Abstract
Background:
Bevacizumab is extensively used in the treatment of advanced non-small-cell lung cancer (NSCLC). Numerous clinical trials have proven the clinical efficacies of bevacizumab biosimilars (BB).
Objective:
Our study aimed to compare the clinical outcomes between bevacizumab reference product (RP) and BB among advanced NSCLC patients in a real-world setting.
Design:
We retrospectively analyzed stage IV metastatic NSCLC patients who were treated with bevacizumab as part of a combination therapy. Patients were categorized into chemotherapy (CT) and epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) groups. We compared the patients’ characteristics, treatment efficacy, and adverse events between RP and BB in the two treatment groups.
Methods:
From January 2020 to July 2022, a total of 171 patients who underwent combination therapy with bevacizumab were screened. Seventy-nine of these patients met the study’s inclusion criteria and were enrolled in the final analysis. We utilized the Kaplan–Meier method to estimate progression-free survival (PFS) and the log-rank test to compare PFS between groups. The Cox proportional hazards model was used to identify predictors of PFS.
Results:
Within the CT cohort, 34 patients were treated with RP in combination with platinum and pemetrexed, and 25 patients received a combination regimen with BB. The median PFS was 6.9 months in the RP group and 8.9 months in the BB group (p = 0.255). Within the EGFR-TKI cohort, 20 patients with EGFR-mutant NSCLC received first-line treatment with EGFR-TKI plus bevacizumab. Of these patients, 9 were treated with a combination regimen that included RP, and 11 patients received EGFR-TKI in combination with BB. The median PFS was 18.4 months for the RP group and 13.6 months for the BB group (p = 0.363).
Conclusion:
In our advanced NSCLC patients, we found no difference in clinical outcomes when receiving treatment with RP or BB. Given a combination regimen, BB was as effective as RP together with either CT or EGFR-TKIs.
Keywords: anti-angiogenesis therapy, bevacizumab, biosimilar, chemotherapy, non-small-cell lung cancer
Introduction
The most recent GLOBOCAN report indicates that lung cancer continues to be the global predominant cause of death from cancer. 1 A better understanding of the molecular pathogenesis of non-small-cell lung cancer (NSCLC) and new treatment options have led to improved outcomes. 2 For patients with advanced NSCLC, standard treatments include chemotherapy (CT), targeted therapy, and immunotherapy. The treatment regimen is typically tailored based on histological cell type and the findings from molecular testing. In 2000, Hanahan and Weinberg proposed the original hallmarks of cancer, including sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. 3 Nowadays, these cancer hallmarks have increased to 10 concepts, with induced angiogenesis being a critical factor in tumorigenesis. 4 Of particular note, vascular endothelial growth factors (VEGFs), especially VEGF-A, not only facilitate angiogenesis but also play pivotal roles in tumor growths independent of angiogenesis. These roles include the direct stimulation of cell proliferation and promotion of immune tolerance, minimizing tumor detection by the immune system.5,6
Bevacizumab (Avastin®), a humanized monoclonal antibody that inhibits VEGF, was the first medication with anti-angiogenic action to be approved by the U.S. Food and Drug Administration (FDA) in 2004 for metastatic colorectal cancer in combination with CT. 7 Subsequently, the FDA extended its approval to include bevacizumab for treating metastatic NSCLC, after publishing the E4599 trial. That study demonstrated that adding bevacizumab to CT with a carboplatin and paclitaxel regimen improves patient survival when compared with CT alone. 8 Furthermore, the NEJ026 study confirmed that the combination of first-generation epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor (TKI) and bevacizumab enhances progression-free survival (PFS) in advanced EGFR-mutant NSCLC patients compared with using erlotinib alone. 9 The IMpower150 trial demonstrated that the regimen of atezolizumab, bevacizumab, plus carboplatin and paclitaxel results in better PFS and overall survival (OS) compared with bevacizumab plus carboplatin and paclitaxel, regardless of the presence of programmed death-ligand 1 expression or genetic alterations of EGFR or ALK (anaplastic lymphoma kinase). Such survival benefits are likely associated with the immunomodulatory effects of bevacizumab, augmenting the efficacy of atezolizumab. 10
The expiration of patents sparked the use of biosimilars globally. 11 These agents are analogous to their reference biologics, exhibiting high similarity though not being identical. Biosimilars are subject to a rigorous evaluation process, including analytical, animal, and clinical studies, to affirm that there are no significant differences in efficacy, safety, or purity compared to the original biologic. 12 The growing availability of biosimilars in the market not only reduces costs but also widens patient access to diverse treatment options.13,14
ABP 215 was the first biosimilar to bevacizumab (Avastin®) and was developed by Amgen Inc. Extensive analytical studies confirmed the high resemblance of ABP 215 to bevacizumab in terms of the following characteristic: its primary and higher-order structures, as well as its particle composition, aggregates, product-related substances, impurities, thermal forced degradation, biological activities, general properties, and process-related impurities. 15 In a phase I study led by Markus et al., ABP 215 was found to have pharmacokinetic properties that are consistent with both the US and EU versions of bevacizumab in healthy adult men. 16 The phase III MAPLE study revealed that ABP 215, when used in combination with carboplatin and paclitaxel, delivers therapeutic outcomes that are on par with the reference product (RP) for treating advanced NSCLC. 17 Despite comparable clinical efficacy between the RP and its biosimilars, research studies demonstrating the tangible benefits of biosimilars in real-world settings are limited, particularly with respect to lung cancer. 18
Here, we investigated the treatment efficacy of MVASI®, a bevacizumab biosimilar (BB), when used in conjunction with either EGFR-TKI or CT regimen comprising platinum and pemetrexed.
Materials and methods
Patients
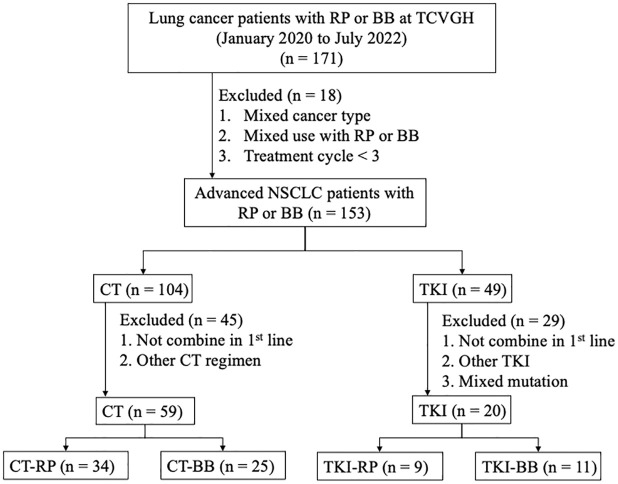
This retrospective, single-center study was approved by the Institutional Review Board of Taichung Veterans General Hospital (IRB CE24146A). We enrolled lung cancer patients who were diagnosed and treated at Taichung Veterans General Hospital between January 2020 and July 2022. The patients’ inclusion criteria were as follows: (a) aged 20 years or older, (b) with cytologically or pathologically confirmed stage IV non-squamous NSCLC, (c) received combination therapy in the first-line treatment with bevacizumab including the reference drug (Avastin®) or the biosimilar (MVASI®), and (d) those who received ⩾3 cycles of anti-angiogenic agent therapy. The exclusion criteria were patients who (a) had mixed types of cancer, (b) received <3 cycles of anti-angiogenic agent therapy, (c) were treated with both the bevacizumab reference drug and its biosimilar, or (d) received angiogenesis inhibitors beyond the first line of treatment. For the final analysis, we studied only those in the CT group treated with a combination of platinum and pemetrexed, and those in the EGFR-TKI group who received gefitinib, erlotinib, or afatinib as the first-line treatment. The flowchart of patient enrollment is shown in Figure 1. The reporting of this study conforms to the STROBE statement.
Figure 1.
Patient collection flowchart.
BB, bevacizumab biosimilar; CT, chemotherapy; NSCLC, non-small-cell lung cancer; RP, reference product; TCVGH, Taichung Veterans General Hospital; TKI, tyrosine kinase inhibitor.
Data for analysis
Our data were extracted from patients’ medical records. The data included the following patient characteristics and treatment specifics: age, gender, smoking status, Eastern Cooperative Oncology Group performance status (ECOG PS), driver mutation status, tumor stage, type of anti-angiogenic agents used, combination treatment regimens, number of treatment cycles, adverse events (AEs) associated with anti-angiogenic therapy, and follow-up data on survival. The staging of lung cancer followed the TNM (tumor, node, metastasis) system as outlined in the 8th edition of the American Joint Committee on Cancer criteria. In addition, evaluations of lung cancer status and response to treatment were one-dimensional measurements in accordance with the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. 19
Driver mutation analysis
We investigated three pivotal oncogenic drivers: EGFR, ALK, and ROS1 (ROS proto-oncogene 1). The presence of the mutation in EGFR was evaluated using either the Cobas EGFR Mutation Test v2 (Roche Molecular Systems, Pleasanton, CA, USA) or the matrix-assisted laser desorption ionization–time of flight mass spectrometry. To detect ALK fusion mutations, a fully automated immunohistochemistry assay was employed utilizing the Ventana system with a specific anti-ALK (D5F3) rabbit monoclonal antibody. The assessment of ROS1 fusion mutations was conducted through fluorescence in situ hybridization.
Efficacy evaluation
Our primary endpoint in the study was PFS, defined as the interval from the start of the first treatment cycle to the earliest recorded event of disease progression or death, in accordance with the RECIST guidelines (version 1.1). Secondary endpoints were the objective response rate (ORR) and the disease control rate (DCR) within the study period. The ORR included the proportion of patients achieving the best overall response of complete response (CR) or partial response (PR), while DCR was the proportion of patients attaining the best overall response of CR, PR, or stable disease, as per the RECIST (version 1.1) criteria. For those who were alive without disease progression at the study’s cutoff date (April 30, 2023), PFS was censored at their last follow-up, indicating no disease progression.
Statistical methods
Categorical variables were expressed as frequency and percentage. For categorical variables, differences between the two groups (one receiving the reference drug and the other treated with biosimilar) were compared with the Chi-square test. The Kaplan–Meier method was utilized to estimate PFS, and the log-rank test was applied to compare PFS between groups. To identify predictors of PFS, we used the Cox proportional hazards model. All statistical analyses were conducted with the SPSS software, version 23.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined at p < 0.05, with a two-tailed test.
Results
Patient’s demographic and clinical features
From January 2020 to July 2022, we enrolled 79 patients for final analysis. Among them, 59 received CT, and 20 were treated with TKIs, both in combination with either bevacizumab reference drug (Avastin®) or its biosimilar (MVASI®). These patients were further divided into four subgroups receiving: (a) chemotherapy with the bevacizumab reference product (CT-RP, n = 34), (b) chemotherapy with the bevacizumab biosimilar (CT-BB, n = 25), (c) TKIs with the bevacizumab reference product (TKI-RP, n = 9), and (d) TKIs with the bevacizumab biosimilar (TKI-BB, n = 11) (Figure 1). Baseline characteristics and demographic data of the overall patient population are summarized in Supplemental Table 1. The data for the CT group are presented in Table 1, while those for patients receiving TKIs are detailed in Table 2.
Table 1.
Patient characteristics of the CT group.
| Characteristics | Total, n (%) | CT-BB, n (%) | CT-RP, n (%) | p Value a |
|---|---|---|---|---|
| Age | 0.828 | |||
| ≧65 | 25 (42.4) | 11 (44.0) | 14 (41.2) | |
| <65 | 34 (57.6) | 14 (56.0) | 20 (58.8) | |
| Gender, n (%) | 0.687 | |||
| Male | 23 (39.0) | 9 (36.0) | 14 (41.2) | |
| Female | 36 (61.0) | 16 (64.0) | 20 (58.8) | |
| Smoking status, n (%) | 0.861 | |||
| Never-smokers | 37 (62.7) | 16 (64.0) | 21 (61.8) | |
| Former or current smokers | 22 (37.3) | 9 (36.0) | 13 (38.2) | |
| ECOG PS, n (%) | 0.215 | |||
| 0–1 | 51 (86.4) | 20 (80.0) | 31 (91.2) | |
| 2–4 | 8 (13.6) | 5 (20.0) | 3 (8.8) | |
| Stage, n (%) | 0.471 | |||
| Stage 4A | 22 (37.3) | 8 (32.0) | 14 (41.2) | |
| Stage 4B | 37 (62.7) | 17 (68.0) | 20 (58.8) | |
| Regimen | 0.296 | |||
| Cisplatin | 42 (71.2) | 16 (64.0) | 26 (76.5) | |
| Carboplatin | 17 (28.8) | 9 (36.0) | 8 (23.5) | |
| Driver mutation (EGFR/ALK/ROS1) | 0.708 | |||
| Yes | 30 (50.8) | 12 (48.0) | 18 (52.9) | |
| No | 29 (49.2) | 13 (52.0) | 16 (47.1) | |
| Objective response rate | 32.8% | 42.4% | 0.579 | |
| Disease control rate | 78.3% | 75.8% | 0.667 |
By Fisher’s exact test.
ALK, anaplastic lymphoma kinase; BB, bevacizumab biosimilar; CT, chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; ROS1, ROS proto-oncogene 1; RP, reference product.
Table 2.
Patient characteristics of the EGFR-TKIs group.
| Characteristics | Total, n (%) | TKI-BB, n (%) | TKI-RP, n (%) | p Value a |
|---|---|---|---|---|
| Age | 0.127 | |||
| ≧65 | 5 (25.0) | 1 (9.1) | 4 (44.4) | |
| <65 | 15 (75.0) | 10 (90.9) | 5 (55.6) | |
| Gender, n (%) | 0.028 | |||
| Male | 8 (40.0) | 7 (63.6) | 1 (11.1) | |
| Female | 12 (60.0) | 4 (36.4) | 8 (88.9) | |
| Smoking status, n (%) | 0.157 | |||
| Never smokers | 14 (70.0) | 6 (54.5) | 8 (88.9) | |
| Former or current smokers | 6 (30.0) | 5 (45.5) | 1 (11.1) | |
| ECOG PS, n (%) | 1.000 | |||
| 0–1 | 17 (85.0) | 9 (81.8) | 8 (88.9) | |
| 2–4 | 3 (15.0) | 2 (18.2) | 1 (11.1) | |
| Stage, n (%) | 0.189 | |||
| Stage 4A | 2 (10.0) | 0 (0.0) | 2 (22.2) | |
| Stage 4B | 18 (90.0) | 11 (100.0) | 7 (77.8) | |
| Baseline EGFR mutation status, n (%) | 0.525 | |||
| Exon 19 deletions | 7 (35.0) | 3 (27.3) | 4 (44.4) | |
| Exon 21 L858R | 12 (60.0) | 7 (63.6) | 5 (55.6) | |
| G719X + L861Q | 1 (5.0) | 1 (9.1) | 0 (0.0) | |
| Objective response rate | 80% | 71.4% | 0.659 | |
| Disease control rate | 90% | 100% | 0.497 |
By Fisher’s exact test.
BB, bevacizumab biosimilar; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; RP, reference product; TKI, tyrosine kinase inhibitor.
For the entire cohort, the median age was 60.4 years, with 60.8% being female, 64.6% identifying as never smokers, and 86.1% having an ECOG PS of 0–1. Overall, 44.3% of patients had brain metastasis, and 49.4% had malignant pleural effusion. In Supplemental Table 1, we categorized these patients into those receiving the bevacizumab biosimilar (BB group) and those receiving the bevacizumab reference product (RP group). There were no significant differences in demographic or baseline disease characteristics between the BB and RP groups.
In the CT cohort, the overall median age was 61.5 years, with 61.0% females, 62.7% never smokers, and 86.4% with an ECOG PS of 0–1. Stage 4B disease was prevalent in 62.7% of the cohort, with the majority (71.2%) receiving a cisplatin-based combination regimen. Half of the patients (50.8%) exhibited driver mutations such as EGFR, ALK, and ROS-1. The demographic and baseline disease characteristics were statistically similar between the CT-RP and CT-BB groups. The median number of anti-angiogenic therapy cycles was 7 (3–30) for the CT-RP group and 7 (3–25) for the CT-BB group (p = 0.627).
In the TKI cohort, the overall median age was 56.3 years. Among those treated with TKIs, the median age in the TKI-RP and TKI-BB subgroups were 61.7 and 55.4 years, respectively. Females represented 60.0% of the cohort, 70.0% were never-smokers, and 85.0% presented with an ECOG PS of 0–1. The majority had stage 4B disease (90.0%). Regarding driver mutations, 7 patients exhibited Exon 19 deletions, 12 had Exon 21 L858R mutations, and 1 patient had G719X + L861Q mutations. The median number of anti-angiogenic therapy cycles was 16 (4–50) for the TKI-RP subgroup and 12 (3–21) for the TKI-BB subgroup (p = 0.295). A significant difference was noted in gender distribution between the TKI-RP and TKI-BB subgroups. This difference could be related to the small sample size. Apart from this, we found no significant difference in demographic or clinical characteristics between the two treatment subgroups.
Clinical efficacy in the overall population
The BB and RP groups demonstrated similar PFS, with a median PFS of 9.9 months (95% confidence interval (CI) 5.1–14.6) in the BB group and 8.4 months (95% CI 6.3–10.5) in the RP group (p = 0.416), as shown in Supplemental Figure 1. Among patients with brain metastasis, BB and RP provided comparable PFS (p = 0.217). Furthermore, there was no difference in PFS between the BB and RP groups in patients with malignant pleural effusion (p = 0.211).
Clinical efficacy in the CT group
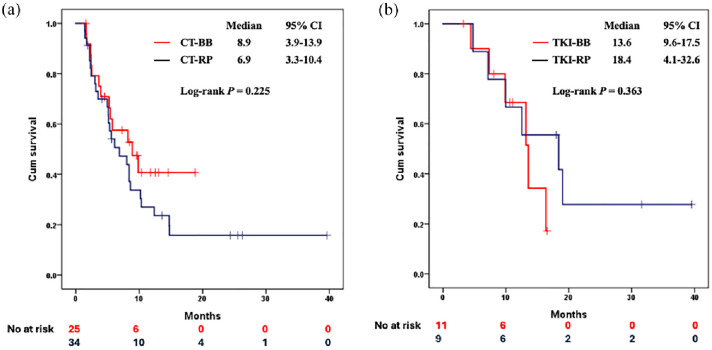
The CT-BB and CT-RP groups demonstrated similar PFS, with a median PFS of 8.9 months (95% CI 3.9–13.9) in the CT-BB group, and 6.9 months (95% CI 3.3–10.4) in the CT-RP group (p = 0.255), as illustrated in Figure 2a. The CT-BB group exhibited ORR and DCR that were similar to those observed in the CT-RP group (Table 1). Two patients in the CT-BB group and one in the CT-RP group had lesions that were not evaluable, and these three patients were therefore excluded from the ORR and DCR assessments. For the other evaluable patients, 34.8% in the CT-BB group and 41.2% in the CT-RP group achieved objective responses. Disease control was achieved by 78.3% in the CT-BB group and 75.8% in the CT-RP group.
Figure 2.
The PFS of patients received (a) first-line platinum-pemetrexed combined with bevacizumab RP or its biosimilar and (b) first-line EGFR-TKIs combined with bevacizumab RP or its biosimilar.
BB, bevacizumab biosimilar; CT, chemotherapy; EGFR-TKI, epidermal growth factor receptor-tyrosine kinase inhibitor; PFS, progression-free survival; RP, reference product.
According to the univariate and multivariate analyses presented in Table 3, the only variable that had successfully predicted PFS was the tumor stage at the start of treatment. Patients with stage 4B disease faced a significantly higher risk of disease progression (adjusted hazard ratio 2.80, 95% CI 1.31–5.99) compared with patients with stage 4A disease. After adjusting for confounders, the type of anti-angiogenic drug—either the reference or biosimilar—did not significantly affect PFS.
Table 3.
Univariate and multivariate analyses of PFS in CT group.
| Variables | Univariate | p Value a | Multivariate | p Value a |
|---|---|---|---|---|
| HR (95% CI) | aHR (95% CI) | |||
| Age | 0.321 | 0.556 | ||
| ≧65 | Reference | |||
| <65 | 1.393 (0.722–2.687) | 1.249 (0.597–2.613) | ||
| Gender, n (%) | 0.947 | 0.304 | ||
| Male | Reference | |||
| Female | 1.022 (0.535–1.951) | 1.689 (0.622–4.585) | ||
| Smoking status, n (%) | 0.714 | 0.545 | ||
| Never smokers | Reference | |||
| Former or current smokers | 1.129 (0.589–2.166) | 1.367 (0.497–3.761) | ||
| ECOG PS, n (%) | 0.924 | 0.483 | ||
| 0–1 | Reference | |||
| 2–4 | 0.995 (0.373–2.448) | 1.448 (0.515–4.073) | ||
| Stage, n (%) | 0.042 | 0.008 | ||
| Stage 4A | Reference | |||
| Stage 4B | 2.046 (1.027–4.080) | 2.799 (1.309–5.985) | ||
| Regimen | 0.274 | 0.194 | ||
| Cisplatin | Reference | |||
| Carboplatin | 0.647 (0.297–1.412) | 0.553 (0.227–1.350) | ||
| Driver mutation (EGFR/ALK/ROS1) | 0.305 | 0.071 | ||
| Yes | Reference | |||
| No | 1.391 (0.741–2.613) | 1.979 (0.943–4.153) | ||
| Bevacizumab | 0.259 | 0.120 | ||
| RP | Reference | |||
| BB | 0.679 (0.347–1.329) | 0.571 (0.282–1.156) |
By Cox proportional hazard model.
aHR, adjusted hazard ratio; ALK, anaplastic lymphoma kinase; BB, bevacizumab biosimilar; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; HR, hazard ratio; PFS, progression-free survival; ROS1, ROS proto-oncogene 1; RP, reference product.
Clinical efficacy in the TKI group
In our TKI cohort, the PFS was comparable between the two subgroups. Specifically, the TKI-BB subgroup had a median PFS of 13.6 months (95% CI 9.6–17.5), compared with a median PFS of 18.4 months (95% CI 4.1–32.6) in the TKI-RP subgroup. The difference was not statistically significant (p = 0.363, see Figure 2b). The TKI-BB and TKI-RP subgroups showed similar ORR and DCR. One patient in the TKI-BB subgroup and two patients in the TKI-RP subgroup were excluded from the final response evaluation due to non-evaluable lesions. Among the evaluable patients, 80% in the TKI-BB subgroup and 71.4% in the TKI-RP subgroup achieved an ORR. Furthermore, 90% in the TKI-BB subgroup and 100% in the TKI-RP subgroup achieved a DCR.
According to the univariate and multivariate analyses as presented in Table 4, we found no predictor for PFS, including the choice of anti-angiogenesis, age, gender, smoking status, ECOG PS, disease stage, or baseline EGFR mutation status.
Table 4.
Univariate and multivariate analyses of PFS in EGFR-TKI group.
| Variables | Univariate | p Value a | Multivariate | p Value a |
|---|---|---|---|---|
| HR (95% CI) | aHR (95% CI) | |||
| Age | 0.383 | 0.781 | ||
| ≧65 | Reference | |||
| <65 | 1.967 (0.430–8.999) | 1.369 (0.149–12.594) | ||
| Gender, n (%) | 0.876 | 0.380 | ||
| Male | Reference | |||
| Female | 0.912 (0.286–2.909) | 2.167 (0.386–12.163) | ||
| Smoking status, n (%) | 0.456 | 0.343 | ||
| Never smokers | Reference | |||
| Former or current smokers | 1.550 (0.490–4.904) | 2.384 (0.396–14.343) | ||
| ECOG PS, n (%) | 0.917 | 0.770 | ||
| 0–1 | Reference | |||
| 2–4 | 1.086 (0.229–5.144) | 1.314 (0.210–8.223) | ||
| Stage, n (%) | 0.631 | 0.567 | ||
| Stage 4A | Reference | |||
| Stage 4B | 1.670 (0.207–13.493) | 2.408 (0.119–48.813) | ||
| Baseline EGFR mutation status, n (%) | 0.211 | 0.108 | ||
| Exon 19 deletions | Reference | |||
| Exon 21 L858R | 0.456 (0.133–1.560) | 0.286 (0.062–1.314) | ||
| Bevacizumab | 0.369 | 0.801 | ||
| RP | Reference | |||
| BB | 1.800 (0.499–6.491) | 1.241 (0.230–6.687) |
By Cox proportional hazard model.
aHR, adjusted hazard ratio; BB, bevacizumab biosimilar; CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; HR, hazard ratio; PFS, progression-free survival; RP, reference product; TKI, tyrosine kinase inhibitor.
Adverse events
AEs related to the study treatments are detailed in Supplementary Table 2. In the CT cohort, 31 patients (52.5%) experienced hypertension of various grades: 26 grade 1, 5 grade 2, and 0 grade 3. Proteinuria of various grades occurred in 23 patients (39.0%): 14 grade 1, 9 grade 2, and 0 grade 3. No significant difference in rates of hypertension or proteinuria was observed between the CT-BB and CT-RP subgroups. Each subgroup had one case of hemoptysis. There were three incidents of thrombosis: one pulmonary embolism in each subgroup and one deep vein thrombosis in the CT-RP subgroup.
In the TKI cohort, hypertension of various grades was reported in 10 patients (50.0%): 7 grade 1, 3 grade 2, and 0 grade 3. Proteinuria of various grades was reported in nine patients (45.0%): 3 grade 1, 6 grade 2, and 0 grade 3. Incidences of hypertension and proteinuria were similar between the TKI-BB and TKI-RP subgroups. There were no reports of hemorrhage or thrombosis in the TKI treatment cohort.
Discussion
In the present study, we found that for stage IV NSCLC patients undergoing treatment with pemetrexed and platinum-based CT, PFS, and ORR were similar regardless of whether they had received the original bevacizumab drug or its biosimilar. In addition, no notable difference in the PFS and ORR was found in patients treated with the first- or second-generation EGFR-TKIs in combination with either form of bevacizumab. Frequencies of AE such as hypertension, proteinuria, hemorrhage, and thrombotic episodes were also similar between those receiving the bevacizumab reference drug and the biosimilar. This is the first study to evaluate the outcome of treatment regimens that include the BB for patients diagnosed with stage IV non-squamous NSCLC.
Bevacizumab is a significant treatment option for patients with non-squamous NSCLC. According to the E4599 study, patients with advanced NSCLC treated with paclitaxel and carboplatin along with bevacizumab experienced a median PFS of 6.2 months, compared with 4.5 months for those who underwent CT alone (HR 0.66, 95% CI 0.57–0.77; p < 0.001). 8 In terms of OS, the bevacizumab group had a better median OS of 12.3 months compared with 10.3 months for the CT-only group (HR 0.79, 95% CI 0.67–0.92; p = 0.003). Adding bevacizumab to CT also showed an enhanced response rate, with 35% in the group receiving paclitaxel, carboplatin, and bevacizumab, versus 15% in the group treated with CT alone (p < 0.001). Based on the above findings, the paclitaxel–carboplatin–bevacizumab group saw an improvement in OS and PFS of around 2 months compared with those receiving CT only. Patel et al. reported that advanced non-squamous NSCLC patients treated with a combination of pemetrexed, carboplatin, and bevacizumab, followed by maintenance therapy with pemetrexed and bevacizumab had a median PFS and ORR of 6.0 months and 34.1%, respectively. 20 The aforementioned study also confirmed that the carboplatin–pemetrexed–bevacizumab regimen slightly improved PFS and had a similar ORR when compared with the paclitaxel–carboplatin–bevacizumab regimen. In a real-world study, Li et al. reported a median PFS of 7.4 months among Chinese advanced lung adenocarcinoma patients receiving pemetrexed–platinum–bevacizumab. 21
In a phase III trial MAPLE study, the efficacies of the bevacizumab RP (Avastin®) and its biosimilar (MVASI®) were compared. Patients who received carboplatin and paclitaxel in combination with bevacizumab or its biosimilar achieved similar treatment outcomes. In the ABP 215 group, ORR was 39.0% which was comparable with 41.7% in the reference drug group. The two groups also had similar PFS, with an estimated HR of 1.03 (90% CI 0.83–1.29). 17 In our CT cohort, the overall median PFS was 8.0 months (95% CI 5.1–11.0) and ORR was 39.2%. Our results are similar to the aforementioned studies. The median PFS rates of CT-RP and CT-BB were 6.9 and 8.9 months, respectively (p = 0.225). The ORR rates of CT-RP and CT-BB were 42.4% and 32.8%, respectively (p = 0.579). In the multivariate analysis, choosing either the bevacizumab RP or its biosimilar did not affect the PFS.
Moreover, earlier clinical trials established the advantages of combining EGFR-TKIs with anti-angiogenic agents in treating patients with advanced EGFR-mutant non-squamous NSCLC. Specifically, the NEJ026 study in Japan found that patients with EGFR-positive advanced non-squamous NSCLC treated with erlotinib in combination with bevacizumab had a median PFS of 16.9 months. This was notably superior to the 13.3 months PFS for those treated with erlotinib only (HR 0.605, 95% CI 0.417–0.877; p = 0.016). Furthermore, the ORR and DCR for patients receiving combined treatment of erlotinib and bevacizumab were 72% and 95%, respectively. 9 In the RELAY study, patients with advanced EGFR-mutant NSCLC treated with erlotinib in conjunction with ramucirumab, a human IgG1 vascular endothelial growth factor receptor 2 (VEGFR2) antagonist, showed a significantly improved PFS of 19.4 months compared with 12.4 months PFS in those receiving a placebo alongside erlotinib (HR 0.59, 95% CI 0.46–0.76; p < 0.0001). 22 Thus, the combined inhibition of EGFR-TKIs and VEGFs is an attractive therapeutic approach for these patients. In a real-world setting, research by Tsai et al. reported an enhanced PFS for a first-line regimen that combined an EGFR-TKI with anti-angiogenic therapy for patients with advanced EGFR-mutant NSCLC, when compared with treatment using TKI alone (17.0 months vs 11.0 months; HR 0.48, 95% CI 0.30–0.77; p = 0.002). 23 Huang et al. reported a median PFS of 16.4 months in patients with advanced EGFR-mutant lung adenocarcinoma treated with an EGFR-TKI in combination with bevacizumab. 24 The overall ORR and DCR were 77.8% and 94.4%, respectively. In addition, the study indicated that different EGFR-TKIs, such as erlotinib and afatinib, had comparable therapeutic effectiveness when used in conjunction with bevacizumab.
In the present study, the median PFS of the TKI-RP group was 18.4 months, and the TKI-BB group had a PFS of 13.6 months, with the difference not reaching statistical significance (p = 0.363). The ORR and DCR for the TKI-RP group were 71.4% and 100%, respectively. Meanwhile, the TKI-BB group had an ORR of 80% and a DCR of 90%. There was no significant difference in ORR or DCR between the two groups. The multivariate analysis also demonstrated that there was no difference between the bevacizumab reference drug and its biosimilar in terms of their effect on PFS. Previously, no study had evaluated the treatment efficacy, including PFS, between the bevacizumab RP and its biosimilar in combination with EGFR-TKIs among stage IV EGFR-mutant non-squamous NSCLC patients.
In our analysis of AEs, we focused on AEs associated with bevacizumab, including hypertension, proteinuria, thrombosis, and hemorrhage. Data from the NEJ026 study showed that the rates of hypertension, proteinuria, thrombosis, and hemorrhage were 46%, 32%, 2%, and 28%, respectively. 9 In the present study, hypertension occurred in 52.5% of patients undergoing CT and in 50% of those treated with TKIs. Proteinuria was observed in 39.0% of the CT group and 45.0% of the TKI group. As there was a limited number of participants, hemorrhage and thrombosis were reported in only two and three patients, respectively. In terms of anti-angiogenic-related AEs, we found no significant difference between the bevacizumab RP or its biosimilar, whether used in combination with CT or EGFR-TKIs.
With advances in recombinant DNA technology, biologics have become one of the essential medications in cancer treatment. They include cell therapies, cytokine or growth factors, monoclonal antibodies, and monoclonal antibody–drug toxin combinations. In 2019, 27% of the new approvals for cancer drugs in the EU, United States, and Japan were biologics.25,26 Over the past 20 years, biological agents have contributed to significant developments in oncology and other fields. However, these drugs are very expensive. The cost of cancer treatment has also increased, with the United States experiencing a 10% annual increase in cancer treatment costs between 1995 and 2013. 27 With patents expiring, the development of biosimilars not only ensures similar clinical treatment efficacy but also has the potential to reduce healthcare expenditure. Biosimilars are typically priced between 70% and 85% of the RPs. In China, in 2022, the BB was priced at 74% of the RPs. Globally, biosimilars for the treatment of cancer are typically discounted by 30% in Europe, 10%–33% in the United States, and 30% in Japan. 14 Biosimilars help to reduce medical expenses and also expand access to treatment for patients with cancer.
There were some limitations in this study. First, this investigation employed a retrospective design, and the data were collected at a single center. There may have been greater bias when compared with prospectively designed studies. Despite this, we endeavored to collect comprehensive data for each case, including characteristics, treatment courses, and outcome evaluations. Second, there was a relatively low number of cases, partly because bevacizumab is not covered by Taiwan’s National Health Insurance program, which meant that patients had limited access to the drug. However, this highlights the crucial role of lower-cost biosimilars in making the medication potentially more accessible to a broader patient population. Third, only Taiwanese patients were eligible for analysis. Therefore, it might not be possible to apply our results to different ethnic groups. Fourth, the choice of RP and BB was based on the shared decision-making between the physicians and patients without clearly defined criteria. According to the statistical analysis, there were no significant differences in patient characteristics between the RP and BB groups. Finally, most of our patients were administered bevacizumab at a dose of 7.5 mg/kg. Hence, it was not possible to determine the effect of 15 mg/kg dosage in our analysis. However, evidence from the AVAil study demonstrated similar treatment efficacy between bevacizumab at a dose of 7.5 or 15 mg/kg when combined with CT. 28 Mok et al. conducted a subgroup analysis of an Asian population in the AVAil study and found similar results, that is, that Asian patients with advanced non-squamous NSCLC receiving bevacizumab at 7.5 mg/kg in combination with CT showed similar treatment efficacy compared with those receiving bevacizumab at 15 mg/kg. 29 The rate of hypertension, the most common AE of special interest, was lower in the 7.5 mg/kg group when compared with the 15 mg/kg group. Therefore, we believe that opting for the lower dosage did not compromise our treatment outcomes.
Conclusion
Our research confirmed the clinical benefit of combining the BB with a platinum-pemetrexed regimen for treating patients with stage IV or recurrent metastatic non-squamous NSCLC. It was also effective when combined with EGFR-TKIs in EGFR-mutant lung adenocarcinoma cases. In summary, patients receiving combination treatments of CT or EGFR-TKIs with either bevacizumab RP or its biosimilars had similar outcomes in terms of ORR, DCR, and PFS.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241290718 for Real-world clinical efficacy of bevacizumab biosimilar in patients with advanced non-small-cell lung cancer by Wei-Fan Ou, Kuo-Hsuan Hsu, Jeng-Sen Tseng, Po-Hsin Lee, Kun-Chieh Chen, Yen-Hsiang Huang, Gee-Chen Chang and Tsung-Ying Yang in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
ORCID iDs: Kuo-Hsuan Hsu  https://orcid.org/0000-0002-8187-904X
https://orcid.org/0000-0002-8187-904X
Jeng-Sen Tseng  https://orcid.org/0000-0003-2341-4495
https://orcid.org/0000-0003-2341-4495
Yen-Hsiang Huang  https://orcid.org/0000-0002-0465-5800
https://orcid.org/0000-0002-0465-5800
Gee-Chen Chang  https://orcid.org/0000-0002-1802-417X
https://orcid.org/0000-0002-1802-417X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wei-Fan Ou, Department of Chest Medicine, Taichung Veterans General Hospital, Taichung, Taiwan.
Kuo-Hsuan Hsu, Department of Chest Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; Lung Cancer Comprehensive Care and Research Center, Taichung Veterans General Hospital, Taichung, Taiwan.
Jeng-Sen Tseng, Department of Chest Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; Lung Cancer Comprehensive Care and Research Center, Taichung Veterans General Hospital, Taichung, Taiwan; Department of Post-Baccalaureate Medicine, College of Medicine, National Chung Hsing University, Taichung, Taiwan; Institute of Biomedical Sciences, National Chung Hsing University, Taichung, Taiwan; School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan.
Po-Hsin Lee, Department of Chest Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan; Doctoral Program in Translational Medicine, National Chung Hsing University, Taichung, Taiwan; Rong Hsing Translational Medicine Research Center, National Chung Hsing University, Taichung, Taiwan.
Kun-Chieh Chen, Division of Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan; Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan; School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
Yen-Hsiang Huang, Department of Chest Medicine, Taichung Veterans General Hospital, No. 1650, Sect. 4, Taiwan Boulevard, Taichung 407, Taiwan; Lung Cancer Comprehensive Care and Research Center, Taichung Veterans General Hospital, No. 1650, Sect. 4, Taiwan Boulevard, Taichung 407, Taiwan; School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, No. 155, Sect. 2, Linong St., Taipei 112, Taiwan.
Gee-Chen Chang, Institute of Biomedical Sciences, National Chung Hsing University, Taichung, Taiwan; School of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan; Division of Pulmonary Medicine, Department of Internal Medicine, Chung Shan Medical University Hospital, Taichung, Taiwan; Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan; School of Medicine, Chung Shan Medical University, Taichung, Taiwan.
Tsung-Ying Yang, Department of Chest Medicine, Taichung Veterans General Hospital, Taichung, Taiwan; Lung Cancer Comprehensive Care and Research Center, Taichung Veterans General Hospital, Taichung, Taiwan; Doctoral Program in Translational Medicine, National Chung Hsing University, Taichung, Taiwan; Rong Hsing Translational Medicine Research Center, National Chung Hsing University, Taichung, Taiwan.
Declarations
Ethics approval and consent to participate: Approval was obtained from the Institutional Review Board (IRB) of Taichung Veterans General Hospital (TCVGH) in Taiwan (IRB No. CE24146A). The requirement for informed consent was waived by the IRB of TCVGH due to the study’s retrospective design. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent for publication: Not applicable.
Author contributions: Wei-Fan Ou: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Software; Writing – original draft; Writing – review & editing.
Kuo-Hsuan Hsu: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Jeng-Sen Tseng: Conceptualization; Data curation; Investigation; Methodology; Project administration; Software; Writing – original draft; Writing – review & editing.
Po-Hsin Lee: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Kun-Chieh Chen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Yen-Hsiang Huang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Gee-Chen Chang: Conceptualization; Data curation; Investigation; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Tsung-Ying Yang: Conceptualization; Data curation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Funding: This study was partially supported by grants from Taichung Veterans General Hospital (TCVGH-1133201B)
The authors declare that there is no conflict of interest.
Availability of data and materials: Data archiving is not mandated but data will be made available on reasonable request.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71(3): 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Howlader N, Forjaz G, Mooradian MJ, et al. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med 2020; 383(7): 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100(1): 57–70. [DOI] [PubMed] [Google Scholar]
- 4. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov 2022; 12(1): 31–46. [DOI] [PubMed] [Google Scholar]
- 5. Li YL, Zhao H, Ren XB. Relationship of VEGF/VEGFR with immune and cancer cells: staggering or forward?. Cancer Biol Med 2016; 13(2): 206–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Garcia J, Hurwitz HI, Sandler AB, et al. Bevacizumab (Avastin®) in cancer treatment: a review of 15 years of clinical experience and future outlook. Cancer Treat Rev 2020; 86: 102017. [DOI] [PubMed] [Google Scholar]
- 7. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004; 350(23): 2335–2342. [DOI] [PubMed] [Google Scholar]
- 8. Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355(24): 2542–2550. [DOI] [PubMed] [Google Scholar]
- 9. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol 2019; 20(5): 625–635. [DOI] [PubMed] [Google Scholar]
- 10. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378(24): 2288–2301. [DOI] [PubMed] [Google Scholar]
- 11. Joshi D, Khursheed R, Gupta S, et al. Biosimilars in oncology: latest trends and regulatory status. Pharmaceutics 2022; 14(12): 2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. U.S. Department of Health and Human Services. Scientific considerations in demonstrating biosimilarity to a reference product. Guidance for industry, https://www.fda.gov/regulatory-information/search-fda-guidance-documents/scientific-considerations-demonstrating-biosimilarity-reference-product (2015, accessed 14 October 2024).
- 13. Camacho LH. Current status of biosimilars in oncology. Drugs 2017; 77(9): 985–997. [DOI] [PubMed] [Google Scholar]
- 14. Luo X, Du X, Li Z, et al. Clinical benefit, price, and uptake for cancer biosimilars vs reference drugs in china: a systematic review and meta-analysis. JAMA Netw Open 2023; 6(10): e2337348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seo N, Polozova A, Zhang M, et al. Analytical and functional similarity of Amgen biosimilar ABP 215 to bevacizumab. MAbs 2018; 10(4): 678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Markus R, Chow V, Pan Z, et al. A phase I, randomized, single-dose study evaluating the pharmacokinetic equivalence of biosimilar ABP 215 and bevacizumab in healthy adult men. Cancer Chemother Pharmacol 2017; 80(4): 755–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thatcher N, Goldschmidt JH, Thomas M, et al. Efficacy and safety of the biosimilar ABP 215 compared with bevacizumab in patients with advanced nonsquamous non-small cell lung cancer (MAPLE): a randomized, double-blind, phase III study. Clin Cancer Res 2019; 25(7): 2088–2095. [DOI] [PubMed] [Google Scholar]
- 18. Jin R, Ogbomo AS, Accortt NA, et al. Real-world outcomes among patients with metastatic colorectal cancer treated first line with a bevacizumab biosimilar (bevacizumab-awwb). Ther Adv Med Oncol 2023; 15: 17588359231182386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45(2): 228–247. [DOI] [PubMed] [Google Scholar]
- 20. Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol 2013; 31(34): 4349–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li X, Huang J, Qiu Y, et al. Pemetrexed-platinum with or without bevacizumab for Chinese chemo-naive advanced lung adenocarcinoma patients: a real-world study. Front Pharmacol 2021; 12: 649222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(12): 1655–1669. [DOI] [PubMed] [Google Scholar]
- 23. Tsai JS, Su PL, Yang SC, et al. EGFR-TKI plus bevacizumab versus EGFR-TKI monotherapy for patients with EGFR mutation-positive advanced non-small cell lung cancer-A propensity score matching analysis. J Formos Med Assoc 2021; 120(9): 1729–1739. [DOI] [PubMed] [Google Scholar]
- 24. Huang YH, Hsu KH, Chin CS, et al. The clinical outcomes of different first-line EGFR-TKIs plus bevacizumab in advanced EGFR-mutant lung adenocarcinoma. Cancer Res Treat 2022; 54(2): 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bennett CL, Schoen MW, Hoque S, et al. Improving oncology biosimilar launches in the EU, the USA, and Japan: an updated policy review from the southern network on adverse reactions. Lancet Oncol 2020; 21(12): e575–e588. [DOI] [PubMed] [Google Scholar]
- 26. Bennett CL, Chen B, Hermanson T, et al. Regulatory and clinical considerations for biosimilar oncology drugs. Lancet Oncol 2014; 15(13): e594–e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Howard DH, Bach PB, Berndt ER, et al. Pricing in the market for anticancer drugs. J Econ Perspect 2015; 29(1): 139–162. [DOI] [PubMed] [Google Scholar]
- 28. Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009; 27(8): 1227–1234. [DOI] [PubMed] [Google Scholar]
- 29. Mok TS, Hsia TC, Tsai CM, et al. Efficacy of bevacizumab with cisplatin and gemcitabine in Asian patients with advanced or recurrent non-squamous non-small cell lung cancer who have not received prior chemotherapy: a substudy of the Avastin in Lung trial. Asia Pac J Clin Oncol 2011; 7(Suppl 2): 4–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241290718 for Real-world clinical efficacy of bevacizumab biosimilar in patients with advanced non-small-cell lung cancer by Wei-Fan Ou, Kuo-Hsuan Hsu, Jeng-Sen Tseng, Po-Hsin Lee, Kun-Chieh Chen, Yen-Hsiang Huang, Gee-Chen Chang and Tsung-Ying Yang in Therapeutic Advances in Medical Oncology