Abstract
Background:
In Denmark, cost savings motivate mandatory biosimilar switches. In 2018, patients switched from originator to biosimilar adalimumab, that is, to GP2017 in Eastern and to SB5 in Western Denmark. However, concerns were raised about additional costs covering, that is, an increased number of outpatient visits due to patient education, treatment monitoring, and patient concerns.
Objectives:
To investigate whether the switch led to increased total healthcare costs, defined as costs related to in- and outpatient contacts in hospitals and the primary sector and use of prescription medicine (excluding biological treatment).
Design:
Observational cohort study with geographical cluster pseudo-randomization.
Methods:
Patients with rheumatoid arthritis, psoriatic arthritis (PsA), and axial spondyloarthritis (AxSpA), who switched were identified in the nationwide DANBIO registry. Total healthcare costs 9 months before and after the switch were captured from the National Patient and Prescription registries. The difference between pre- and post-switch costs was estimated by a generalized estimation equations (GEE) model.
Results:
Overall, 1316 patients switched to GP2017 (n = 621) or SB5 (n = 695). Total healthcare costs were mainly driven by hospital costs. The monthly fluctuations of hospital costs 9 months before and after the switch were largely similar or decreased. In the adjusted analyses (GEE), hospital costs decreased after the switch (by approximately 15%) for GP2017 switchers, especially PsA (estimate = 0.83; 95% CI 0.75–0.92) and AxSpA patients (estimate = 0.85; 0.77–0.93), with no significant changes for SB5 switchers.
Conclusion:
We found no increase in total healthcare costs in 9 months following a nationwide mandatory adalimumab originator to biosimilar switch. Our findings were strengthened by similar results for GP2017 and SB5.
Keywords: inflammatory arthritis, biosimilar switches, health-economic outcomes, adalimumab
Introduction
Biological disease-modifying antirheumatic drugs (bDMARDs) have improved the treatment of inflammatory rheumatic diseases, including rheumatoid arthritis (RA), psoriatic arthritis (PsA), and axial spondyloarthritis (AxSpA). 1 Associated medication costs, however, pose a major economic burden, also limiting patient access around the world. During the last decade, the patents of several biologics have expired leading to the marketing of less expensive biosimilars.2,3 For adalimumab, several biosimilars are available based on results from randomized clinical trials (RCTs) demonstrating non-inferiority among patients switching from the originator to a biosimilar.4–8 In 2022, the European Union (EU) medicines regulatory network recommended biosimilars to be considered interchangeable meaning that they can replace the reference product or other biosimilars of the same reference product. 9 The use of biosimilars has further been recommended in international guidelines and consensus statements, including the EULAR recommendations for the management of RA.10,11 This has created financial incentives among stakeholders to implement switching for cost savings (=non-medical switch) and thus potentially increased patient access.12,13
Policies for biosimilar uptake vary between European countries and around the world. In Denmark, national guidelines for the use of biological and targeted synthetic DMARDs are issued annually based on a national tender system. This has resulted in several non-medical switches since 2015 and thus a high biosimilar uptake.14–16
In the year 2018, the Danish national guidelines mandated the switch of all patients with inflammatory rheumatic diseases treated with originator adalimumab to biosimilar adalimumab based on geographical residence.14,17 Based on the nationwide tendering process, two different adalimumab biosimilars were available. Patients in Eastern regions (the Capital Region and Region Zealand) were switched to GP2017 (Hyrimoz®) and those in Western regions of Denmark (Region North, Middle, and South) to SB5 (Imraldi®). This strategy provided a unique opportunity for a pseudo-randomized study, and we have previously demonstrated that the switch had no impact on disease activity and treatment retention. 17 The economic benefit was expected to be 34%–49% of the annual adalimumab medication costs (corresponding to approximately 50 million euros annually).17–19 However, concerns were raised regarding benefits being outweighed by additional costs covering, that is, increased numbers of outpatient visits or hospital contacts due to patient education, treatment monitoring, and patient concerns. This could potentially be driven by factors such as changes in brand name, differences in devices, use of excipients, etc.19–22
Health-economic studies of adalimumab switching have mainly focused on the treatment costs of the biologic drug,18,19 whereas costs associated with healthcare utilization have not yet been addressed. Thus, the primary aim of this study was to investigate whether the switch from originator to either of the adalimumab biosimilars (GP2017/SB5) led to increased total healthcare costs. These were defined as all costs related to in- and outpatient contacts in hospitals and the primary sector and costs of prescription medicine (excluding biological treatment). In addition, it was investigated whether there was a change in healthcare utilization, and if selected patient and disease characteristics affected costs.
Methods
Study design
This was a population-based, observational cohort study with surrogate cluster (i.e., geographical) pseudo-randomization, as previously described in Nabi et al. 17 We emulated a pragmatic trial, in which eligible participants were patients with RA, PsA, or AxSpA monitored in the DANBIO registry and treated with originator adalimumab, who switched to either GP2017 or SB5 according to the national guideline (=switchers). Total healthcare costs before and after switching were estimated with patients serving as their controls in the statistical analyses of healthcare use before and after the switch.
Data sources
Eligible patients were identified in the Danish DANBIO quality registry, which was established in the year 2000, with a prospective follow-up of >95% of all Danish adults with rheumatic disease treated with bDMARDs in routine care.23,24 For the current study, data in DANBIO were censored by December 2020. By use of the unique civil registration numbers, which each Danish citizen receives at birth, DANBIO data were enriched with patient-level information from the Danish National Patient Registry (DNPR), the Danish Health Insurance Register, and The Danish National Prescription Registry. Thus, we identified comorbidities and utilization of healthcare services, including hospital admissions and duration (hospital days), outpatient visits, primary sector contacts, and prescription medication. The DNPR has virtually complete data on in- and outpatient contacts including information on the ICD-10 diagnosis for the contact. 25 The Danish Health Insurance Register contains information on the settlement and use of health insurance services between the hospital owners (Danish Regions) and providers covered by the health insurance, that is, general practitioners (GPs), practicing specialists, dentists, psychologists, etc. This information was used to estimate the costs of healthcare utilization. 26 The Danish National Prescription registry has individual-level information on all dispensed prescriptions, including ATC-codes 27 (Supplemental Table 1). All findings have been reported in accordance with the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) statement. 28
Study population and study period
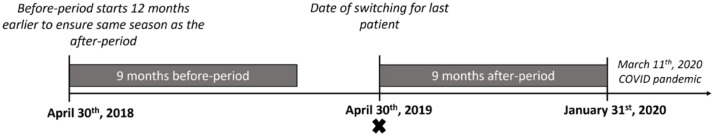
The study included originator-treated patients with a clinical diagnosis in DANBIO of RA, PsA, or AxSpA, who switched to either biosimilar GP2017 or SB5 between November 1st, 2018 and April 30th, 2019. 17 The baseline (=index date) was the date of the switch. In Denmark, the first COVID-19 lockdown started March 11th, 2020. To minimize the impact of the COVID-19 lockdown on the analyses, the duration of the before- and after-switch periods was restricted to 9 months, that is, the after-switch period ended (for the last patient) on January 31st, 2020 (Figure 1). To ensure that each switcher had comparable calendar seasons in the before- and after-period, the before-period was set to start on the same date the year before (index date for the individual patient minus 12 months; Figure 1).
Figure 1.
Overview of study periods. The figure illustrates the dates for the last patient in the cohort. The X indicates the index date (date of switch).
Outcomes
Primary outcome: Total healthcare costs
Total healthcare costs in the above-defined 9 months of before- and after-switch period (the latter including the index date) were estimated and compared. The cost analysis was divided into costs for hospital contacts, primary sector contacts, and prescription medication. Costs for hospital contacts were calculated according to the activity-based fees (the so-called Diagnosis Related Groups tariffs) and were divided into costs related to (1) the musculoskeletal system and connective tissue (ICD10 codes M00–M99) and (2) other diseases (Supplemental Table 2). 29
The primary sector included GPs, other specialist doctors, physiotherapists, chiropractors, and foot specialists.
Prescription medicine was grouped into prescribed pain medication (including paracetamol, aspirin, nonsteroid anti-inflammatory drugs (ATC-codes M01A, N02B) and opioids (N02A)) and other medications. Medication costs were estimated according to the defined daily dose (DDD) of medication total costs. Biological drugs were not included, as they are provided directly to the patients through hospitals and pricing information is not publicly available.
The analysis of total healthcare costs before and after the switch was divided into three parts: (1) an estimation of average costs before and after the switch, (2) an analysis of change in costs after (compared to before) the switch, and (3) investigation of whether patient characteristics affected changes. All analyses were stratified according to biosimilar drug and further by indication (RA, PsA, and AxSpA).
Secondary outcome: Healthcare utilization
Due to changes in the registration practice of treatment days for in- and outpatient care in the DNPR from 2019 and onwards, it was not possible to include information regarding healthcare utilization in the hospitals (hospital admissions, hospital days, and outpatient visits). 30 Primary sector healthcare utilization was measured as the number of unique dates with a contact with a GP, other specialist doctor, physiotherapist, chiropractor, foot specialist, or other based on data from the Danish Health Insurance Register. The use of medication was estimated as DDD. The analysis of utilization before and after the switch was divided into three parts, similar to the cost analysis above.
Clinical variables
Clinical characteristics and DMARD treatment history were retrieved from DANBIO and included the following covariates: age (years), sex (female/male), disease duration (years), use of concomitant methotrexate (MTX; yes/no), treatment duration for originator adalimumab (above/below 6 years), and whether biosimilar adalimumab treatment was withdrawn within 180 days after the switch (yes/no). Previous comorbidities for each patient were identified in the DNPR and coded according to the ICD-10 chapters (21 groups, Supplemental Table 2).
Ethics
The study was approved by the regional GDPR authority (Capital Region Denmark, RH-2015-209, 04145). In Denmark, registry studies neither require patient consent nor ethical approval. 31
Statistical analyses
All analyses were performed (using SAS V.9.4, SAS Institute Inc) for the overall population stratified by biosimilar drug and further stratified by diagnosis. A p value <0.05 was considered statistically significant. Clinical characteristics and disease activity are presented as medians (interquartile range) for continuous variables and frequencies (percentages) for categorical variables.
Total healthcare costs before and after the switch
Total healthcare costs before and after the switch were estimated by a two-step generalized estimation equations (GEE) regression model 32 with a gamma distribution and a log-link function, where we controlled for age, sex, biosimilar treatment withdrawal within 180 days, treatment duration of originator treatment, and previous comorbidities (Supplemental Table 2). For each patient, previous comorbidities were measured according to the 21 comorbidity groups but excluding musculoskeletal diseases. A GEE model was used since the costs before and after included repeated measures in the same patients and the costs at the two timepoints were correlated. 33
From the estimates from the GEE model, costs were predicted before and after the switch for a standard patient (defined as being female, 55 years old, not stopped treatment within 180 days, previous duration of originator adalimumab treatment >6 years, and with no comorbidities (excluding musculoskeletal) 12 months before the switch). To estimate whether there were changes in costs after the switch, we included a before–after dummy variable (after = 1) in the GEE model. The estimate for this dummy showed whether there were changes over time (before-minus-after).
For the analyses testing the influence of patient characteristics on changes in total healthcare costs, the statistical model was extended to include interaction terms for the before–after dummy (after = 1) and the variable for the respective patient characteristics (after*independent variable). Thus, it included the following interaction terms: after*sex, after*age, after*previous originator adalimumab treatment, and after*biosimilar withdrawal within 180 days. All estimates reported from the GEE model were on the original scale (the coefficients obtained were exponentiated).
Healthcare utilization before and after the switch
The healthcare utilization (treatment days, number of contacts in the primary sector, and medication usage as DDD) before and after the switch was analyzed using a Poisson regression model similarly including a before–after dummy variable (after = 1) and controlling for the above-mentioned clinical variables. The utilization was predicted before and after according to the same patient characteristics (standard patient) as in the cost analysis.
Results
In total, 1318 patients switched to either GP2017 or SB5 during the inclusion period. Two patients, who were not Danish residents at the index date, were excluded; thus 1316 patients were included in the analyses. Baseline characteristics at the time of switch for both the GP2017 (n = 621) and SB5 treatment groups (n = 695) are provided in Table 1. In general, patients had established disease (median disease duration 14 years), and the majority of patients (>70%) had been treated with originator adalimumab for >6 years before the index date, as previously described. 17 During 180 days of follow-up, 33 patients (5.3%) in the GP2017 group and 40 patients (5.8%) in the SB5 group withdrew treatment.
Table 1.
Characteristics of patients who switched from originator adalimumab to biosimilar GP2017 or SB5, overall and stratified by diagnosis.
| Clinical characteristics | All patients (n = 1316) | GP2017 (n = 621) | SB5 (n = 695) | |||||
|---|---|---|---|---|---|---|---|---|
| GP2017 | SB5 | PsA | RA | AxSpA | PsA | RA | AxSpA | |
| Number of patients (N) | 621 | 695 | 146 | 213 | 262 | 173 | 253 | 269 |
| Age a , years (median, IQR) | 55 (44–64) | 56 (47–65) | 55 (47–62) | 63 (56–70) | 46 (39–57) | 55 (48–63) | 64 (57–71) | 49 (42–57) |
| Female, n (%) | 320 (52) | 328 (47) | 80 (55) | 158 (74) | 82 (31) | 58 (34) | 181 (72) | 89 (33) |
| Disease duration a , years (median, IQR) | 14 (9–20) | 14 (9–21) | 13 (9–17) | 17 (12–23) | 12 (8–18) | 14 (9–20) | 16 (11–24) | 12 (8–18) |
| Concomitant methotrexate a , n (%) | 337 (54) | 377 (54) | 87 (60) | 178 (84) | 73 (28) | 109 (63) | 213 (84) | 55 (20) |
| Duration of original bio-treatment before the switch (n, %) | ||||||||
| 1 year | 25 (4) | 42 (6) | 7 (5) | 4 (2) | 14 (5) | 13 (8) | 7 (3) | 22 (8) |
| 2–3 years | 76 (12) | 43 (6) | 20 (14) | 24 (11) | 32 (12) | 7 (4) | 9 (4) | 27 (10) |
| 4–5 years | 73 (12) | 53 (8) | 16 (11) | 31 (15) | 26 (10) | 16 (9) | 18 (7) | 19 (7) |
| 6+ years | 447 (72) | 557 (80) | 103 (71) | 154 (72) | 190 (73) | 137 (79) | 219 (86) | 201 (75) |
| Stopped biosimilar treatment within 180 days (n, %) | 33 (5) | 40 (6) | 10 (7) | 8 (4) | 15 (6) | 6 (4) | 18 (7) | 16 (6) |
Source: Reprinted from Nabi et al. 17 with permission.
At baseline.
AxSpA, axial spondyloarthritis; IQR, interquartile range; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
Healthcare costs
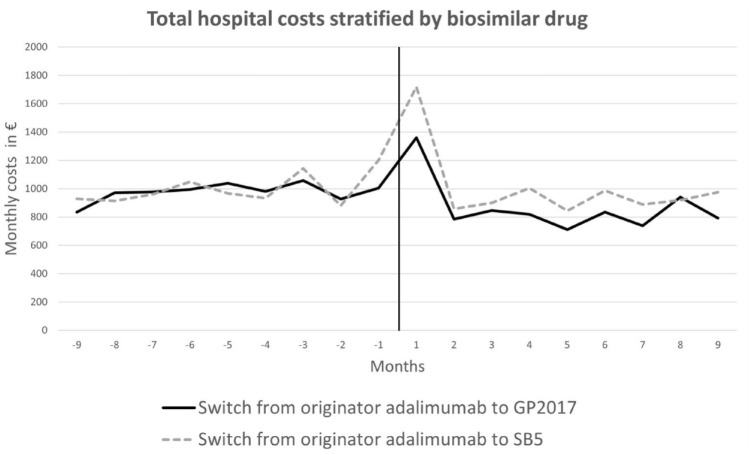
The hospital costs (i.e., all in- and outpatient contacts) represented the largest share of the total healthcare costs before and after switching, corresponding to approximately 90% of all costs (Table 2). The majority of these were related to diseases of the musculoskeletal system and connective tissue. The average monthly hospital costs 9 months before and after the switch remained largely similar or decreased after the switch (Figure 2). For both treatment groups, an increase was observed in the first month after the switch, which included the date of switching. Healthcare costs in the primary sector fluctuated throughout the 18-month period with no clear pattern (results not shown). The estimated health costs (in €) before and after the switch for a “standard patient” are given in Table 2 for both GP2017 and SB5 treatment cohorts. Results from the fully adjusted regression analyses regarding changes in costs are provided in Table 3. For the GP2017-switch cohort, a statistically significant decrease of approximately 15% in total health costs was observed for PsA (estimate = 0.85; 95% CI (0.78–0.93)) and AxSpA patients (0.86 (0.79–0.94)), mainly driven by reduced hospital costs. On the other hand, there was a 26% increase in costs of prescribed non-pain medication (PsA patients) and a 14% increase in primary sector costs (AxSpA; Table 3). For the SB5-switch cohort, significant changes were seen for costs related to prescription medication in RA patients (estimate = 1.11, 95% CI (1.01–1.23)) and other primary sector costs in AxSpA patients (0.74 (0.56–0.97), Table 3). Overall, sex, age, type of arthritis diagnosis, previous comorbidities, and adalimumab treatment history did not influence changes in healthcare costs, whereas type of biosimilar drug (GP2017 or SB5) had a significant impact. Results were similar in the total treatment cohort (Supplemental Table 3) and stratified by diagnosis (results not shown).
Table 2.
Adjusted average healthcare costs for a standard patient for GP2017 and SB5 stratified by diagnosis.
| Healthcare service | GP2017 | SB5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PsA | RA | AxSpA | PsA | RA | AxSpA | |||||||
| Health costs (predicted) | Health costs (predicted) | Health costs (predicted) | Health costs (predicted) | Health costs (predicted) | Health costs (predicted) | |||||||
| Before (€) | After (€) | Before (€) | After (€) | Before (€) | After (€) | Before (€) | After (€) | Before (€) | After (€) | Before (€) | After (€) | |
| Hospital costs | ||||||||||||
| Musculoskeletal system and the connective tissue | 7.018 | 5.880 | 7.311 | 8.058 | 7.142 | 6.641 | 7.696 | 8.003 | 7.603 | 7.851 | 6.877 | 7.767 |
| Other secondary sector treatment costs | 824 | 614 | 313 | 310 | 520 | 1.070 | 446 | 500 | 442 | 677 | 640 | 476 |
| Hospital total treatment costs | 8.048 | 6.236 | 7.446 | 8.051 | 8.010 | 7.534 | 7.903 | 8.054 | 7.799 | 8.357 | 7.565 | 8.026 |
| Primary sector | ||||||||||||
| General practitioner | 123 | 103 | 116 | 98 | 94 | 91 | 111 | 111 | 120 | 120 | 112 | 105 |
| Specialist | 70 | 124 | 81 | 56 | 58 | 108 | 56 | 81 | 40 | 40 | 81 | 54 |
| Physiotherapist, chiropractor, and foot specialist | 296 | 291 | 204 | 247 | 173 | 226 | 435 | 523 | 376 | 382 | 299 | 206 |
| Other | 52 | 48 | 47 | 42 | 31 | 80 | 58 | 43 | 40 | 41 | 56 | 51 |
| Primary sector total costs | 542 | 580 | 451 | 437 | 353 | 524 | 595 | 643 | 565 | 563 | 549 | 427 |
| Prescription medication | ||||||||||||
| Pain medication (DDD) | 59 | 42 | 18 | 18 | 14 | 30 | 46 | 36 | 29 | 29 | 58 | 43 |
| Not pain medication (DDD) | 209 | 234 | 128 | 130 | 172 | 312 | 255 | 223 | 124 | 192 | 86 | 74 |
| Prescription medication total costs (DDD) | 273 | 275 | 148 | 151 | 186 | 335 | 311 | 274 | 149 | 220 | 142 | 110 |
| Total healthcare costs | 8.856 | 7.083 | 8.048 | 8.662 | 8.603 | 8.346 | 8.859 | 9.013 | 8.511 | 9.110 | 8.103 | 8.378 |
Adjusted average healthcare costs, given in euro (€), estimated for a “standard patient” defined as a 55-year-old female, with no previous comorbidities, with previous duration of originator adalimumab >6 years and not withdrawn biosimilar GP2017/SB5 within 180 days.
AxSpA, axial spondyloarthritis; DDD, defined daily dose; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
Figure 2.
Monthly average hospital costs per patient 9 months before and after the switch from originator to biosimilar adalimumab. The black dotted line indicates the date of the switch. y-axis: mean cost per patient per month (unadjusted). Number of patients in each biosimilar treatment group: GP2017 (n = 621) and SB5 (n = 695).
Table 3.
Estimated healthcare costs after versus before switch stratified by diagnosis and biosimilar drug.
| PsA | RA | AxSpA | ||||
|---|---|---|---|---|---|---|
| After | After | After | ||||
| Estimate (95% CI) | p | Estimate (95% CI) | p | Estimate (95% CI) | p | |
| GP2017 | ||||||
| Hospital costs | ||||||
| Musculoskeletal system and the connective tissue | 0.77 (0.69–0.85) | <0.001 | 0.92 (0.79–1.05) | 0.22 | 0.82 (0.76–0.89) | <0.001 |
| Other hospital costs | 0.98 (0.68–1.41) | 0.92 | 1.25 (0.82–1.90) | 0.29 | 1.73 (1.15–2.60) | 0.008 |
| Total hospital costs | 0.83 (0.75–0.92) | <0.001 | 0.99 (0.86–1.14) | 0.87 | 0.85 (0.77–0.93) | <0.001 |
| Primary sector | ||||||
| General practitioner | 0.94 (0.79–1.11) | 0.46 | 0.87 (0.77–0.99) | 0.04 | 1.14 (1.00–1.31) | 0.06 |
| Specialist | 1.04 (0.72–1.49) | 0.85 | 1.02 (0.76–1.37) | 0.91 | 1.58 (1.11–2.25) | 0.01 |
| Physiotherapist, chiropractor, and foot specialist | 1.05 (0.82–1.35) | 0.71 | 1.16 (1.03–1.30) | 0.01 | 1.05 (0.87–1.27) | 0.62 |
| Other | 0.93 (0.73–1.19) | 0.58 | 0.89 (0.72–1.10) | 0.27 | 0.73 (0.59–0.90) | 0.003 |
| Primary sector total costs | 1.00 (0.87–1.16) | 0.97 | 1.02 (0.93–1.12) | 0.64 | 1.14 (1.00–1.29) | 0.04 |
| Prescription medication | ||||||
| Pain medication | 0.96 (0.73–1.27) | 0.77 | 0.92 (0.74–1.14) | 0.43 | 1.02 (0.84–1.25) | 0.83 |
| Not pain medication | 1.26 (1.07–1.48) | 0.005 | 1.05 (0.91–1.23) | 0.49 | 1.03 (0.88–1.21) | 0.69 |
| Prescription medication total costs | 1.20 (1.04–1.39) | 0.01 | 1.04 (0.91–1.19) | 0.56 | 1.04 (0.90–1.19) | 0.62 |
| Total healthcare costs | 0.85 (0.78–0.93) | <0.001 | 0.99 (0.87–1.13) | 0.89 | 0.86 (0.79–0.94) | <0.001 |
| SB5 | ||||||
| Hospital costs | ||||||
| Musculoskeletal system and the connective tissue | 1.05 (0.96–1.15) | 0.26 | 1.00 (0.94–1.07) | 0.99 | 1.00 (0.93–1.07) | 0.92 |
| Other hospital costs | 1.02 (0.73–1.45) | 0.89 | 1.08 (0.71–1.64) | 0.71 | 0.95 (0.54–1.67) | 0.86 |
| Total hospital costs | 1.03 (0.95–1.13) | 0.46 | 1.00 (0.93–1.08) | 0.97 | 1.01 (0.91–1.12) | 0.92 |
| Primary sector | ||||||
| General practitioner | 0.96 (0.83–1.11) | 0.54 | 0.97 (0.87–1.07) | 0.49 | 1.01 (0.89–1.16) | 0.84 |
| Specialist | 1.02 (0.58–1.81) | 0.93 | 0.94 (0.71–1.26) | 0.69 | 0.94 (0.60–1.46) | 0.78 |
| Physiotherapist, chiropractor, and foot specialist | 0.98 (0.83–1.17) | 0.84 | 1.09 (0.98–1.21) | 0.10 | 0.95 (0.86–1.04) | 0.26 |
| Other | 0.87 (0.74–1.03) | 0.11 | 0.97 (0.86–1.11) | 0.68 | 0.74 (0.56–0.97) | 0.03 |
| Primary sector total costs | 0.99 (0.88–1.12) | 0.90 | 1.02 (0.96–1.10) | 0.49 | 0.94 (0.86–1.02) | 0.15 |
| Prescription medication | ||||||
| Pain medication (DDD) | 0.92 (0.83–1.02) | 0.10 | 0.98 (0.88–1.10) | 0.76 | 0.98 (0.85–1.12) | 0.73 |
| Not pain medication (DDD) | 1.02 (0.93–1.11) | 0.74 | 1.13 (1.02–1.26) | 0.02 | 0.96 (0.87–1.06) | 0.40 |
| Prescription medication total costs (DDD) | 1.00 (0.93–1.08) | 0.95 | 1.11 (1.01–1.23) | 0.03 | 0.96 (0.88–1.04) | 0.31 |
| Total healthcare costs | 1.03 (0.95–1.11) | 0.48 | 1.01 (0.94–1.08) | 0.85 | 1.00 (0.91–1.10) | 0.99 |
Results of the GLM model (after = 1) adjusted for sex, age, number of years treated with originator drug (>6 years = 1), stopped the medication within 180 days (yes–no), and comorbidity (number of WHO 21 chapters excluding WHO 13 ICD-10 M (musculoskeletal diseases)). Values in bold indicate statistically significant results.
AxSpA, axial spondyloarthritis; DDD, defined daily dose; PsA, psoriatic arthritis; RA, rheumatoid arthritis.
Healthcare utilization before and after the switch
Results from the analyses of healthcare utilization in the primary sector and prescribed medication before and after the switch are given in Supplemental Tables 4 to 5. There were slight fluctuations in utilization across treatment groups with no clear pattern in relation to the switch.
Discussion
In this nationwide study of >1300 Danish real-world patients with inflammatory arthritis, we investigated the health-economic consequences of switching from originator to biosimilar adalimumab (GP2017 or SB5, based on geographical residence) following a mandatory nationwide guideline. Overall, we found no negative impact of the switch on total healthcare costs on a range of healthcare services (hospital contacts, primary sector visits, and prescribed medication) during a 9-month period. On the contrary, when stratified by biosimilar drug, we found a significant decrease of approximately 15% in total healthcare costs after the switch mainly for GP2017 switchers with PsA or AxSpA.
Several studies, both RCTs and real-world observational studies, have suggested biosimilar switching to have no negative impact on treatment effect, effectiveness, safety, and immunogenicity.1,5,7,10,17,34 However, the use of biosimilars varies around the world with a significantly lower uptake in, for example, the United States, Japan, and Canada compared to some European countries. 35 In Denmark, the biosimilar uptake is high due to mandated switch procedures and national treatment guidelines that favor the use of biosimilars.15,16,18,36 Although the use of biosimilars may significantly reduce the financial burden of bDMARDs on the healthcare system, 13 the full impact of biosimilar switching on healthcare utilization and the associated costs is complex to assess. 19 Most previous studies have focused on the direct drug costs and found substantial savings (up to 83% for adalimumab), 18 but it is important to consider the costs associated with healthcare utilization (e.g., additional consultations). 37 Thus, it is reassuring that we found no increased healthcare costs following this nationwide mandatory adalimumab switch.
Previous studies exploring healthcare costs following a biosimilar switch have mainly been done for infliximab and etanercept 19 ; among these, only a few have included a before–after switch period or compared to patients, who did not switch. A European survey among rheumatology specialists found increased use of healthcare services (visits and procedures) at 0–3 and 4–6 months in >1200 patients who switched to etanercept biosimilar. The study mainly included patients with stable RA disease and compared switchers versus non-switchers. 38 The study was limited by the survey format and did not take comorbidities or seasonal variations into account. Other infliximab and etanercept biosimilar switch studies based on medical records and registry data report either no major changes or marginal increases in the number of outpatient visits 6 months post-switch.39–41 However, these experiences cannot be readily extrapolated to adalimumab for several reasons: the administration routes may differ (intravenous for infliximab), the drugs may be used by patients with different demographic and disease characteristics, and the perception of biosimilars among healthcare professionals/patients may change over time.42,43
In this study, we provide data regarding the average healthcare costs and utilization before and after the switch for both GP2017 and SB5 and further stratified by diagnosis. For both biosimilars, the costs either remained unchanged or decreased compared to before the switch throughout the 9 months of the after-period. The initial increase in hospital costs after the switch is likely due to consultations in relation to the switching—a pattern previously seen also for etanercept. 39 Stratified by drug, we found reduced overall healthcare costs for GP2017 switchers. A difference between GP2017 and SB5 was supported by the test of patient characteristics associated with changes in healthcare costs where the type of adalimumab biosimilar drug had a significant influence. We have previously demonstrated higher 1-year treatment retention and disease remission rates among GP2017 switchers compared to SB5. 17 This may be due to changes in the device; however, geographical differences or residual confounding could potentially impact these results, as previously discussed in Nabi et al. 17 Patients were mainly informed about the switch by nurses in the outpatient clinics, and no specific patient material was distributed. Differences in the transition or communication strategy in the different regions of Denmark should be considered, but we have no data to explore this further. Also, neither patients nor healthcare professionals were blinded by the switch, and the presence of a nocebo effect due to negative expectations may potentially have affected outcomes.22,44–46
The study is based on data from DANBIO linked with other Danish nationwide registries. Strengths include the nationwide prospective follow-up, high data completeness of the registries, and a high (89%) compliance to the nationwide switch guideline, 17 which provides a unique opportunity for monitoring outcomes in large cohorts. It is also a strength that patients served as their controls in the statistical analyses of use before and after the switch. Patients were switched according to their geographical residence; thus, for the comparisons of GP2017 versus SB5, confounding by indication is expected to be minimal. A limitation is the lack of adalimumab medication costs in the estimates. The drug prices fluctuated over time and were not publicly available due to the Danish tender process. Others have, however, reported a reduction in adalimumab costs by up to 80% when switching from an originator to a biosimilar.18,47 Our study focused on costs in the healthcare sector. It was beyond the scope to investigate patient-related costs or indirect costs (productivity loss, etc.).
Conclusion
In conclusion, we found no increased total healthcare costs in 9 months following a nationwide mandatory adalimumab originator to biosimilar switch. Our findings were strengthened by similar results for GP2017 and SB5.
Supplemental Material
Supplemental material, sj-docx-1-tab-10.1177_1759720X241289391 for Counting the costs: a nationwide study on healthcare use following an adalimumab biosimilar switch in >1300 inflammatory arthritis patients by Hafsah Nabi, Rikke Ibsen, Michael Ibsen, Jakob Kjellberg, Merete Lund Hetland and Bente Glintborg in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
We are thankful to all patients and Danish departments of rheumatology for reporting to the DANBIO registry. Also, the work of IT consultants Niels Steen Krogh and Zitelab Aps, who extracted data from DANBIO is acknowledged. The authors acknowledge AbbVie for supporting the work. This work was presented as a poster at the EULAR Congress 2023.
Footnotes
ORCID iD: Hafsah Nabi  https://orcid.org/0000-0001-6331-7927
https://orcid.org/0000-0001-6331-7927
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hafsah Nabi, DANBIO and Copenhagen Center for Arthritis Research (COPECARE), Center for Rheumatology and Spine Diseases, Centre of Head and Orthopedics, Copenhagen University Hospital Rigshospitalet, Valdemar Hansens Vej 17, Glostrup 2600, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Rikke Ibsen, i2minds, Aarhus, Denmark.
Michael Ibsen, i2minds, Aarhus, Denmark.
Jakob Kjellberg, The Danish Centre for Social Science Research, Copenhagen, Denmark.
Merete Lund Hetland, DANBIO and Copenhagen Center for Arthritis Research (COPECARE), Center for Rheumatology and Spine Diseases, Centre of Head and Orthopedics, Copenhagen University Hospital Rigshospitalet, Glostrup, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Bente Glintborg, DANBIO and Copenhagen Center for Arthritis Research (COPECARE), Center for Rheumatology and Spine Diseases, Centre of Head and Orthopedics, Copenhagen University Hospital Rigshospitalet, Glostrup, Denmark; Department of Clinical Medicine, Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark.
Declarations
Ethics approval and consent to participate: The study has been approved by the regional GDPR authority (Capital Region Denmark, RH-2015-209, 04145). In Denmark, registry studies neither require patient consent nor ethical approval.
Consent for publication: Not applicable.
Author contributions: Hafsah Nabi: Conceptualization; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Rikke Ibsen: Conceptualization; Data curation; Formal analysis; Methodology; Software; Visualization; Writing – review & editing.
Michael Ibsen: Conceptualization; Formal analysis; Investigation; Methodology; Resources; Software; Writing – review & editing.
Jakob Kjellberg: Conceptualization; Methodology; Resources; Writing – review & editing.
Merete Lund Hetland: Conceptualization; Investigation; Data curation; Funding acquisition; Methodology; Project administration; Supervision; Writing – review & editing.
Bente Glintborg: Conceptualization; Investigation; Data curation; Funding acquisition; Methodology; Project administration; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Research grant from AbbVie, who had no on the data collection, statistical analyses, or decision to submit.
Hafsah Nabi: Research grant from AbbVie and Sandoz.
Merete Lund Hetland: AbbVie, Biogen, BMS, Celtrion, Eli Lilly Denmark A/S, Janssen Biologics B.V, Lundbeck Fonden, MSD, Pfizer, Roche, Samsung Bioepis, Sandoz. Co-chair EuroSpA, which generates real-world evidence of treatment of psoriatic arthritis and axial spondyloarthritis based on secondary data and is partly funded by Novartis.
Bente Glintborg: BMS, Pfizer, Sandoz (research grants) Furthermore, chair of the steering committee of the Danish Rheumatology Quality Registry (DANBIO), which receives public funding from the hospital owners and funding from pharmaceutical companies.
Remaining authors: none declared.
Availability of data and materials: In this study, data are combined from several Danish nationwide registries and hosted at Statistics Denmark. Data cannot be shared publicly due to requirements of the involved register holders and the general data protection regulation, to protect the privacy of individuals.
Patient and public involvement: Thank you to the patient partners within the DANBIO steering committee.
References
- 1. Schulze-Koops H, Skapenko A. Biosimilars in rheumatology: a review of the evidence and their place in the treatment algorithm. Rheumatology 2017; 56(Suppl_4): iv30–iv48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Putrik P, Ramiro S, Kvien TK, et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis 2014; 73(1): 198–206. [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Goncalves J, Quinn M, et al. Era of biosimilars in rheumatology: reshaping the healthcare environment. RMD Open 2019; 5(1): e000900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weinblatt ME, Baranauskaite A, Niebrzydowski J, et al. Phase III randomized study of SB5, an adalimumab biosimilar, versus reference adalimumab in patients with moderate-to-severe rheumatoid arthritis. Arthritis Rheumatol 2018; 70(1): 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cohen SB, Alonso-Ruiz A, Klimiuk PA, et al. Similar efficacy, safety and immunogenicity of adalimumab biosimilar BI 695501 and Humira reference product in patients with moderately to severely active rheumatoid arthritis: results from the phase III randomised VOLTAIRE-RA equivalence study. Ann Rheum Dis 2018; 77(6): 914–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Genovese MC, Glover J, Greenwald M, et al. FKB327, an adalimumab biosimilar, versus the reference product: results of a randomized, Phase III, double-blind study, and its open-label extension. Arthritis Res Ther 2019; 21(1): 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhao S, Chadwick L, Mysler E, et al. Review of biosimilar trials and data on adalimumab in rheumatoid arthritis. Curr Rheumatol Rep 2018; 20(10): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Blauvelt A, Lacour JP, Fowler JF, et al. Phase III randomized study of the proposed adalimumab biosimilar GP2017 in psoriasis: impact of multiple switches. Br J Dermatol 2018; 179(3): 623–631. [DOI] [PubMed] [Google Scholar]
- 9. Statement on the scientific rationale supporting interchangeability of biosimilar medicines in the EU, www.ema.europa.eu/contact (2022, accessed 16 September 2024). [DOI] [PMC free article] [PubMed]
- 10. Kay J, Schoels MM, Dörner T, et al. Consensus-based recommendations for the use of biosimilars to treat rheumatological diseases. Ann Rheum Dis 2018; 77(2): 165–174. [DOI] [PubMed] [Google Scholar]
- 11. Smolen JS, Landewé RBM, Bergstra SA, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis 2023; 82(1): 3–18. [DOI] [PubMed] [Google Scholar]
- 12. Troein P, Newton M, Scott K. The impact of biosimilar competition in Europe, https://ec.europa.eu/health/system/files/2021-01/biosimilar_competition_en_0.pdf (2020, accessed 16 September 2024).
- 13. Kvien TK, Patel K, Strand V. The cost savings of biosimilars can help increase patient access and lift the financial burden of health care systems. Semin Arthritis Rheum 2022; 52: 151939. [DOI] [PubMed] [Google Scholar]
- 14. Medicinrådet. Implementation of biosimilar Adalimumab, Denmark [Internet], https://medicinraadet.dk/media/svab0lxx/medicinraadets-vurdering-af-ibrugtagning-af-biosimilaer-adalimumab-version-10_adlegacy.pdf (2018, accessed 16 September 2024).
- 15. Jensen TB, Bartels D, Sædder EA, et al. The Danish model for the quick and safe implementation of infliximab and etanercept biosimilars. Eur J Clin Pharmacol 2020; 76(1): 35–40. [DOI] [PubMed] [Google Scholar]
- 16. Glintborg B, Loft AG, Omerovic E, et al. To switch or not to switch: results of a nationwide guideline of mandatory switching from originator to biosimilar etanercept. One-year treatment outcomes in 2061 patients with inflammatory arthritis from the DANBIO registry. Ann Rheum Dis 2019; 78(2): 192–200. [DOI] [PubMed] [Google Scholar]
- 17. Nabi H, Georgiadis S, Loft AG, et al. Comparative effectiveness of two adalimumab biosimilars in 1318 real-world patients with inflammatory rheumatic disease mandated to switch from originator adalimumab: nationwide observational study emulating a randomised clinical trial. Ann Rheum Dis 2021; 80(11): 1400–1409. [DOI] [PubMed] [Google Scholar]
- 18. Jensen TB, Kim SC, Jimenez-Solem E, et al. Shift from adalimumab originator to biosimilars in Denmark. JAMA Intern Med 2020; 180(6): 902–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hillhouse E, Mathurin K, Bibeau J, et al. The economic impact of originator-to-biosimilar non-medical switching in the real-world setting: a systematic literature review. Adv Ther 2022; 39(1): 455–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kristensen LE, Alten R, Puig L, et al. Non-pharmacological effects in switching medication: the Nocebo effect in switching from originator to biosimilar agent. BioDrugs 2018; 32(5): 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. The Lancet Rheumatology. Biosimilars and the era of interchangeability. Lancet Rheumatol 2023; 5(9): e495. [DOI] [PubMed] [Google Scholar]
- 22. Rezk MF, Pieper B. Treatment outcomes with biosimilars: be aware of the Nocebo effect. Rheumatol Ther 2017; 4(2): 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ibfelt EH, Sørensen J, Jensen D V, et al. Validity and completeness of rheumatoid arthritis diagnoses in the nationwide DANBIO clinical register and the Danish National Patient Registry. Clin Epidemiol 2017; 9: 627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hetland ML. DANBIO—powerful research database and electronic patient record. Rheumatology 2011; 50(1): 69–77. [DOI] [PubMed] [Google Scholar]
- 25. Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol 2015; 7: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sahl Andersen J, De Fine Olivarius N, Krasnik A. The Danish National Health Service Register. Scand J Public Health 2011; 39(7): 34–37. [DOI] [PubMed] [Google Scholar]
- 27. Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, et al. Existing data sources for clinical epidemiology: the Danish National Database of Reimbursed Prescriptions. Clin Epidemiol 2012; 4(1): 303–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61(4): 344–349. [DOI] [PubMed] [Google Scholar]
- 29. DRG-takster 2019—Sundhedsdatastyrelsen, https://sundhedsdatastyrelsen.dk/da/afregning-og-finansiering/takster-drg/takster-2019 (2019, accessed 16 September 2024).
- 30. Sundhedsdatastyrelsen. Datakvalitetsrapport om LPR 2019-overgangen fra LPR2 til LPR3, https://sundhedsdatastyrelsen.dk/da/registre-og-services/om-de-nationale-sundhedsregistre/sygdomme-laegemidler-og-behandlinger/landspatientregisteret/landspatientregisteret-moderniseres (2020, accessed 16 September 2024).
- 31. Overblik over anmeldelsespligten | De Videnskabsetiske Komitéer, https://videnskabsetik.dk/ansoegning-til-etisk-komite/overblik-over-anmeldelsespligten (2024, accessed 16 September 2024).
- 32. Buntin MB, Zaslavsky AM. Too much ado about two-part models and transformation? Comparing methods of modeling Medicare expenditures. J Health Econ 2004; 23(3): 525–542. [DOI] [PubMed] [Google Scholar]
- 33. Wang M. Generalized estimating equations in longitudinal data analysis: a review and recent developments. Adv Stat 2014; 2014(1): 303728. [Google Scholar]
- 34. Thomas D, Strand V, Cornes P, et al. The changing landscape of biosimilars in rheumatology. Ann Rheum Dis 2016; 75(6): 974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Alnaqbi KA, Bellanger A, Brill A, et al. An international comparative analysis and roadmap to sustainable biosimilar markets. Front Pharmacol 2023; 14: 1188368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Glintborg B, Sørensen IJ, Loft AG, et al. A nationwide non-medical switch from originator infliximab to biosimilar CT-P13 in 802 patients with inflammatory arthritis: 1-year clinical outcomes from the DANBIO registry. Ann Rheum Dis 2017; 76(8): 1426–1431. [DOI] [PubMed] [Google Scholar]
- 37. Liu Y, Yang M, Garg V, et al. Economic impact of non-medical switching from originator biologics to biosimilars: a systematic literature review. Adv Ther 2019; 36(8): 1851–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tarallo M, Onishchenko K, Alexopoulos ST. Costs associated with non-medical switching from originator to biosimilar etanercept in patients with rheumatoid arthritis in the UK. J Med Econ 2019; 22(11): 1162–1170. [DOI] [PubMed] [Google Scholar]
- 39. Glintborg B, Ibsen R, Bilbo REQ, et al. Does a mandatory non-medical switch from originator to biosimilar etanercept lead to increase in healthcare use and costs? A Danish register-based study of patients with inflammatory arthritis. RMD Open 2019; 5(2): e001016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glintborg B, Sørensen J, Hetland ML. Does a mandatory non-medical switch from originator to biosimilar infliximab lead to increased use of outpatient healthcare resources? A register-based study in patients with inflammatory arthritis. RMD Open 2018; 4(2): e000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gibofsky A, Skup M, Yang M, et al. Short-term costs associated with non-medical switching in autoimmune conditions. Clin Exp Rheumatol 2019; 37(1): 97–105. [PubMed] [Google Scholar]
- 42. Leonard E, Wascovich M, Oskouei S, et al. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm 2019; 25(1): 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gasteiger C, Lobo M, Dalbeth N, et al. Patients’ beliefs and behaviours are associated with perceptions of safety and concerns in a hypothetical biosimilar switch. Rheumatol Int 2021; 41(1): 163–171. [DOI] [PubMed] [Google Scholar]
- 44. Tweehuysen L, van den Bemt BJF, van Ingen IL, et al. Subjective complaints as the main reason for biosimilar discontinuation after open-label transition from reference infliximab to biosimilar infliximab. Arthritis Rheumatol 2018; 70(1): 60–68. [DOI] [PubMed] [Google Scholar]
- 45. Fleischmann R, Jairath V, Mysler E, et al. Nonmedical switching from originators to biosimilars: does the Nocebo effect explain treatment failures and adverse events in rheumatology and gastroenterology? Rheumatol Ther 2020; 7(1): 35–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Odinet JS, Day CE, Cruz JL, et al. The biosimilar Nocebo effect? A systematic review of double-blinded versus open-label studies. J Manag Care Spec Pharm 2018; 24(10): 952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coghlan J, He H, Schwendeman AS. Overview of Humira® biosimilars: current European landscape and future implications. J Pharm Sci 2021; 110(4): 1572–1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tab-10.1177_1759720X241289391 for Counting the costs: a nationwide study on healthcare use following an adalimumab biosimilar switch in >1300 inflammatory arthritis patients by Hafsah Nabi, Rikke Ibsen, Michael Ibsen, Jakob Kjellberg, Merete Lund Hetland and Bente Glintborg in Therapeutic Advances in Musculoskeletal Disease