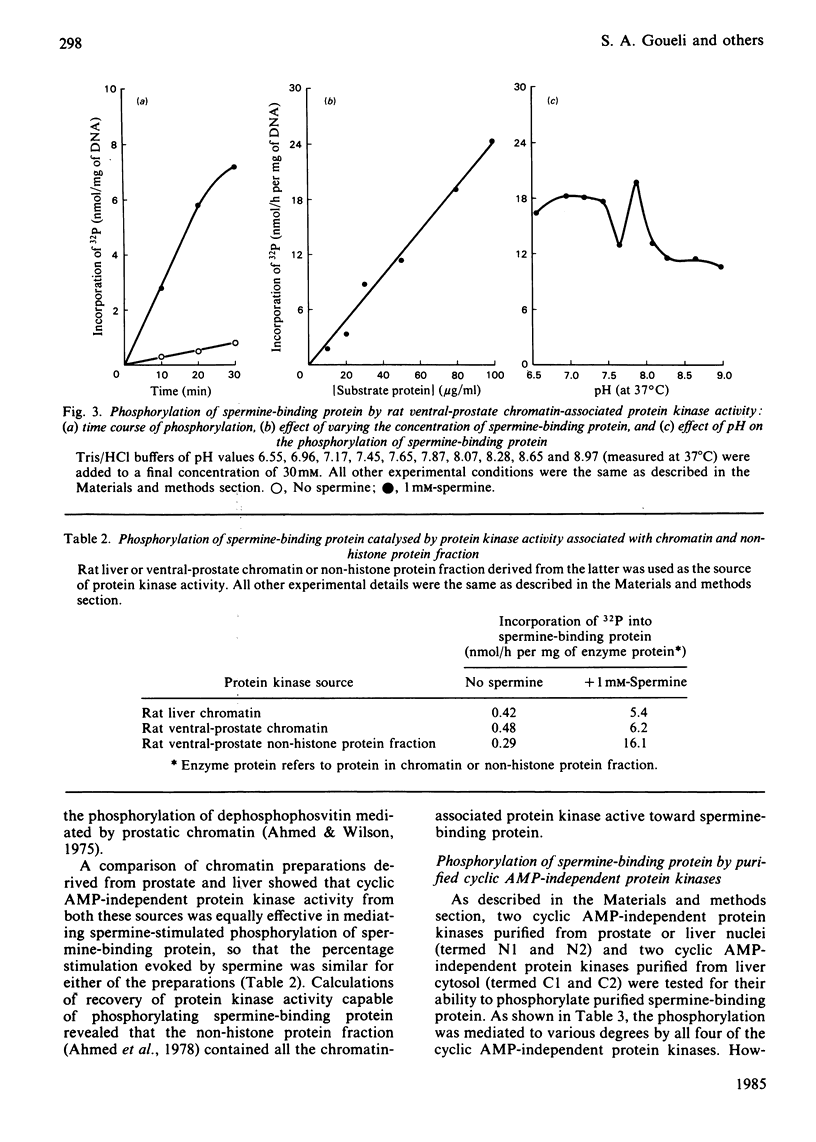
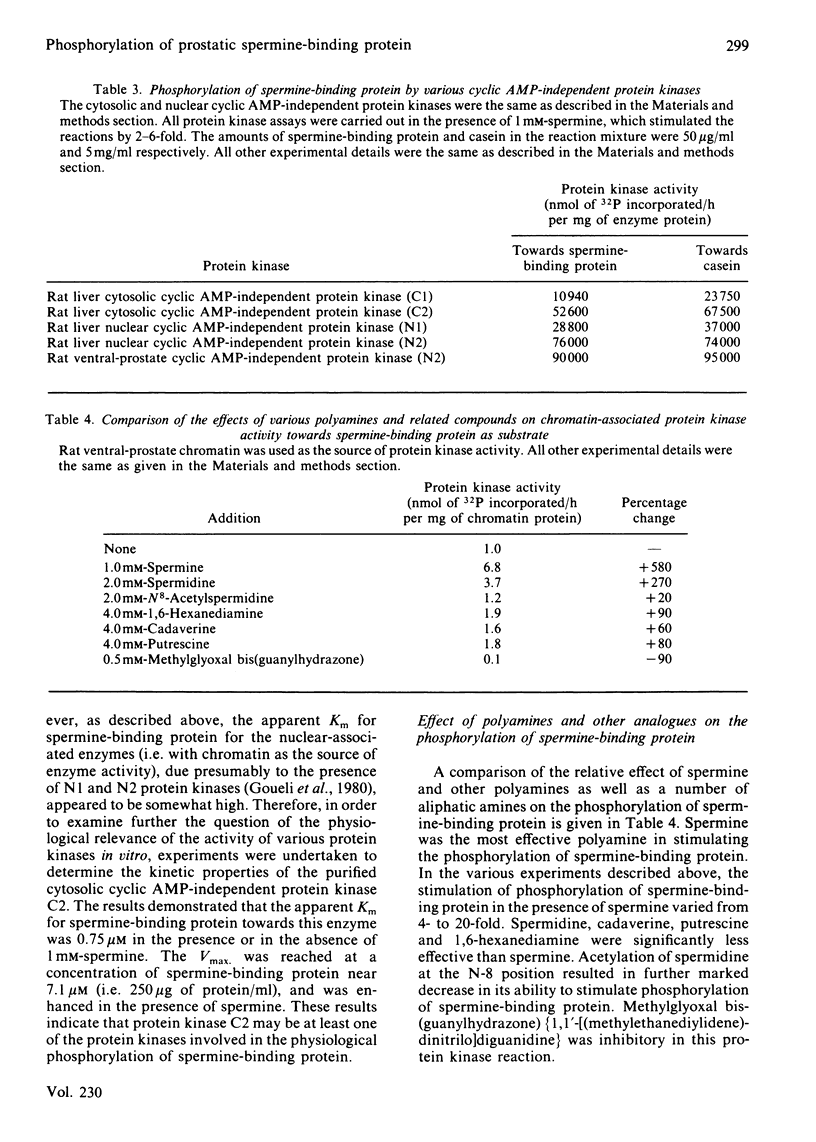
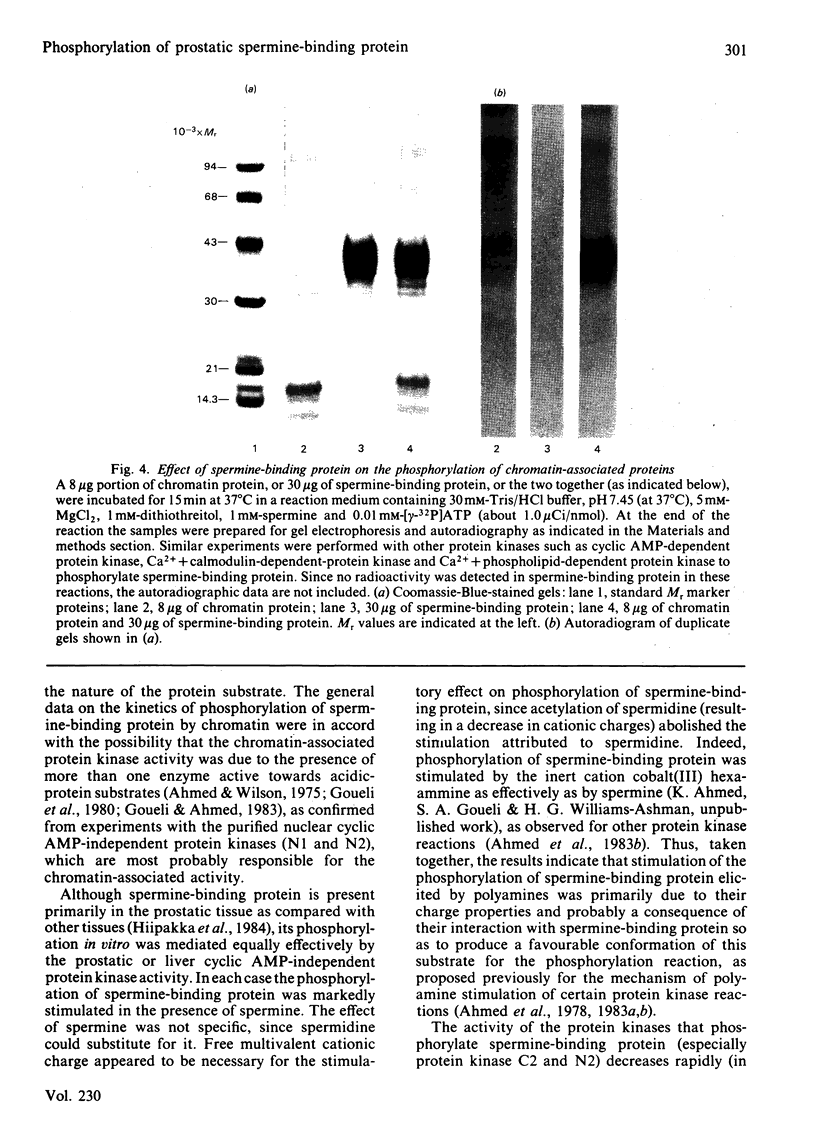
Spermine-binding protein (a rat ventral prostatic protein with high affinity for spermine) was phosphorylated in situ through the action of intrinsic cellular protein kinase(s), suggesting it to be a phosphoprotein in vivo. The purified protein served as a substrate in a number of cyclic AMP-independent protein kinase reactions in vitro, but not for cyclic AMP-dependent, Ca2+ + calmodulin-dependent or Ca2+ + phospholipid-dependent protein kinases. Available data indicate that at least one of the cyclic AMP-independent protein kinases (cytosolic protein kinase C2) may be physiologically relevant in mediating the phosphorylation of this protein. The phosphorylation reaction was stimulated several-fold in the presence of spermine. Spermidine was somewhat less effective, whereas putrescine, cadaverine and 1,6-hexanediamine were minimally active. Phospho amino acid analysis of 32P-labelled spermine-binding protein indicated that phosphoserine was the only labelled phospho amino acid. Spermine-binding protein did not undergo autophosphorylation, or modify the stimulative effect of spermine on the phosphorylation of other substrates such as non-histone proteins. In situ the phosphorylation of spermine-binding protein in tissue from castrated rats was markedly diminished as compared with the normal. Since the phosphorylation of spermine-binding protein appears to be mediated by cyclic AMP-independent protein kinase(s) whose activity in the prostate is under androgenic control, it is suggested that androgen-dependent modulation of the protein kinase(s) exerts a regulatory control (via phosphorylation-dephosphorylation) on the spermine-binding activity and stability of this protein in vivo. Further, since this protein is a substrate for only the cyclic AMP-independent protein kinases, it could serve as a tool for the investigation of such kinases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Davis A. T., Goueli S. A. Differential effects of polyamines on the phosphorylation of chromatin-associated proteins. Biochem J. 1983 Jan 1;209(1):197–205. doi: 10.1042/bj2090197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed K., Goueli S. A., Williams-Ashman H. G. Polyamine-like effects of cobalt (III) hexaammine on various cyclic nucleotide-independent protein phosphokinase reactions. Biochem Biophys Res Commun. 1983 Apr 15;112(1):139–146. doi: 10.1016/0006-291x(83)91808-9. [DOI] [PubMed] [Google Scholar]
- Ahmed K., Ishida H. Effect of testosterone on nuclear phosphoproteins of rat ventral prostate. Mol Pharmacol. 1971 May;7(3):323–327. [PubMed] [Google Scholar]
- Ahmed K., Wilson M. J. Chromatin-associated protein phosphokinases of rat ventral prostate. Characteristics and effects of androgenic status. J Biol Chem. 1975 Mar 25;250(6):2370–2375. [PubMed] [Google Scholar]
- Ahmed K., Wilson M. J., Davis A. T. Phosvitin phosphate content. Implications for protein kinase assay. Biochim Biophys Acta. 1975 Jan 23;377(1):80–83. doi: 10.1016/0005-2744(75)90288-0. [DOI] [PubMed] [Google Scholar]
- Ahmed K., Wilson M. J., Goueli S. A., Williams-Ashman H. G. Effects of polyamines on prostatic chromatin- and non-histone-protein-associated protein kinase reactions. Biochem J. 1978 Dec 15;176(3):739–750. doi: 10.1042/bj1760739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitte L., Kabat D. Isotopic labeling and analysis of phosphoproteins from mammalian ribosomes. Methods Enzymol. 1974;30:563–590. doi: 10.1016/0076-6879(74)30056-0. [DOI] [PubMed] [Google Scholar]
- Cochet C., Job D., Pirollet F., Chambaz E. M. Cyclic nucleotide independent casein kinase (G type) in bovine adrenal cortex: purification and properties of two molecular forms. Biochim Biophys Acta. 1981 Apr 14;658(2):191–201. doi: 10.1016/0005-2744(81)90289-8. [DOI] [PubMed] [Google Scholar]
- Dahmus M. E. Purification and properties of calf thymus casein kinases I and II. J Biol Chem. 1981 Apr 10;256(7):3319–3325. [PubMed] [Google Scholar]
- Erdmann H., Böcher M., Wagner K. G. Two protein kinases from nuclei of cultured tobacco cells with properties similar to the cyclic nucleotide-independent enzymes (NI and NII) from animal tissue. FEBS Lett. 1982 Jan 25;137(2):245–248. doi: 10.1016/0014-5793(82)80359-1. [DOI] [PubMed] [Google Scholar]
- Goueli S. A., Ahmed K. Fractionation and partial purification of rat liver nuclear protein kinases. Int J Biochem. 1983;15(9):1109–1118. doi: 10.1016/0020-711x(83)90225-2. [DOI] [PubMed] [Google Scholar]
- Goueli S. A., Ahmed K. Indomethacin and inhibition of protein kinase reactions. Nature. 1980 Sep 11;287(5778):171–172. doi: 10.1038/287171a0. [DOI] [PubMed] [Google Scholar]
- Goueli S. A., Ahmed K. Phosphorylation of prostatic nuclear matrix proteins is under androgenic control. Arch Biochem Biophys. 1984 Nov 1;234(2):646–650. doi: 10.1016/0003-9861(84)90315-1. [DOI] [PubMed] [Google Scholar]
- Goueli S. A., Steer R. C., Wilson M. J., Ahmed K. Partial purification and differential androgen sensitivity of protein phosphokinases from nuclei of rat ventral prostate. Eur J Biochem. 1980 Dec;113(1):45–51. doi: 10.1111/j.1432-1033.1980.tb06137.x. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Purification of casein kinases. J Biol Chem. 1979 Feb 10;254(3):762–768. [PubMed] [Google Scholar]
- Hiipakka R. A., Chen C., Schilling K., Oberhauser A., Saltzman A., Liao S. Immunochemical characterization of the androgen-dependent spermine-binding protein of the rat ventral prostate. Biochem J. 1984 Mar 1;218(2):563–571. doi: 10.1042/bj2180563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liang T., Mezzetti G., Chen C., Liao S. Selective polyamine-binding proteins. Spermine binding by an androgen-sensitive phosphoprotein. Biochim Biophys Acta. 1978 Sep 6;542(3):430–441. doi: 10.1016/0304-4165(78)90374-4. [DOI] [PubMed] [Google Scholar]
- Mezzetti G., Loor R., Liao S. Androgen-sensitive spermine-binding protein of rat ventral prostate. Purification of the protein and characterization of the hormonal effect. Biochem J. 1979 Nov 15;184(2):431–440. doi: 10.1042/bj1840431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K. M., Bell L. E., Siefken D. A., Jacob S. T. A heparin-sensitive nuclear protein kinase. Purification, properties, and increased activity in rat hepatoma relative to liver. J Biol Chem. 1981 Jul 25;256(14):7468–7477. [PubMed] [Google Scholar]
- Thornburg W., Gamo S., O'Malley A. F., Lindell T. J. Properties of rat liver nuclear protein kinases. Biochim Biophys Acta. 1979 Nov 9;571(1):35–44. doi: 10.1016/0005-2744(79)90222-5. [DOI] [PubMed] [Google Scholar]
- Tse E. Y., Goueli S. A., Ahmed K. Differential androgenic control of prostatic cytosolic cAMP-dependent and -independent protein kinases. Arch Biochem Biophys. 1984 Apr;230(1):39–48. doi: 10.1016/0003-9861(84)90084-5. [DOI] [PubMed] [Google Scholar]