Abstract
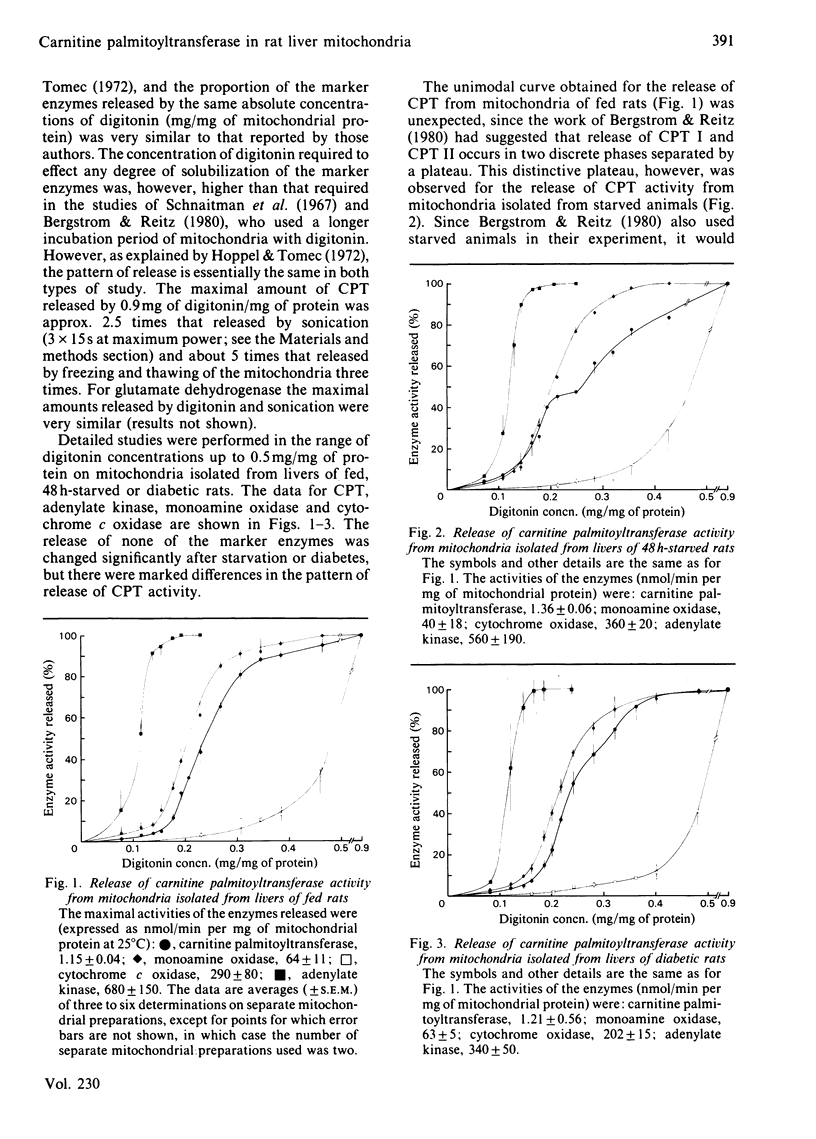
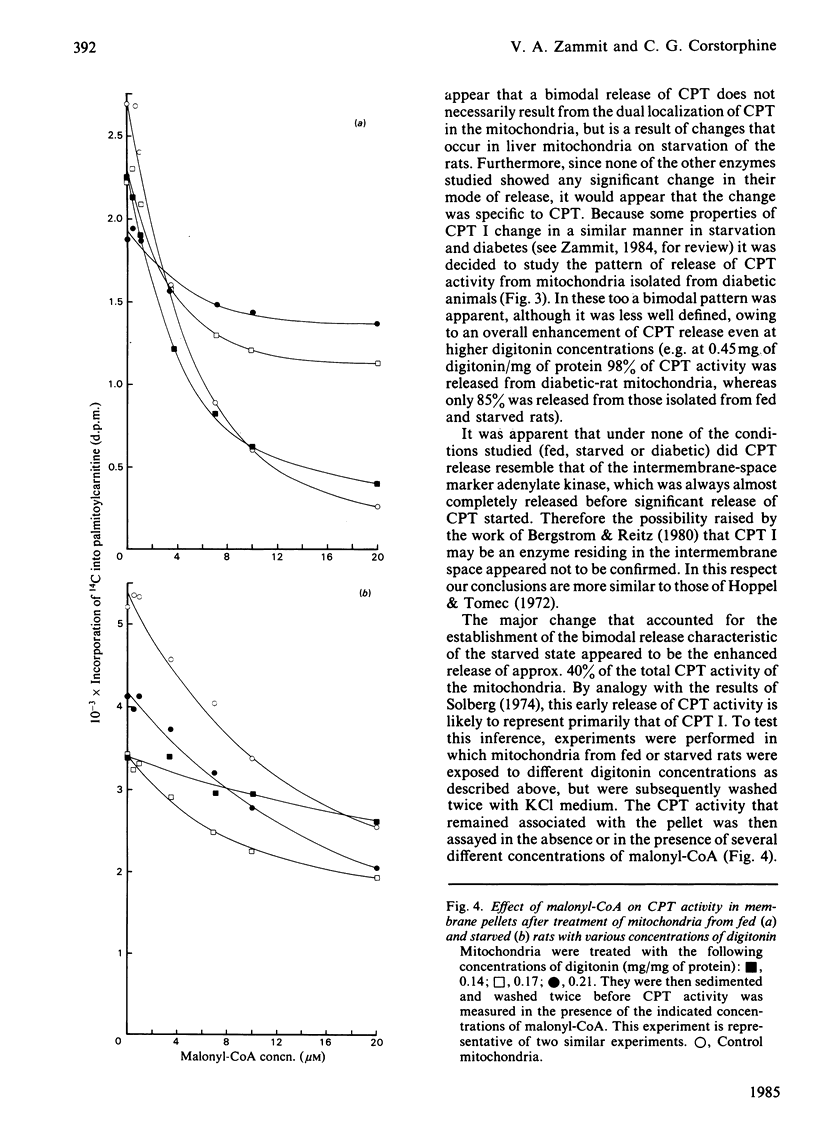
The release of carnitine palmitoyltransferase (CPT) activity from rat liver mitochondria by increasing concentrations of digitonin was studied for mitochondrial preparations from fed, 48 h-starved and diabetic animals. A bimodal release of activity was observed only for mitochondria isolated from starved and, to a lesser degree, from diabetic rats, and it appeared to result primarily from the enhanced release of approx. 40% and 60%, respectively, of the total CPT activity. This change in the pattern of release was specific to CPT among the marker enzymes studied. For all three types of mitochondria there was no substantial release of CPT concurrently with that of the marker enzyme for the soluble intermembrane space, adenylate kinase. These results illustrate that the bimodal pattern of release of CPT reported previously for mitochondria from starved rats [Bergstrom & Reitz (1980) Arch. Biochem. Biophys. 204, 71-79] is not an immutable consequence of the localization of CPT activity on either side of the mitochondrial inner membrane. Sequential loss of CPT I (i.e. the overt form) from the mitochondrial inner membrane did not affect the concentration of malonyl-CoA required to effect fractional inhibition of the CPT I that remained associated with the mitochondria. The results are discussed in relation to the possibility that altered enzyme-membrane interactions may account for some of the altered regulatory properties of CPT I in liver mitochondria of animals in different physiological states.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Takagi S., Sankawa U., Inari S., Saitô H. Saponin-cholesterol interaction in the multibilayers of egg yolk lecithin as studied by deuterium nuclear magnetic resonance: digitonin and its analogues. Biochemistry. 1980 Apr 29;19(9):1904–1911. doi: 10.1021/bi00550a027. [DOI] [PubMed] [Google Scholar]
- BANGHAM A. D., HORNE R. W., GLAUERT A. M., DINGLE J. T., LUCY J. A. Action of saponin on biological cell membranes. Nature. 1962 Dec 8;196:952–955. doi: 10.1038/196952a0. [DOI] [PubMed] [Google Scholar]
- Bergstrom J. D., Reitz R. C. Studies on carnitine palmitoyl transferase: the similar nature of CPTi (inner form) and CPTo (outer form). Arch Biochem Biophys. 1980 Oct 1;204(1):71–79. doi: 10.1016/0003-9861(80)90008-9. [DOI] [PubMed] [Google Scholar]
- Bird M. I., Saggerson E. D. Binding of malonyl-CoA to isolated mitochondria. Evidence for high- and low-affinity sites in liver and heart and relationship to inhibition of carnitine palmitoyltransferase activity. Biochem J. 1984 Sep 15;222(3):639–647. doi: 10.1042/bj2220639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremer J. The effect of fasting on the activity of liver carnitine palmitoyltransferase and its inhibition by malonyl-CoA. Biochim Biophys Acta. 1981 Sep 24;665(3):628–631. doi: 10.1016/0005-2760(81)90282-4. [DOI] [PubMed] [Google Scholar]
- Bremer J., Woldegiorgis G., Schalinske K., Shrago E. Carnitine palmitoyltransferase. Activation by palmitoyl-CoA and inactivation by malonyl-CoA. Biochim Biophys Acta. 1985 Jan 9;833(1):9–16. doi: 10.1016/0005-2760(85)90247-4. [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., Kopec B., Fritz I. B. The localization of carnitine palmitoyltransferase on the inner membrane of bovine liver mitochondria. J Biol Chem. 1973 Jun 10;248(11):4075–4082. [PubMed] [Google Scholar]
- Fiol C. J., Bieber L. L. Sigmoid kinetics of purified beef heart mitochondrial carnitine palmitoyltransferase. Effect of pH and malonyl-CoA. J Biol Chem. 1984 Nov 10;259(21):13084–13088. [PubMed] [Google Scholar]
- Hoppel C. L. Carnitine and carnitine palmitoyltransferase in fatty acid oxidation and ketosis. Fed Proc. 1982 Oct;41(12):2853–2857. [PubMed] [Google Scholar]
- Hoppel C. L., Tomec R. J. Carnitine palmityltransferase. Location of two enzymatic activities in rat liver mitochondria. J Biol Chem. 1972 Feb 10;247(3):832–841. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Mills S. E., Long C. S., Foster D. W. Observations on the affinity for carnitine, and malonyl-CoA sensitivity, of carnitine palmitoyltransferase I in animal and human tissues. Demonstration of the presence of malonyl-CoA in non-hepatic tissues of the rat. Biochem J. 1983 Jul 15;214(1):21–28. doi: 10.1042/bj2140021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeillie E. M., Zammit V. A. Regulation of acetyl-CoA carboxylase in rat mammary gland. Effects of starvation and of insulin and prolactin deficiency on the fraction of the enzyme in the active form in vivo. Biochem J. 1982 Apr 15;204(1):273–280. doi: 10.1042/bj2040273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S. E., Foster D. W., McGarry J. D. Effects of pH on the interaction of substrates and malonyl-CoA with mitochondrial carnitine palmitoyltransferase I. Biochem J. 1984 Apr 15;219(2):601–608. doi: 10.1042/bj2190601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M., Nojima S., Akiyama T., Sankawa U., Inoue K. Interaction of digitonin and its analogs with membrane cholesterol. J Biochem. 1984 Oct;96(4):1231–1239. doi: 10.1093/oxfordjournals.jbchem.a134941. [DOI] [PubMed] [Google Scholar]
- Robinson I. N., Zammit V. A. Sensitivity of carnitine acyltransferase I to malonly-CoA inhibition in isolated rat liver mitochondria is quantitatively related to hepatic malonyl-CoA concentration in vivo. Biochem J. 1982 Jul 15;206(1):177–179. doi: 10.1042/bj2060177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH L. Spectrophotometric assay of cytochrome c oxidase. Methods Biochem Anal. 1955;2:427–434. doi: 10.1002/9780470110188.ch13. [DOI] [PubMed] [Google Scholar]
- Saggerson E. D. Carnitine acyltransferase activities in rat liver and heart measured with palmitoyl-CoA and octanoyl-CoA. Latency, effects of K+, bivalent metal ions and malonyl-CoA. Biochem J. 1982 Feb 15;202(2):397–405. doi: 10.1042/bj2020397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shany S., Bernheimer A. W., Grushoff P. S., Kim K. S. Evidence for membrane cholesterol as the common binding site for cereolysin, streptolysin O and saponin. Mol Cell Biochem. 1974 May 30;3(3):179–186. doi: 10.1007/BF01686643. [DOI] [PubMed] [Google Scholar]
- Solberg H. E. Acyl group specificity of mitochondrial pools of carnitine acyltransferases. Biochim Biophys Acta. 1974 Aug 22;360(2):101–112. doi: 10.1016/0005-2760(74)90160-x. [DOI] [PubMed] [Google Scholar]
- Topping D. L., Targ M. E. Time-course of changes in blood glucose and ketone bodies, plasma lipids and liver fatty acid composition in streptozotocin-diabetic male rats. Horm Res. 1975;6(3):129–137. doi: 10.1159/000178670. [DOI] [PubMed] [Google Scholar]
- West D. W., Chase J. F., Tubbs P. K. The separation and properties of two forms of carnitine palmitoyltransferase from ox liver mitochondria. Biochem Biophys Res Commun. 1971 Mar 5;42(5):912–918. doi: 10.1016/0006-291x(71)90517-1. [DOI] [PubMed] [Google Scholar]
- Zammit V. A., Corstorphine C. G., Gray S. R. Changes in the ability of malonyl-CoA to inhibit carnitine palmitoyltransferase I activity and to bind to rat liver mitochondria during incubation in vitro. Differences in binding at 0 degree C and 37 degrees C with a fixed concentration of malonyl-CoA. Biochem J. 1984 Sep 1;222(2):335–342. doi: 10.1042/bj2220335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Increased sensitivity of carnitine palmitoyltransferase I activity to malonyl-CoA inhibition after preincubation of intact rat liver mitochondria with micromolar concentrations of malonyl-CoA in vitro. Biochem J. 1983 Mar 15;210(3):953–956. doi: 10.1042/bj2100953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zammit V. A. Mechanisms of regulation of the partition of fatty acids between oxidation and esterification in the liver. Prog Lipid Res. 1984;23(1):39–67. doi: 10.1016/0163-7827(84)90005-5. [DOI] [PubMed] [Google Scholar]
- Zammit V. A. The effect of glucagon treatment and starvation of virgin and lactating rats on the rates of oxidation of octanoyl-L-carnitine and octanoate by isolated liver mitochondria. Biochem J. 1980 Aug 15;190(2):293–300. doi: 10.1042/bj1900293. [DOI] [PMC free article] [PubMed] [Google Scholar]


