Abstract
Most monitoring programs next to large per- and polyfluoroalkyl substances (PFAS) sources focus on drinking water contamination near source zones. However, less is understood about how these sources affect downgradient hydrological systems and food webs. Here, we report paired PFAS measurements in water, sediment, and aquatic biota along a hydrological gradient away from source zones contaminated by the use of legacy aqueous film-forming foam (AFFF) manufactured using electrochemical fluorination. Clustering analysis indicates that the PFAS composition characteristic of AFFF is detectable in water and fishes >8 km from the source. Concentrations of 38 targeted PFAS and extractable organofluorine (EOF) decreased in fishes downgradient of the AFFF-contaminated source zones. However, PFAS concentrations remained above consumption limits at all locations within the affected watershed. Perfluoroalkyl sulfonamide precursors accounted for approximately half of targeted PFAS in fish tissues, which explain >90% of EOF across all sampling locations. Suspect screening analyses revealed the presence of a polyfluoroketone pharmaceutical in fish species, and a fluorinated agrochemical in water that likely does not accumulate in biological tissues, suggesting the presence of diffuse sources such as septic system and agrochemical inputs throughout the watershed in addition to AFFF contamination. Based on these results, monitoring programs that consider all hydrologically connected regions within watersheds affected by large PFAS sources would help ensure public health protection.
Keywords: per- and polyfluoroalkyl substances (PFAS), perfluoroalkyl sulfonamides (FASA), aquatic biota, fish, shellfish, aqueous film-forming foam (AFFF), source zones, contamination
Short abstract
PFAS contamination extends beyond source zones and accumulates in fish in downgradient watersheds, highlighting the need for comprehensive watershed monitoring programs.
1. Introduction
Human exposure to per- and polyfluoroalkyl substances (PFAS) has been associated with an increased risk of various cancers, immune dysfunction, and metabolic disorders, among other adverse effects.1,2 Consumption of fish and shellfish is an important source of PFAS exposure for many individuals.3,4 Next to PFAS source zones across the United States (U.S.), even one meal of freshwater fishes may be sufficient to exceed recommended daily exposure limits for perfluorooctanesulfonate (PFOS).5 Many studies have characterized PFAS contamination and composition in surface water and groundwater surrounding source zones.6−9 However, implications of these sources for wildlife and recreational/subsistence fisheries in downgradient watersheds are less understood.10,11 Here, we examine how the magnitude and composition of PFAS in fishes and other aquatic biota change along a hydrological gradient away from source zones contaminated by the use of aqueous film-forming foams (AFFF), predominantly manufactured using electrochemical fluorination (ECF).7,12
Design of appropriate monitoring programs for PFAS requires an understanding of how concentrations in surface water and aquatic biota are attenuated downgradient of large source areas. One challenge is that standard analytical methods only measure a small subset of PFAS present in environmental samples.13,14 For example, in our past work, we showed that perfluoroalkyl sulfonamides (FASA) are frequently detected in water and fish tissues, but are not yet included in routine monitoring next to AFFF-contaminated sites, despite the availability of analytical standards facilitating detection.15 FASA are a class of precursor PFAS formed during biotransformation of abundant perfluoroalkyl sulfonamido compounds in legacy ECF AFFF.16,17 Field-measured bioaccumulation factors (BAF) for FASA in fish tissues were 1–3 orders of magnitude higher than their terminal perfluoroalkyl acids (PFAA), reinforcing the need for additional characterization of potential exposure risks.15
Bulk organofluorine measurements such as extractable organofluorine (EOF) are useful for evaluating a greater portion of the organofluorine mass budget.7,18,19 Prior work on AFFF and at an AFFF-contaminated source zone has shown that all of the EOF can be explained by the sum of targeted PFAS and oxidizable precursors.12,20 Suspect screening and nontargeted analysis using high-resolution mass spectrometry (HRMS) offer complementary information by confirming the presence of specific PFAS and for assigning probable structures to unknown PFAS.21 Combining these analytical methods allows for a more comprehensive understanding of chemical composition and how concentrations in water and biological tissues change in hydrological systems downgradient of large PFAS sources.
In downgradient watersheds, attributing the importance of diffuse PFAS sources that enter the flow path represents another challenge. This information is important for determining benefits from remediation and potential liability for cleanup. Statistical clustering techniques and data on PFAS composition can provide information on predominant sources of contamination,7,22 and past work suggests such an approach can be extended to fish.11,23−25 Source attribution may be confounded by transformation of many precursor PFAS in AFFF and other sources along the flow path, changing the original PFAS composition.7,9,17 In biota, metabolism of precursors and differing propensity of PFAS for accumulation in biological tissues can also alter the source signature.26−28
The main objective of this work was to better understand how PFAS composition and concentrations in surface water and aquatic biota change with hydrological distance from source zones. To do this, we collected paired water and sediment samples concurrently with fish, eel, shellfish, and other aquatic fauna along a hydrological transect away from source zones within an ECF AFFF-impacted watershed on Cape Cod, Massachusetts (MA), U.S. We constructed a mass budget for EOF in multiple aquatic species, including those commonly consumed by local fishers, by comparing EOF to the sum of targeted PFAS and suspect screening using HRMS. These data provide insights into the appropriate spatial extent of watershed monitoring programs and fish consumption advisories near contaminated sites.
2. Methods and Materials
2.1. Field Sampling
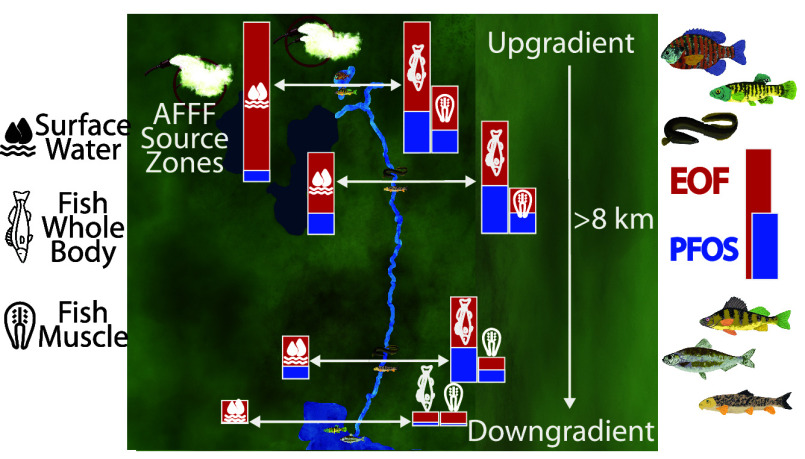
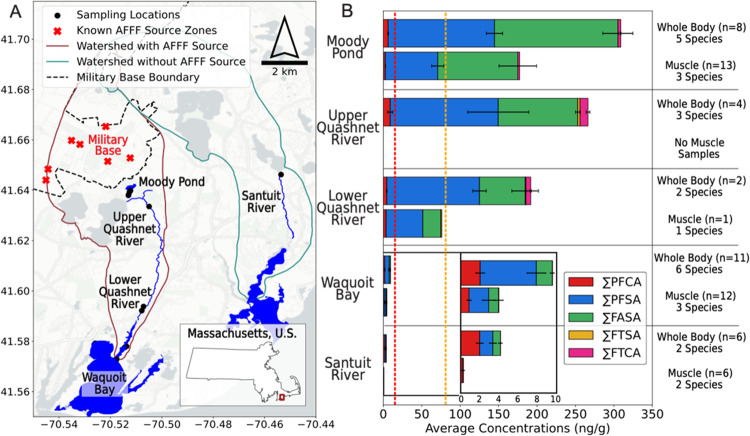
Figure 1A shows sampling locations for paired surface water, sediment, and aquatic biota on Cape Cod, MA, U.S. The predominant PFAS source within the watershed is an upgradient military base that historically used AFFF.7,9,29 Sampling locations were selected along a hydrological gradient away from the military base, beginning with a groundwater-fed pond (Moody Pond), followed by the downgradient river (Quashnet River) that extends ∼8 km before reaching the downgradient estuary (Waquoit Bay). Moody Pond and the Quashnet River are both freshwater sites (salinity <1) (Table S1). At the estuarine site (Waquoit Bay), salinity ranged from 17 to 28 across sampling locations (Table S1). Samples were also collected from the Santuit River (salinity <1), which does not have known PFAS point sources within its watershed (Figure 1A).
Figure 1.
Overview of sampling locations and concentrations of targeted per- and polyfluoroalkyl substances (PFAS) in fishes. (A) Sampling locations (black circles) on Cape Cod, MA, U.S. Known aqueous film-forming foam (AFFF) source zones identified on the military base are shown by red “X” markers.40 Watershed boundaries shown are from the Cape Cod Commission.41 (B) Average targeted PFAS concentrations (ng g–1 wet-weight) in fishes (whole-body or muscle tissue) across sampling locations. The inset shows a zoomed-in version of concentrations for samples from Waquoit Bay and Santuit River. PFAS subclasses shown include perfluorocarboxylates (∑PFCA), perfluorosulfonates (∑PFSA), perfluoroalkyl sulfonamides (∑FASA), fluorotelomer sulfonates (∑FTSA), and fluorotelomer carboxylates (∑FTCA). Error bars indicate the standard error of averages for each subclass. State of MA fish consumption guidelines are shown by the vertical orange (sensitive population do not consume >81 ng g–1 perfluorooctanesulfonate (PFOS)) and red (general population 1 meal/month >15 ng g–1 PFOS) dashed lines.
Surface water grab samples were collected in duplicate (n = 26) along with field blanks (n = 7) in 1 L bottles (Table S1). Sediment samples (n = 11) were collected using polypropylene sediment push cores (6.3 cm diameter; 25 cm length) from the same locations as the surface water samples. Sediment cores were sectioned into 5 cm depth profiles and freeze-dried. Common fish species (n = 19), an eel species (n = 1), shellfish (n = 2), and other aquatic fauna (n = 2) were collected concurrently with water and sediment sampling by collaborators at the MA Division of Fisheries and Wildlife, the MA Division of Marine Fisheries, and the Town of Mashpee Department of Natural Resources (Table S2). All methods and fish handling followed protocols specified in permits issued by MA Department of Fish and Game. All field samples were stored frozen at −20 °C prior to analysis. Additional details on field sampling are provided in the Supporting Information (SI Section 1.1).
2.2. Sample Extraction
Water samples (300 mL) were spiked with 3.75 ng isotopically labeled internal standards followed by offline weak anion exchange (WAX) solid phase extraction (SPE) with dispersive Envi-Carb cleanup, following established methods.13,29 Freeze-dried sediment samples (1 g dry-weight) were spiked with 3.75 ng internal standard, extracted in 1% ammonium hydroxide (NH4OH) in methanol, followed by dispersive Envi-Carb cleanup, following established methods.13,30
For fishes with fork length >14 cm and eel >25 cm fork length, we extracted PFAS from muscle fillets to best capture the fraction consumed by recreational fishers. We measured whole-body concentrations for fishes ≤14 cm and eel ≤25 cm, and composite samples for fishes with <10 cm fork length. For shellfish and gastropods, whole-body soft tissue concentrations were determined. Tissue samples were homogenized using a hand-held OMNI International TH homogenizer. Homogenized tissue (0.5 g wet-weight) was fortified with 2.25 ng internal standard. Following methods developed and detailed in prior work,15 we used an acetonitrile extraction using a bead blender (MP Biomedicals FastPrep-24) and 4.8 mm stainless steel beads for further homogenization with WAX SPE cleanup, modified from established methods.13,31,32 Additional details on sample preparation are provided in SI Sections 1.2–1.3.
2.3. Targeted Analysis
Extracts from water, sediment, and biological tissues were analyzed at Harvard University for targeted PFAA (n = 18) and precursors (n = 19) using an Agilent (Santa Clara, CA) 6460 triple quadrupole liquid chromatography-tandem mass spectrometer (LC-MS/MS) in negative electrospray ionization (ESI–) mode, following previously published methods (SI Section 1.4).27,29 Targeted PFAS were quantified using isotopic dilution and extracted internal standards with 11- to 15-point calibration curves. For PFAS without matched internal standards, the standard closest in retention time or within the same functional group class was used for quantification (Table S3). Linear and branched isomers of perfluorohexanesulfonate (PFHxS), PFOS, and N-methyl and N-ethyl sulfonamidoacetic acids were quantified separately using available isomeric standards. For FASA and several other PFAA, we quantified the sum of both isomers using the linear isomer calibration curves (SI Section 1.5; Figures S1, S2). Results are discussed as the sum of isomers unless indicated.
Details on blanks (Table S4), duplicates, procedural and matrix spike recoveries (Table S5), and internal standard recoveries (Table S6) are provided in SI Section 1.6. Average procedural and matrix spike recoveries for all targeted PFAS present in samples generally fell within 70–130% (Table S5). Method detection limits (MDLs) were calculated as three times the sample signal-to-noise ratio multiplied by the sample dilution volumes or weights. Only values >MDLs are reported (SI Section 1.7; Table S7). Method trueness was assessed using the National Institute of Standards and Technology Standard Reference Material (NIST SRM) 1947 (SI Section 1.8; Table S8).
2.4. Suspect Screening and Nontargeted Analysis
Surface water and biological tissue extracts were analyzed at Harvard University for suspect PFAS using a Vanquish Flex ultrahigh-performance liquid chromatograph (ThermoFisher) coupled with a quadrupole Orbitrap Exploris 120 MS instrument (ThermoFisher) in ESI– mode (UHPLC-HRMS). Data were acquired using full scan MS1 mode with resolution of 60,000 and a scan range of 200–800 m/z and data-dependent analysis mode with resolution of 30,000 for MS2 confirmation (Table S9). Data were preprocessed using Compound Discoverer 3.3 (ThermoFisher) with peak intensity removal <10,000, mass tolerance <5 ppm, retention time tolerance (±0.2 min), and inclusion of various PFAS mass lists described in the SI. Confidence levels of 2a, 2b, and 3 were assigned accordingly.33,34 Identification of perfluoropentane sulfonamide (FPeSA) with a newly available analytical standard led to additional quantification of this compound on the UHPLC-HRMS. Additional details are provided in SI Section 1.9.
2.5. Extractable Organofluorine Analysis
We analyzed extractable organofluorine (EOF) in selected surface water, sediment, and biological tissue samples at Harvard University (SI Section 1.10). Extractions followed the methods used for targeted PFAS analysis, but internal standards were not added prior to extraction. All sample extractions included a WAX SPE cleanup step with a 0.01% NH4OH in Milli-Q water rinse for inorganic fluorine removal.
Protocols for EOF analysis followed those described in prior work.19 Extracts were split for analysis of EOF and targeted PFAS. EOF was analyzed by combustion ion chromatography (CIC) using a Metrohm CIC with combustion unit from Analytik Jena (Jena, Germany), 920 Absorber Module, and 930 Compact IC Flex ion chromatograph from Metrohm (Herisau, Switzerland). Sample extracts (100 μL) were injected into the combustion unit at 1050 °C, and anions were separated with an ion exchange column (Metrosep A Supp 5-150/4) operated at 30 °C, with sodium carbonate–bicarbonate buffer as the eluent and isocratic elution. The fluoride (F–) concentration was measured via ion conductivity. To determine the fraction of EOF accounted for by targeted PFAS, the other extract split was spiked with internal standard following extraction and analyzed by LC-MS/MS, and PFAS concentrations were converted to fluorine-equivalents for direct comparison.
Concentrations above the instrumental background were corrected by subtracting the extraction blank average. MDLs were calculated as 3 times the standard deviation of the average matrix extraction blank adjusted by the dilution factor. We evaluated removal of inorganic fluorine using a sodium fluoride spike (average: 95 ± 10%, Table S10) and organofluorine recovery using a PFAS mixture spike (average: 83 ± 19%, Tables S11 and S12) in procedural and matrix samples across all sample types.
2.6. Stable Isotope Analysis
Samples of dorsal muscle (eel and fish species) and soft tissue (invertebrates) were homogenized, dried at 60 °C for at least 48 h, and ground into a fine homogeneous powder.35 Samples (520 ± 120 μg) were weighed in tin capsules, and carbon and nitrogen stable isotopes (δ13C and δ15N) and elemental composition (% C and % N) were determined on an isotope ratio mass spectrometer (Thermo Flash 2000) coupled to an elemental analyzer (EA-irMS) at Harvard University. Carbon and nitrogen isotopic composition were expressed as per mil (‰) relative to Vienna PeeDee Belemnite limestone and atmospheric nitrogen, respectively. All δ13C values were normalized using the carbon-to-nitrogen ratio (C:N) to account for variable lipid content.35 Additional details on isotope analyses and δ13C normalization are provided in SI Section 1.11.
2.7. Statistical Analyses
All statistical analyses were performed in Python version 3.7.4 using SciPy36 and statsmodels.37 Targeted PFAS with <70% detection frequency were excluded from statistical summaries. For analytes with ≥70% detection frequency, we replaced values <MDL using simple imputation (MDL/√2). Detectable PFAS concentrations in surface water and biological tissue samples were used to calculate field-measured bioaccumulation factors (BAF) (μg of PFAS kg–1 wet-weight biota tissue/μg of PFAS L–1 water). Detectable PFAS concentrations in surface sediment and benthic tissue samples were used to calculate field-measured biota-sediment accumulation factors (BSAF) (μg of PFAS kg–1 wet-weight biota tissue/μg of PFAS kg–1 sediment normalized to the fraction of organic carbon) (SI Section 1.12).
We used principal component analysis (PCA) and hierarchical clustering to examine changes in the composition of PFAS in water and aquatic biota at various distances away from the AFFF-contaminated source zones. Results from the PCA are based on center-log ratio transformed molar compositional scaled data with nondetects <60% detection frequency imputed using MDL/√2, following prior studies.7,22,38 We tested for statistically significant differences among PFAS concentrations by location and species using one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference (HSD) test for surface water and sediment samples and Kruskal–Wallis followed by the Dunn–Bonferroni post hoc test for tissue samples (SI Section 1.12, Table S13A–C).
3. Results and Discussion
3.1. Fishes throughout AFFF-Impacted Watershed Exceed Consumption Guidelines
Concentrations of targeted PFAS in water and fish tissues decreased downgradient of the AFFF-impacted source zones (Figures 1B and S3). The sum of targeted PFAS concentrations in surface water was significantly higher at sites closest to the AFFF source zones (140–580 ng L–1) compared to the downgradient sites (11–75 ng L–1, Dunn, p < 0.05, Table S13A). Similarly, tissue PFAS concentrations were highest for fishes collected from the upgradient sites closer to the AFFF-impacted source zones (60.9–416 ng g–1 in Moody Pond and the Quashnet River, Table S14). In the downgradient estuary (Waquoit Bay) and background watershed (Santuit River), the sum of targeted PFAS concentrations in fish tissues was significantly lower (0.11–17 ng g–1, Dunn, p < 0.05, Table S13B) than the upgradient sites. Concentrations of the sum of targeted PFAS in sediment were low (<MDL-21 ng g–1) throughout the AFFF-impacted watershed (Table S14), likely reflecting the low organic carbon content sediment characteristic of the Cape Cod aquifer, which was <5% at all sites except the upper Quashnet River (Table S1). Accordingly, PFAS concentrations in fish tissues were more strongly associated with water concentrations (r = 0.8–0.9, p < 0.05) than sediment concentrations (r = 0.1–0.4, p > 0.05, Figure S4).
We measured both muscle tissue and whole-body PFAS concentrations for some fish species and eel, which are reported separately here (Table S2). Whole-body PFAS concentrations were significantly higher than in muscle tissues (Kruskal–Wallis, p < 0.05, Figures S5 and S6), but PFAS composition was similar (Figure S7). Levanduski et al.39 suggested whole-body to muscle fillet conversion factors of 0.50–0.55 for PFOS and the n = 9–11 perfluorinated carbon (C9–C11) perfluorocarboxylates (PFCA), and 0.52 for total PFAS based on the combination of these four analytes. We calculated slightly lower values (range: 0.33–0.48) for these same PFAS compared to the prior study and a higher total PFAS value (0.62 ± 0.11) when including all additional analytes measured by targeted analysis (Figure S8). Lower values in this study for the four targeted PFAS likely reflect the smaller sample size in our study, as well as variability among species and sites. The higher value for total PFAS in this study does overlap with the prior study when considering the standard error of the slope, but additionally reflects inclusion of analytes such as FASA that have a high propensity for bioaccumulation,15 and may partition differently among organs. When available, such conversions may be better captured by direct measurements.
At the upgradient sites (Moody Pond/Quashnet River), 14 targeted PFAS were detected in all biological samples including: PFHxS (C6 PFSA), PFOS (C8 PFSA), perfluorodecanesulfonate (C10 PFSA), perfluorononanoate (PFNA) to perfluorotetradecanoate (PFTeDA) (C8–C13 PFCA), C4–C6 and C8 FASA, and 7:3 fluorotelomer carboxylate (FTCA) (Table S15). A maximum of 22 of 38 targeted PFAS were detected in eel and two fish species samples. PFOS accounted for the greatest fraction (range: 13–90%, average: 53 ± 22%) of the targeted PFAS measured in eel and other fish tissues from Moody Pond and the Quashnet River (Figure S7), with concentrations ranging between 21 and 220 ng g–1 in both muscle and whole-body samples (Table S14). All fish muscle tissue and whole-body samples exceeded the 1 meal/month limit of 15 ng g–1 PFOS for the general population set by the state of Massachusetts in 2021 (Figure 1B).42 Muscle tissue concentrations in two species (yellow perch: Perca flavescens and American eel: Anguilla rostrata) exceeded the 1 meal/6-month limit (91 ng g–1 PFOS) for the general population and the do-not-consume limit (81 ng g–1 PFOS) for sensitive populations (Table S16).42
High concentrations of FASA (10–230 ng g–1) were detected in fish species from the upgradient sites in the AFFF-impacted watershed (Figure 1B, Table S14) and accounted for 16–85% (average: 46 ± 20%) of targeted PFAS.15 C4–C6 FASA are stable intermediate transformation products of C4–C6 perfluoroalkyl sulfonamido precursors that make up large fractions of the PFAS in ECF AFFF.12,43 Persistence of FASA in the downgradient ecosystem is consistent with their stability against oxidation and slow transformation.9,12 No regulatory thresholds have been established for FASA despite preliminary data showing potential toxicity and bioaccumulation potential.44,45 These results highlight an urgent need for advisory programs to consider FASA when developing fish consumption guidelines.15
In the estuary (Waquoit Bay), all fishes and eel contained detectable concentrations of PFOS and the C10–C13 PFCA. A maximum of 15 of 38 targeted PFAS were detected in four fish species (Table S15). Estuarine fish tissues had significantly lower PFOS concentrations (0.65–11 ng g–1; Dunn, p < 0.05, Table S13B) compared to freshwater fish tissues from the upgradient sites (Figure 1B, Table S14). Fishes and eels from the downgradient sites exceeded the ≤0.50 ng g–1 limit considered suitable for unlimited consumption.42 Estuarine fishes had a lower proportion of FASA and a greater proportion of C10–C13 PFCA present compared to upgradient freshwater fishes (Figure S7). Similarly, PFOS and >C10 PFCA accounted for the greatest proportion of targeted PFAS in fish tissues from the background watershed (Santuit River) (Figure S7). Background PFAS concentrations in fishes may reflect diffuse sources such as agrochemical use,46 atmospheric deposition,47,48 and septic system inputs,49 the primary wastewater management and nutrient source in the study region.50−52
Among estuarine shellfish, PFOS and the C8 and C10–C13 PFCA were detected in ≥80% of blue crab (Callinectes sapidus) samples but most quahog (Mercenaria mercenaria) samples did not contain detectable PFAS (Table S15). Quahogs are filter feeders that burrow into sediment and obtain food from settling particles in the water,53 whereas blue crabs opportunistically consume a combination of bivalves, crustaceans, and fishes, as well as plants and detritus (Table S2).54−56 Both the sediment (0.10–1.5 ng g–1) and unfiltered surface water (11–33 ng L–1) from the estuary had low PFAS concentrations (Table S14). This may partially reflect the lower uptake of PFAS in bivalves, which has also been observed in past work.57−59
3.2. Targeted PFAS Account for Most Extractable Organofluorine (EOF) in Biological Tissues
In past work on surface waters from the same AFFF-impacted watershed,7 the fraction of unexplained organofluorine (EOF minus targeted PFAA and oxidizable precursors) increased from 37% at sites closest to AFFF source zones to 39–76% at sites closest to the estuarine mouth of the river. The authors hypothesized that this may predominately reflect diffuse releases of fluorinated pharmaceuticals from domestic and municipal wastewater disposal and agrochemicals that are infrequently measured alongside PFAS in fluorine mass budgets.7,60
In this study, additional analytical standards were available for FASA, which improved and expanded the quantification of precursor concentrations. With this improvement in analytical precision, we found unexplained organofluorine measured in surface water samples from the same locations decreased to 29–40% at sites closest to the AFFF source zones and 24% at sites closest to the estuarine mouth of the river (Table S17, Figure S9). Targeted PFAS and EOF concentrations were strongly correlated in the water samples (Figure S9). Differences between studies in unexplained organofluorine fractions may also reflect temporal/seasonal variations in septic system and agrochemical inputs into the downgradient watershed. For example, fluazifop-p-butyl, an active ingredient with a −CF3 group used in herbicides to control perennial grasses, was identified using suspect screening with greater peak area abundance in surface waters downgradient in the AFFF-impacted and background watershed (Table S18, Figure S10).61 In both studies, EOF concentrations in surface waters were highest closest to the AFFF source zones (Moody Pond) and lowest at the downgradient sites near the estuary (Table S17, Figure S9).7
In fishes and eel, EOF concentrations were highest (35–280 ng F g–1) at sampling locations closest to the AFFF source zones and decreased with distance downgradient, analogous to spatial patterns observed for targeted PFAS (Figure 2A). EOF concentrations were below detection in samples from the estuary as well as the background watershed site (Table S17). Targeted PFAS and EOF concentrations were strongly correlated in biological tissues (Figure 2B,C). The terminal PFAA accounted for an average of 44% (95% confidence interval (CI): 37–55%) of the EOF across biological species and sites (Figure 2B). When targeted precursors such as FASA were included, the average fraction of EOF in species accounted for by the targeted PFAS measurements increased to 93% (95% CI: 79–110%) (Figure 2C). These data indicate there is little unexplained organofluorine in biological tissues in the AFFF-impacted watershed and that precursors account for a substantial portion of their tissue burdens.
Figure 2.
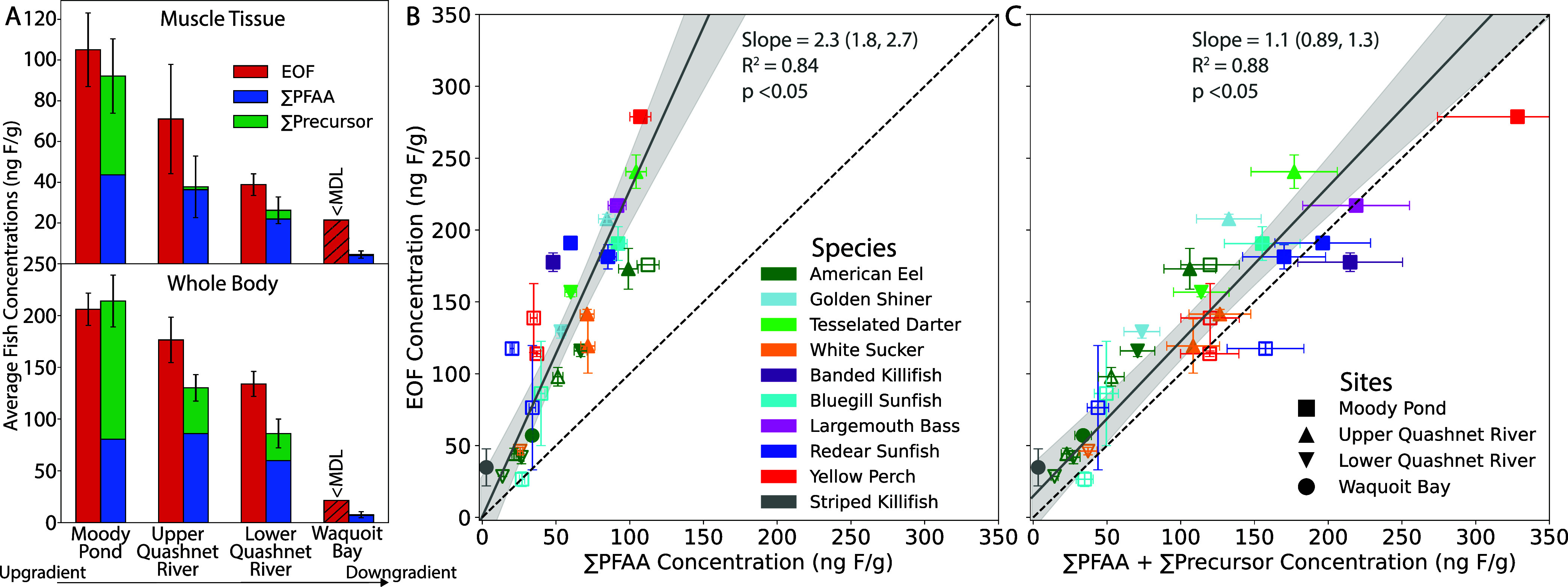
Extractable organofluorine (EOF) mass budget in biological tissue samples. (A) Comparison of EOF concentrations (ng F g–1) in eel and fish muscle tissue (n = 2–7) and whole-body (n = 3–10) samples to summed concentrations (ng F g–1) of targeted per- and polyfluoroalkyl substances (PFAS) (Table S17) grouped by perfluoroalkyl acids (∑PFAA) and precursors (∑Precursor), which includes perfluoroalkyl sulfonamides (FASA), fluorotelomer sulfonates (FTSA), and fluorotelomer carboxylates (FTCA). In panel (A), hatched bars indicate >70% of samples were below the method detection limit (MDL). Error bars represent the standard error in biota samples grouped by site. (B) Comparison of ∑PFAA to EOF concentrations in aquatic biota. (C) Comparison of ∑PFAA + ∑Precursor to EOF concentrations in aquatic biota. Each marker represents an average by species, tissue type, and location. EOF error bars represent the standard deviation of replicate measurements, and targeted PFAS error bars represent the average weighted error based on the relative percent difference between sample method replicates. Marker type denotes sample site collection, marker color denotes species, and open markers represent muscle tissue samples, whereas closed markers represent whole-body samples. Weighted least-squares linear regression (gray solid line) with 95% confidence intervals (shaded gray area) for aquatic biota are compared to the 1:1 line (black dash).
The proportion of unexplained organofluorine in biological tissues from the AFFF-impacted watershed did not increase with distance from the source zones, consistent with surface water measurements in this study, and instead reflected interindividual variability (Figure 2). The lower proportion of unexplained organofluorine in biological tissues (0–49%) relative to surface water samples (24–40%) suggests some of the unidentified organofluorine present in surface waters does not readily bioaccumulate in tissues or unidentified precursors are metabolized in vivo.
Nontargeted analysis of aquatic biota revealed 14 additional PFAS identified using suspect screening, 7 of which were also identified in surface water samples (Table S18, Figures S10–S12). These included the C3 FASA, the C6 and C8 perfluoroalkyl sulfinic acids, the H-substituted C6, C8, and C10 PFSA, and the unsaturated C8 PFSA. Suspect PFAS only identified in biota samples included the C5 FASA with a methylated sulfonamide headgroup, the C7 FASA, a C8 PFSA with a pentafluorosulfide group, an ether-based C9 PFSA, and the longer-chain C10 and C11 PFSA (Table S18). The suspect PFAS identified in aquatic biota were variations of sulfonate-based PFAS and additional FASA. Given that most of the EOF was explained by the targeted analytes (Figure 2C), it is likely that these compounds are present at relatively low concentrations compared with the PFAA and targeted FASA.
All biota samples from the downgradient estuary and the background site contained 1,1,1,2,2-pentafluoro-7-phenylheptan-3-one (FKGK11) (Figures S11 and S12). FKGK11 was identified at a confidence level of 3 and detected in 66% of aquatic biota samples but not in surface water samples (Table S18). This compound contains a −CF2CF3 group and is a type of polyfluoroketone pharmaceutical used as a selective inhibitor for certain phospholipase enzymes.62,63 Similar to other suspect PFAS, the peak area abundance did not change as a function of distance from the AFFF source zones (Figures S11 and S12). Based on this spatial pattern, we speculate that diffuse septic system discharges in the watershed are the source of FKGK11 in biological tissues.
3.3. Attenuation of the AFFF Signature in Downgradient Fish Samples
Past work has shown that the composition of PFAS in environmental and human samples can be useful for identifying predominant PFAS sources.22,64−67 Applying such techniques is helpful downgradient of large source zones, where diffuse sources may mix with the original PFAS contamination profile. In prior work in the same AFFF-impacted watershed, the PFAS composition in downgradient surface water was statistically enriched in C6 ECF precursors compared to watersheds without known AFFF contamination, indicating an ECF AFFF source.7 Principal component analysis (PCA) showed that the AFFF-impacted watershed was distinguishable by elevated loadings of PFHxS, PFOS, and C6 ECF precursors. Background sites contained more abundant PFCA/PFSA ≤ C4, indicating diffuse contamination sources (Figure S13).7
Similarly, in this work the enrichment of C4 PFSA in the downgradient estuary (Figure 3A) and background site (Santuit River) (Figure S13) points to septic system inputs. This is consistent with prior work that identified C4 PFSA as a human waste indicator.49,51 The enrichment of C4 FASA in the background site points to either septic system inputs or atmospheric deposition as this compound is now widely used in certain manufacturing processes, is a known degradation product of other sulfonamido precursors, and has been detected in water and fish from other background sites.25,68,69 The PFAS composition in surface water samples collected closest to the AFFF source zones (Moody Pond) is enriched in PFHxS (C6 PFSA) and C4–C6 FASA (ECF precursors) (Figure 3A), indicative of an ECF AFFF source.
Figure 3.
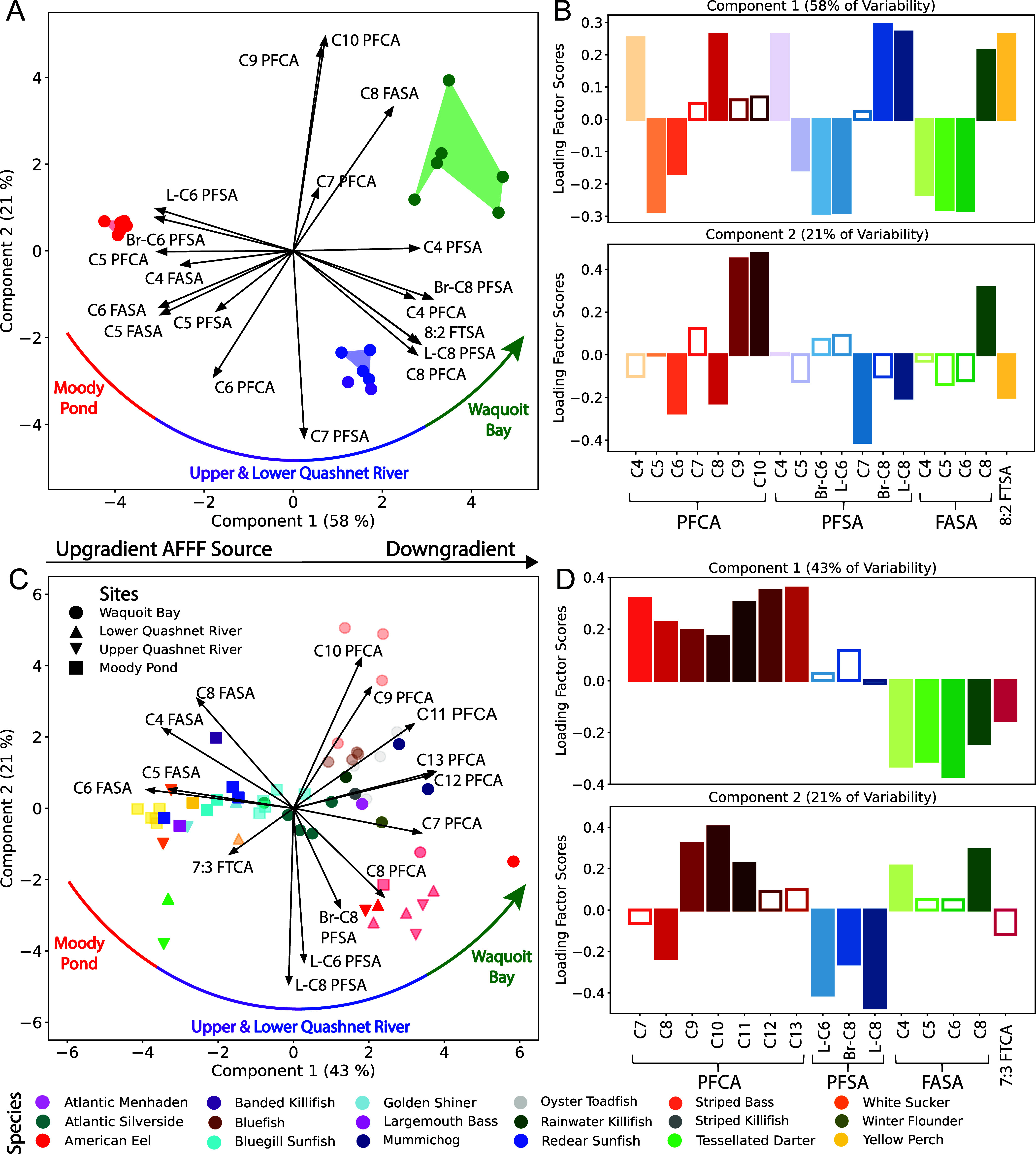
Principal component analysis (PCA) for targeted per- and polyfluoroalkyl substances (PFAS) in (A) surface waters and (C) fish species and eel from locations downgradient of legacy aqueous film-forming foam (AFFF) source zones. PFAS are indicated by their perfluorinated carbon chain length (Cn). Isomers are indicated as branched (Br) and linear (L). Marker type denotes the site of species collection, and lighter shaded markers refer to muscle tissue samples, whereas darker shaded markers refer to whole-body samples. Loading scores are shown for (B) surface water and (D) fish samples with dominant loadings (factor >0.15) shaded. Groupings based on hierarchical clustering are shown in the Supporting Information (SI) and are consistent with the PCA shown here (Figures S14 and S15).
Commercial availability of analytical standards for FASA ≤ C6 since publication of our prior work enabled quantitative detection of different short-chain FASA in this work.7 All three sampling locations (Moody Pond, the Quashnet River, and Waquoit Bay) cluster separately based on their PFAS composition. Along the dominant component (PC1), the Quashnet River falls between Moody Pond and Waquoit Bay, indicating dilution of the dominant AFFF source signature along the flow path of the river (Figures 3A and S14). More diffuse sources are apparent in water samples from the downgradient estuary (Waquoit Bay) that have high loadings of various long-chain PFCA in PC1 and PC2 (Figure 3A,B). Results of this analysis indicate the surface water PFAS composition characteristic of ECF AFFF can persist for substantial distances in downgradient watersheds. In this case, the AFFF signature can be detected until the flow path enters the marine environment (>8 km from the source zones).
For fishes, the PFAS composition similarly shows distinct transformations along the hydrological flow path away from the AFFF source zones (Figure 3C). The dominant component (PC1) shows freshwater fish samples closest to the source zones (Moody Pond) are enriched in FASA compared to downgradient estuarine fish samples that are relatively enriched in the long-chain C9–C13 PFCA (Figure 3C,D). Like the surface waters, fish samples collected from the Quashnet River fall between Moody Pond and Waquoit Bay along PC1 (Figures 3C and S15). Along PC2, fish samples from the Quashnet River are enriched in C6/C8 PFSA (PFHxS and PFOS) compared with fish samples from Moody Pond and Waquoit Bay. PFOS is the dominant terminal PFAA in ECF AFFF, and enrichment in PFHxS likely reflects C6 FASA precursor transformation and accumulation in fish tissues along the flow path away from the AFFF source zones (Figure 3C). Declining C6 FASA composition and increasing PFHxS abundance in fish tissues has previously been reported downgradient of an AFFF source zone.11
Unlike the fish samples, the PFAS composition measured in eel did not change along the hydrological flow path (Figure 3C). PFOS accounted for 73–90% of total targeted PFAS in eel (Figure S16). High relative composition in tissues, combined with low detection of precursors, placed eel as an outlier on the PCA. Eel had similar δ13C isotope values as other fishes from the same surface waters, suggesting they share similar energetic pathways as other benthic and benthopelagic fish species (Table S2, Figure S17).70,71 Apart from the one adult eel captured in Moody Pond, eel from both riverine and estuarine sites had only slightly higher δ15N values (±0.2–1.5‰) relative to other species (Table S2, Figure S18). This suggests no substantial differences in trophic position and that extensive metabolism of precursors by consumed prey is unlikely. The distinct PFAS composition in eel that mainly consists of PFOS and exhibits low FASA concentrations compared to fishes may therefore reflect differences in precursor uptake and metabolism and would be an interesting focus of future work.
The isomer composition of PFOS and PFHxS may also provide clues about source loadings to different sampling locations.72 The ECF manufacturing process for AFFF is known to produce PFHxS and PFOS with approximately 70–80% linear isomers and 20–30% branched isomers.73 The composition of linear isomers of PFHxS (87 ± 0.40%) and PFOS (71 ± 3.7%) measured in surface water samples from this work were similar to values reported in past work for the same AFFF-impacted watershed (85 ± 2.0% for PFHxS and 67 ± 10% for PFOS).7 Isomeric composition did not change with distance for PFHxS, but the fraction of total PFOS present as the linear isomer decreased slightly between Moody Pond (75 ± 0.38%) and the downgradient estuary (67 ± 1.3%) (Table S16). This stable isomeric composition is consistent with the persistence of the ECF AFFF signature in downgradient surface waters.
In fishes and eel samples, linear isomers accounted for 94 ± 5.0% of total PFHxS and PFOS, likely due to more efficient uptake and more limited elimination compared to the branched isomers, as described in prior work.74−77 Variability in isomeric composition was greater across species than across sampling locations (0–20% branched PFHxS and PFOS isomers across biota samples). These results suggest that variability in isomer metabolism across species affects the isomeric profiles more than source composition. Branched isomers for C6 and C8 FASA were abundant in all samples (Table S16), but FASA were integrated and interpreted as the sum of isomers in this study since branched isomer analytical standards were not available. PFAS composition may thus be more useful than the isomer composition for tracking PFAS source signatures in biological tissues.
3.4. Source Proximity and Physicochemical Properties of PFAS Drive Tissue Concentrations
Quantitative data on PFAS discharges to U.S. waterbodies from diverse sources that have been identified or are suspected are not yet readily available.65,67,78 Past studies have therefore used a simple exponential decay function with hydrological distance between sources and affected waterbodies as a first approximation of their relative influence.22,79 However, limited data are available to evaluate the reliability of such approximations.
We used the data collected in this study to examine changes in PFAS concentrations in surface water and eel and fish species throughout the watershed downgradient of the AFFF source zones (Figure 4). In the figure, we show the hydrological distance between the most upstream Quashnet River location and Waquoit Bay. Other parts of the flow path could not be described in this manner due to complex groundwater/surface water exchanges.9,12,29,80 Regional hydrologic studies have confirmed a groundwater connection between Moody Pond and the Quashnet River, but this has not yet been traced in detail.81
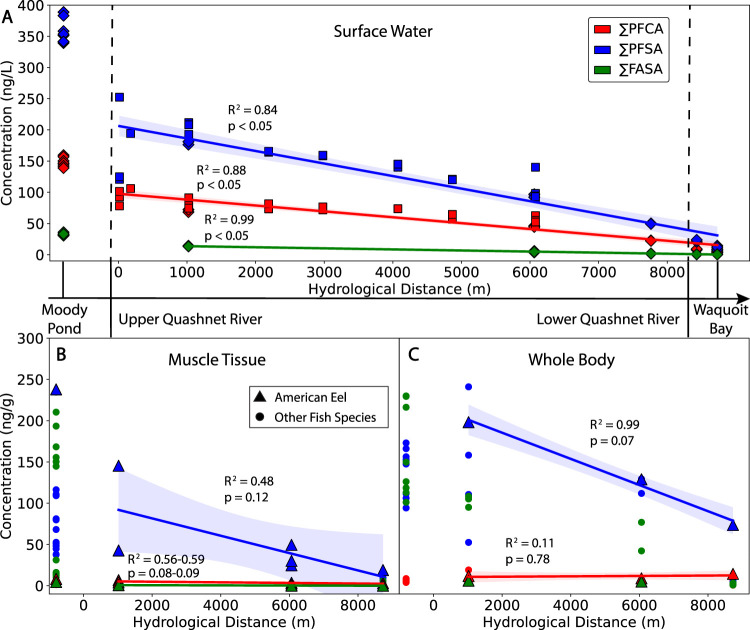
Figure 4.
Decline in targeted sum concentration (ng L–1 or ng g–1 wet-weight) of perfluorocarboxylates (∑PFCA), perfluorosulfonates (∑PFSA), and perfluoroalkyl sulfonamides (∑FASA) by site and with hydrological distance for (A) surface water, (B) fish and eel muscle tissue, and (C) fish and eel whole-body samples. The Quashnet River surface water samples include data from this study (diamonds) and Ruyle et al.7 (squares). Best fit (R2 and p-values) represent weighted least-squares linear regression (colored lines) with 95% confidence intervals (shaded areas) for surface water samples (diamonds/squares) and eel muscle tissue and whole-body samples (triangles) from the river and estuary.
Qualitatively, there is a large decline in targeted PFAS surface water concentrations between Moody Pond and the upgradient Quashnet River (Figure 4A). After this decrease, measured PFAS concentrations along the Quashnet River flow path follow a near-linear decline in targeted PFAS with hydrological distance. A linear decline is suggestive of simple dilution as the dominant factor controlling concentrations along this stretch of the river (Figure 4A). Simplified modeling approaches used in prior work22,79 do not capture the hydrological complexity of this watershed, but do appear to provide a reasonable first approximation of the relative influence of PFAS sources in areas where the true hydrological pathways are not well-defined.
We measured PFAS concentrations in one species (eel) at all sampling locations in this study (Figure 4B,C). In the reaches of the river, concentrations in eel decreased linearly with distance from the AFFF-contaminated source zones, similar to the decrease in water concentrations. There was no significant decline for ∑PFCA or ∑FASA due to low concentrations detected in all of the eel samples. For ∑PFSA, declines in surface water concentrations indicated by the slope of the regression (mean: −0.020, 95% CI: −0.023 to −0.017) overlap with confidence intervals for changes in eel concentrations (mean whole-body: −0.016, 95% CI: −0.038 to 0.007), suggesting the rates of decline are not significantly different. However, a shallower decline in PFAS concentrations in eel and fish species relative to water concentrations across sites likely reflects species migrations throughout the river across gradients in contamination.
Variability in PFAS concentrations and composition in aquatic biota across sites was greater than variability among species (Figures S5 and S6). Within each sampling location, we found minimal significant differences (Dunn, p < 0.05) in PFAS concentrations among species (Table S13B,C). Aquatic species from Moody Pond and the Quashnet River were all within an estimated 0.7 trophic position difference based on their tissue δ15N, suggesting similarity (Figure S18). Trophic position estimates based on tissue bulk δ15N values are sensitive to variable trophic enrichment among species as well as potential baseline differences among trophic pathways (e.g., benthic vs pelagic) and may influence estimates for certain species in this work (Figure S18).82,83 Nonetheless, PFAS concentrations generally did not vary as a function of the established trophic position estimates at these sites (Table S2). The greatest variability in δ15N values among species was observed in the downgradient estuary (Figures S17 and S18), but there was no significant relationship between ∑PFAS and δ15N, δ13C, or fish size (Table S19). These results are consistent with past work showing fish PFAS concentrations depend mainly on proximity to point sources and propensity for partitioning to biological tissues as a function of their physicochemical properties.24,27,84
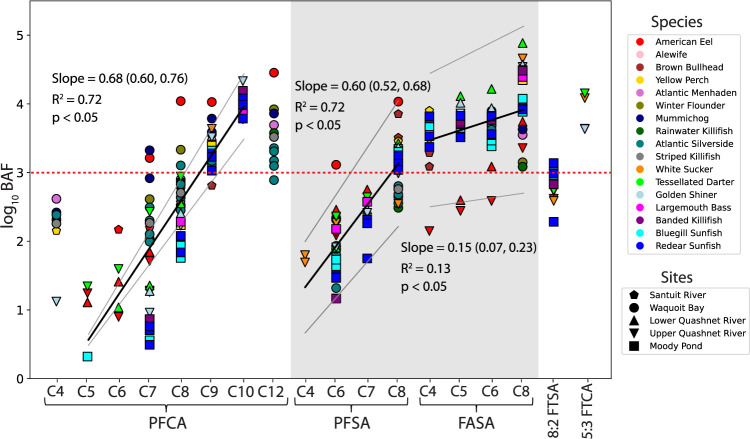
Field-measured log10 bioaccumulation factors (BAF) for PFOS ranged between 2.0 and 4.0 across species and tissue types (Figures 5 and S19, Table S20). For the longer-chain C9–C12 PFCA, log10 BAF ranged from 2.6 to 4.5 across aquatic species but was not a dominant contributor to the overall PFAS burden in biological samples (Figures 5 and S19, S20, Table S20). Field-measured log10 BAF values for FASA (C4–C8) were higher (1.2–4.9) than those for both PFCA and PFSA of equivalent chain length (0.10–4.0) (Figures 5 and S19, Table S20).
Figure 5.
Field-measured bioaccumulation factors (log10 BAF, L kg–1 wet-weight) for perfluorocarboxylates (PFCA), perfluorosulfonates (PFSA), perfluoroalkyl sulfonamides (FASA), and other targeted precursors in whole-body eel and fish samples from sites within the aqueous film-forming foam (AFFF)-impacted and background watersheds. Each marker indicates an individual measurement. Marker type denotes sample site collection, and marker color denotes species. R2 and p-values are based on linear regression (shown as solid lines with 95% confidence intervals) of BAF data for C5–C10 PFCA (PFHxA-PFUnDA), C4–C8 PFSA (PFBS–PFOS), and C4–C8 FASA (FBSA–FOSA). Per- and polyfluoroalkyl substances (PFAS) that have a tendency to bioaccumulate (log10 BAF ≥ 3.0) are indicated above the red dotted line. Data originally presented in Pickard et al.15 are shown in the gray box. Figure S19 shows BAF results for muscle tissue samples, and BAF data are provided in Table S20.
Physicochemical properties of PFAS affect their uptake in food webs and drive a chain-length-dependent relationship with BAF among each class of PFAS. Similar to past work,27 we found a statistically significant linear relationship (p < 0.05) between log BAF and PFCA and PFSA perfluorinated carbon chain length (R2 = 0.50–0.76) (Figures 5 and S19). The slope of both relationships was similar (Figure 5), suggesting that hydrophobicity and molecular weight with increasing perfluorinated carbon chain length were the dominant factors controlling partitioning to biological tissues (proteins/phospholipids).85−87
By contrast, we observed only weak chain-length-dependent patterns in bioaccumulation for FASA (R2 = 0.04–0.13). This suggests molecular weight and hydrophobicity are not as important for accumulation in biological tissues for this class of precursors. Partition coefficients for short-chain FASA are predicted to be much lower than those for PFOS, despite substantially higher field-measured BAF.88 The hydrophilic nature of the sulfonamide headgroup in FASA allows for a greater fraction of neutral FASA to be present in solution, allowing for greater membrane permeability and higher intracellular levels in lipid-rich tissues, as discussed in Pickard et al.15,44 Similar uptake of FASA across different perfluorinated carbon chain lengths are suggestive of specific kinetic processes or facilitated uptake of these compounds that does not depend on passive diffusion or equilibrium chemical partitioning. Further studies on the tissue-specific uptake and partitioning of FASA are needed to fully understand bioaccumulation patterns for this abundant class of precursor PFAS.
Biota-sediment accumulation factors (BSAF, kg organic carbon kg−1 wet-weight) for PFAS were much lower (log10 BSAF = −1.2 to 1.4) than BAF in benthic species (Figures S21 and S22, Table S21). Similar to the review by Burkhard et al.,89 only fishes with PFCA ≥ C8, PFHxS, PFOS, and FASA had log10 BSAF > 0.01, and shellfish had negative log10 BSAF values. BSAF in biota samples showed decreasing trends with perfluorinated carbon chain length for PFCA > C9 (Figure S21), consistent with prior studies, indicating lower bioavailability of long-chain PFCA due to greater sorption to organic carbon.45,57
3.5. Implications
Fish and shellfish are an important dietary PFAS exposure source for humans and wildlife.11,80,90 Prior to 2010, most U.S. fish consumption advisories91 were for methylmercury and persistent organic pollutants (POPs) like polychlorinated biphenyls that strongly biomagnify in food webs.92,93 Trophic level is one of the strongest predictors of tissue burdens for these legacy pollutants. By contrast, PFAS show weak or negative trophic magnification in most aquatic food webs, and mechanisms of accumulation of ionogenic PFAS differ from those of neutral POPs.4,86 We found limited evidence of PFAS accumulation as a function of estimated trophic position (Figure 5). Instead, proximity to contaminated source zones and physicochemical properties of different PFAS that affect partitioning to tissues are better predictors of observed concentrations.
In this study, we showed PFAS concentrations declined linearly in water and aquatic biota with hydrological distance in the downgradient Quashnet River (Figure 4) and qualitatively showed an exponential decline between the upgradient freshwater pond (Moody Pond) and downgradient estuary (Waquoit Bay). Despite these declines, tissue PFAS concentrations exceeded state-level fish consumption guidelines for PFOS throughout the AFFF-impacted watershed, even >8 km away from the source zones (Figure 1). Our mass budget for EOF in biological tissues showed that PFOS accounted for less than half (38 ± 16%) of the EOF throughout the watershed (Figure 2). These findings reinforce the importance of expanding the PFAS analytes currently considered by fish advisory programs.
Our work reinforces that statistical clustering techniques are useful for identifying predominant PFAS sources in both water and aquatic biota. We identified PFAS characteristic of AFFF contamination in both surface waters and biological tissues (C4–C6 FASA and PFHxS) throughout the AFFF-impacted watershed (Figure 3). The AFFF source signature became indistinguishable from the background site in water and biota only when it entered the downgradient estuary and mixed with seawater. Biological monitoring that considers all hydrologically connected regions within PFAS-impacted watersheds could help safeguard public health.
Acknowledgments
Financial support for this work was provided by the National Institute of Environmental Health Sciences Superfund Research Program (P42ES027706). H.M.P. was partially supported by a fellowship from the Natural Sciences and Engineering Research Council of Canada (NSERC). D.R.L. was supported by the Environmental Health Program of the U.S. Geological Survey. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Research was conducted in Waquoit Bay National Estuarine Research Reserve. We thank Steve Hurley, Ashley Fisher, Dale Oakley, James Rossignol, Adam Kautez, Donovan McElligatt, Lara Shtanko, Samuel Logan, and Jennifer Sun for their assistance with field sampling. We thank Molly Gabler-Smith for assistance with fish dissections, Susie Carter for assistance with stable isotope analyses, and Colin Thackray for coding assistance.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.4c07016.
Sample information; supporting descriptions of extraction methods, analyses, and quality assurance/quality control; and tables and figures of peak areas, concentrations, isotopic signatures, and bioaccumulation factor data (PDF)
Locations of biota collection, type of species and corresponding species information, number of samples per species collected, given code names for each species, calculated lengths and weights, and the type of tissue measured for each individual sample or composite of samples (Table S2); range in concentrations for PFCA, PFSA, and targeted precursors in surface water, sediment, and biological samples (Table S14); detection frequencies of targeted PFAS > MDL measured by LC-MS/MS in surface water, sediment and biological samples (Table S15); average (±SD) concentrations for PFCA, PFSA, and targeted precursors in surface water, sediment, and biological samples (Table S16); average EOF concentrations measured in surface water, sediment, and biological samples across all sites (Table S17); suspect PFAS identified in surface water, sediment, and biological tissue (muscle and whole-body) samples (Table S18); range in log BAF for PFCA, PFSA, and targeted precursors (Table S20); and range in log BSAF for PFCA, PFSA, and targeted precursors (Table S21) (XLSX)
The authors declare no competing financial interest.
Supplementary Material
References
- Sunderland E. M.; Hu X. C.; Dassuncao C.; Tokranov A. K.; Wagner C. C.; Allen J. G. A Review of the Pathways of Human Exposure to Poly- and Perfluoroalkyl Substances (PFASs) and Present Understanding of Health Effects. J. Exposure Sci. Environ. Epidemiol. 2019, 29 (2), 131–147. 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton S. E.; Ducatman A.; Boobis A.; DeWitt J. C.; Lau C.; Ng C.; Smith J. S.; Roberts S. M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2021, 40 (3), 606–630. 10.1002/etc.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenk D.; Bignami M.; Bodin L.; Chipman J. K.; del Mazo J.; Grasl-Kraupp B.; Hogstrand C.; Hoogenboom L. R.; Leblanc J.; Nebbia C. S.; Nielsen E.; Ntzani E.; Petersen A.; Sand S.; Vleminckx C.; Wallace H.; Barregård L.; Ceccatelli S.; Cravedi J.; Halldorsson T. I.; Haug L. S.; Johansson N.; Knutsen H. K.; Rose M.; Roudot A.; Van Loveren H.; Vollmer G.; Mackay K.; Riolo F.; Schwerdtle T.; Risk to Human Health Related to the Presence of Perfluoroalkyl Substances in Food. EFSA J. 2020, 18 (9), e06223 10.2903/j.efsa.2020.6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Silva A. O.; Armitage J. M.; Bruton T. A.; Dassuncao C.; Heiger-Bernays W.; Hu X. C.; Kärrman A.; Kelly B.; Ng C.; Robuck A.; Sun M.; Webster T. F.; Sunderland E. M. PFAS Exposure Pathways for Humans and Wildlife: A Synthesis of Current Knowledge and Key Gaps in Understanding. Environ. Toxicol. Chem. 2021, 40 (3), 631–657. 10.1002/etc.4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbo N.; Stoiber T.; Naidenko O. V.; Andrews D. Q. Locally Caught Freshwater Fish across the United States Are Likely a Significant Source of Exposure to PFOS and Other Perfluorinated Compounds. Environ. Res. 2023, 220, 115165 10.1016/j.envres.2022.115165. [DOI] [PubMed] [Google Scholar]
- D’Agostino L. A.; Mabury S. A. Certain Perfluoroalkyl and Polyfluoroalkyl Substances Associated with Aqueous Film Forming Foam Are Widespread in Canadian Surface Waters. Environ. Sci. Technol. 2017, 51 (23), 13603–13613. 10.1021/acs.est.7b03994. [DOI] [PubMed] [Google Scholar]
- Ruyle B. J.; Pickard H. M.; LeBlanc D. R.; Tokranov A. K.; Thackray C. P.; Hu X. C.; Vecitis C. D.; Sunderland E. M. Isolating the AFFF Signature in Coastal Watersheds Using Oxidizable PFAS Precursors and Unexplained Organofluorine. Environ. Sci. Technol. 2021, 55 (6), 3686–3695. 10.1021/acs.est.0c07296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzen-Hanson K. A.; Roberts S. C.; Choyke S.; Oetjen K.; McAlees A.; Riddell N.; McCrindle R.; Ferguson P. L.; Higgins C. P.; Field J. A. Discovery of 40 Classes of Per- and Polyfluoroalkyl Substances in Historical Aqueous Film-Forming Foams (AFFFs) and AFFF-Impacted Groundwater. Environ. Sci. Technol. 2017, 51 (4), 2047–2057. 10.1021/acs.est.6b05843. [DOI] [PubMed] [Google Scholar]
- Tokranov A. K.; LeBlanc D. R.; Pickard H. M.; Ruyle B. J.; Barber L. B.; Hull R. B.; Sunderland E. M.; Vecitis C. D. Surface-Water/Groundwater Boundaries Affect Seasonal PFAS Concentrations and PFAA Precursor Transformations. Environ. Sci.: Processes Impacts 2021, 23, 1893–1905. 10.1039/D1EM00329A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrizo J. C.; Munoz G.; Vo Duy S.; Liu M.; Houde M.; Amé M. V.; Liu J.; Sauvé S. PFAS in Fish from AFFF-Impacted Environments: Analytical Method Development and Field Application at a Canadian International Civilian Airport. Sci. Total Environ. 2023, 879, 163103 10.1016/j.scitotenv.2023.163103. [DOI] [PubMed] [Google Scholar]
- Nilsen E.; Muensterman D.; Carini L.; Waite I.; Payne S.; Field J. A.; Peterson J.; Hafley D.; Farrer D.; Jones G. D. Target and Suspect Per- and Polyfluoroalkyl Substances in Fish from an AFFF-Impacted Waterway. Sci. Total Environ. 2024, 906, 167798 10.1016/j.scitotenv.2023.167798. [DOI] [PubMed] [Google Scholar]
- Ruyle B. J.; Thackray C. P.; Butt C. M.; LeBlanc D. R.; Tokranov A. K.; Vecitis C. D.; Sunderland E. M. Centurial Persistence of Forever Chemicals at Military Fire Training Sites. Environ. Sci. Technol. 2023, 57 (21), 8096–8106. 10.1021/acs.est.3c00675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency . Method 1633 Analysis of Per- and Polyfluoroalkyl Substances (PFAS) in Aqueous, Solid, Biosolids, and Tissue Samples by LC-MS/MS, EPA 821-R-24–001, 2024.
- Yeung L. W. Y.; Mabury S. A. Are Humans Exposed to Increasing Amounts of Unidentified Organofluorine?. Environ. Chem. 2016, 13 (1), 102. 10.1071/EN15041. [DOI] [Google Scholar]
- Pickard H. M.; Haque F.; Sunderland E. M. Bioaccumulation of Perfluoroalkyl Sulfonamides (FASA). Environ. Sci. Technol. Lett. 2024, 11 (4), 350–356. 10.1021/acs.estlett.4c00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook E. K.; Olivares C. I.; Antell E. H.; Yi S.; Nickerson A.; Choi Y. J.; Higgins C. P.; Sedlak D. L.; Alvarez-Cohen L. Biological and Chemical Transformation of the Six-Carbon Polyfluoroalkyl Substance N -Dimethyl Ammonio Propyl Perfluorohexane Sulfonamide (AmPr-FHxSA). Environ. Sci. Technol. 2022, 56 (22), 15478–15488. 10.1021/acs.est.2c00261. [DOI] [PubMed] [Google Scholar]
- Ruyle B. J.; Schultes L.; Akob D. M.; Harris C. R.; Lorah M. M.; Vojta S.; Becanova J.; McCann S.; Pickard H. M.; Pearson A.; Lohmann R.; Vecitis C. D.; Sunderland E. M. Nitrifying Microorganisms Linked to Biotransformation of Perfluoroalkyl Sulfonamido Precursors from Legacy Aqueous Film-Forming Foams. Environ. Sci. Technol. 2023, 57 (14), 5592–5602. 10.1021/acs.est.2c07178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y.; Helbling D. E. Revealing the Factors Resulting in Incomplete Recovery of Perfluoroalkyl Acids (PFAAs) When Implementing the Adsorbable and Extractable Organic Fluorine Methods. Water Res. 2023, 244, 120497 10.1016/j.watres.2023.120497. [DOI] [PubMed] [Google Scholar]
- Ruyle B. J.; Pickard H. M.; Schultes L.; Fredriksson F.; Heffernan A. L.; Knappe D. R. U.; Lord H. L.; Meng P.; Mills M. A.; Ndungu K.; Roesch P.; Rundberget J. T.; Tettenhorst D. R.; Buren J. V.; Vogel C.; Westerman D. C.; Yeung L. W. Y.; Sunderland E. M. Interlaboratory Comparison of Extractable Organofluorine Measurements in Groundwater and Eel (Anguilla Rostrata): Recommendations for Methods Standardization. Environ. Sci. Technol. 2023, 57 (48), 20159–20168. 10.1021/acs.est.3c04560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruyle B. J.; Thackray C. P.; McCord J. P.; Strynar M. J.; Mauge-Lewis K. A.; Fenton S. E.; Sunderland E. M. Reconstructing the Composition of Per- and Polyfluoroalkyl Substances in Contemporary Aqueous Film-Forming Foams. Environ. Sci. Technol. Lett. 2021, 8 (1), 59–65. 10.1021/acs.estlett.0c00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynar M.; McCord J.; Newton S.; Washington J.; Barzen-Hanson K.; Trier X.; Liu Y.; Dimzon I. K.; Bugsel B.; Zwiener C.; Munoz G. Practical Application Guide for the Discovery of Novel PFAS in Environmental Samples Using High Resolution Mass Spectrometry. J. Expo Sci. Environ. Epidemiol 2023, 33 (4), 575–588. 10.1038/s41370-023-00578-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.; Lohmann R.; Dassuncao C.; Hu X. C.; Weber A. K.; Vecitis C. D.; Sunderland E. M. Source Attribution of Poly- and Perfluoroalkyl Substances (PFASs) in Surface Waters from Rhode Island and the New York Metropolitan Area. Environ. Sci. Technol. Lett. 2016, 3 (9), 316–321. 10.1021/acs.estlett.6b00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults J. F.; Higgins C. P.; Helbling D. E. Integration of Per- and Polyfluoroalkyl Substance (PFAS) Fingerprints in Fish with Machine Learning for PFAS Source Tracking in Surface Water. Environ. Sci. Technol. Lett. 2023, 10 (11), 1052–1058. 10.1021/acs.estlett.3c00278. [DOI] [Google Scholar]
- Langberg H. A.; Hale S. E.; Breedveld G. D.; Jenssen B. M.; Jartun M. A Review of PFAS Fingerprints in Fish from Norwegian Freshwater Bodies Subject to Different Source Inputs. Environ. Sci.: Processes Impacts 2022, 24, 330–342. 10.1039/D1EM00408E. [DOI] [PubMed] [Google Scholar]
- Kaboré H. A.; Goeury K.; Desrosiers M.; Vo Duy S.; Liu J.; Cabana G.; Munoz G.; Sauvé S. Novel and Legacy Per- and Polyfluoroalkyl Substances (PFAS) in Freshwater Sporting Fish from Background and Firefighting Foam Impacted Ecosystems in Eastern Canada. Sci. Total Environ. 2022, 816, 151563 10.1016/j.scitotenv.2021.151563. [DOI] [PubMed] [Google Scholar]
- Langberg H. A.; Breedveld G. D.; Slinde G. Aa.; Grønning H. M.; Høisæter Å.; Jartun M.; Rundberget T.; Jenssen B. M.; Hale S. E. Fluorinated Precursor Compounds in Sediments as a Source of Perfluorinated Alkyl Acids (PFAA) to Biota. Environ. Sci. Technol. 2020, 54 (20), 13077–13089. 10.1021/acs.est.0c04587. [DOI] [PubMed] [Google Scholar]
- Pickard H. M.; Ruyle B. J.; Thackray C. P.; Chovancova A.; Dassuncao C.; Becanova J.; Vojta S.; Lohmann R.; Sunderland E. M. PFAS and Precursor Bioaccumulation in Freshwater Recreational Fish: Implications for Fish Advisories. Environ. Sci. Technol. 2022, 56 (22), 15573–15583. 10.1021/acs.est.2c03734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink W. A.; Bignert A.; Berger U. Perfluoroalkyl Acids (PFAAs) and Selected Precursors in the Baltic Sea Environment: Do Precursors Play a Role in Food Web Accumulation of PFAAs?. Environ. Sci. Technol. 2016, 50 (12), 6354–6362. 10.1021/acs.est.6b01197. [DOI] [PubMed] [Google Scholar]
- Weber A. K.; Barber L. B.; LeBlanc D. R.; Sunderland E. M.; Vecitis C. D. Geochemical and Hydrologic Factors Controlling Subsurface Transport of Poly- and Perfluoroalkyl Substances, Cape Cod, Massachusetts. Environ. Sci. Technol. 2017, 51 (8), 4269–4279. 10.1021/acs.est.6b05573. [DOI] [PubMed] [Google Scholar]
- Shojaei M.; Kumar N.; Guelfo J. L. An Integrated Approach for Determination of Total Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Technol. 2022, 56 (20), 14517–14527. 10.1021/acs.est.2c05143. [DOI] [PubMed] [Google Scholar]
- Schultes L.; Sandblom O.; Broeg K.; Bignert A.; Benskin J. P. Temporal Trends (1981–2013) of Per- and Polyfluoroalkyl Substances and Total Fluorine in Baltic Cod (Gadus Morhua). Environ. Toxicol. Chem. 2020, 39 (2), 300–309. 10.1002/etc.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassuncao C.; Hu X. C.; Zhang X.; Bossi R.; Dam M.; Mikkelsen B.; Sunderland E. M. Temporal Shifts in Poly- and Perfluoroalkyl Substances (PFASs) in North Atlantic Pilot Whales Indicate Large Contribution of Atmospheric Precursors. Environ. Sci. Technol. 2017, 51 (8), 4512–4521. 10.1021/acs.est.7b00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonnet J. A.; McDonough C. A.; Xiao F.; Schwichtenberg T.; Cao D.; Kaserzon S.; Thomas K. V.; Dewapriya P.; Place B. J.; Schymanski E. L.; Field J. A.; Helbling D. E.; Higgins C. P. Communicating Confidence of Per- and Polyfluoroalkyl Substance Identification via High-Resolution Mass Spectrometry. Environ. Sci. Technol. Lett. 2022, 9 (6), 473–481. 10.1021/acs.estlett.2c00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymanski E. L.; Jeon J.; Gulde R.; Fenner K.; Ruff M.; Singer H. P.; Hollender J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48 (4), 2097–2098. 10.1021/es5002105. [DOI] [PubMed] [Google Scholar]
- Logan J. M.; Jardine T. D.; Miller T. J.; Bunn S. E.; Cunjak R. A.; Lutcavage M. E. Lipid Corrections in Carbon and Nitrogen Stable Isotope Analyses: Comparison of Chemical Extraction and Modelling Methods. J. Anim. Ecol. 2008, 77 (4), 838–846. 10.1111/j.1365-2656.2008.01394.x. [DOI] [PubMed] [Google Scholar]
- Virtanen P.; Gommers R.; Oliphant T. E.; Haberland M.; Reddy T.; Cournapeau D.; Burovski E.; Peterson P.; Weckesser W.; Bright J.; van der Walt S. J.; Brett M.; Wilson J.; Millman K. J.; Mayorov N.; Nelson A. R. J.; Jones E.; Kern R.; Larson E.; Carey C. J.; Polat İ.; Feng Y.; Moore E. W.; VanderPlas J.; Laxalde D.; Perktold J.; Cimrman R.; Henriksen I.; Quintero E. A.; Harris C. R.; Archibald A. M.; Ribeiro A. H.; Pedregosa F.; van Mulbregt P.; et al. SciPy 1.0: Fundamental Algorithms for Scientific Computing in Python. Nat. Methods 2020, 17 (3), 261–272. 10.1038/s41592-019-0686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabold S.; Perktold J. In Statsmodels: Econometric and Statistical Modeling with Python, Proceedings of the 9th Python in Science Conference (SciPy 2010), 2010; pp 92–96.
- Aitchison J. The Statistical Analysis of Compositional Data. J. R. Stat. Soc., Series B: Methodol. 1982, 44 (2), 139–177. 10.1111/j.2517-6161.1982.tb01195.x. [DOI] [Google Scholar]
- Levanduski E.; Richter W.; Becker J.; Hassanzadeh Y.; Razavi N. R. Two for the Price of One: Deriving Per- and Polyfluoroalkyl Substances (PFAS) Fillet and Whole-Body Conversion Equations in Fish. Environ. Sci. Technol. Lett. 2024, 11 (6), 511–517. 10.1021/acs.estlett.4c00033. [DOI] [Google Scholar]
- Gill K.; O’Reilly M.. Air Force Civil Engineer Center (AFCEC) Emerging Contaminants Update - JBCC Cleanup Team Meeting, 2024. http://jbcc-iagwsp.org/community/impact/presentations/EC_Update_JBCCCT_April_2024.pdf.
- Cape Cod Commission . Embayments - Cape Cod Commission. https://gis-cccommission.opendata.arcgis.com/datasets/CCCommission::embayments/about (accessed May 28, 2024).
- Massachusetts Department of Public Health . Evaluation of PFAS in Recreational Waterbodies in Massachusetts. https://www.mass.gov/doc/technical-basis-for-issuing-fish-advisories-0/download (accessed Dec 05, 2023).
- Nickerson A.; Rodowa A. E.; Adamson D. T.; Field J. A.; Kulkarni P. R.; Kornuc J. J.; Higgins C. P. Spatial Trends of Anionic, Zwitterionic, and Cationic PFASs at an AFFF-Impacted Site. Environ. Sci. Technol. 2021, 55 (1), 313–323. 10.1021/acs.est.0c04473. [DOI] [PubMed] [Google Scholar]
- Rericha Y.; Cao D.; Truong L.; Simonich M. T.; Field J. A.; Tanguay R. L. Sulfonamide Functional Head on Short-Chain Perfluorinated Substance Drives Developmental Toxicity. iScience 2022, 25 (2), 103789 10.1016/j.isci.2022.103789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz G.; Mercier L.; Duy S. V.; Liu J.; Sauvé S.; Houde M. Bioaccumulation and Trophic Magnification of Emerging and Legacy Per- and Polyfluoroalkyl Substances (PFAS) in a St. Lawrence River Food Web. Environ. Pollut. 2022, 309, 119739 10.1016/j.envpol.2022.119739. [DOI] [PubMed] [Google Scholar]
- Ogawa Y.; Tokunaga E.; Kobayashi O.; Hirai K.; Shibata N. Current Contributions of Organofluorine Compounds to the Agrochemical Industry. iScience 2020, 23 (9), 101467 10.1016/j.isci.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.; Pike K. A.; Gray R.; Sprankle J. W.; Faust J. A.; Edmiston P. L. Non-Targeted Identification and Semi-Quantitation of Emerging per- and Polyfluoroalkyl Substances (PFAS) in US Rainwater. Environ. Sci.: Processes Impacts 2023, 25, 1771–1787. 10.1039/D2EM00349J. [DOI] [PubMed] [Google Scholar]
- D’Ambro E. L.; Pye H. O. T.; Bash J. O.; Bowyer J.; Allen C.; Efstathiou C.; Gilliam R. C.; Reynolds L.; Talgo K.; Murphy B. N. Characterizing the Air Emissions, Transport, and Deposition of Per- and Polyfluoroalkyl Substances from a Fluoropolymer Manufacturing Facility. Environ. Sci. Technol. 2021, 55 (2), 862–870. 10.1021/acs.est.0c06580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M.; Phelps W.; Masarik K.; Burke K.; Zhang C.; Schwartz A.; Wang M.; Nitka A. L.; Schutz J.; Trainor T.; Washington J. W.; Rheineck B. D. Prevalence and Source Tracing of PFAS in Shallow Groundwater Used for Drinking Water in Wisconsin, USA. Environ. Sci. Technol. 2023, 57 (45), 17415–17426. 10.1021/acs.est.3c02826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiela I.; Collins G.; Kremer J.; Lajtha K.; Geist M.; Seely M.; Brawley J.; Sham C. H. Nitrogen Loading from Coastal Watersheds to Receiving Estuaries: New Method and Application. Ecol. Appl. 1997, 7 (2), 358–380. 10.1890/1051-0761(1997)007[0358:NLFCWT]2.0.CO;2. [DOI] [Google Scholar]
- Schaider L. A.; Ackerman J. M.; Rudel R. A. Septic Systems as Sources of Organic Wastewater Compounds in Domestic Drinking Water Wells in a Shallow Sand and Gravel Aquifer. Sci. Total Environ. 2016, 547, 470–481. 10.1016/j.scitotenv.2015.12.081. [DOI] [PubMed] [Google Scholar]
- Howes B.; Samimy R.; Schlezinger D.; Eichner E.; Kelley S.; Ramsey J.. Linked Watershed-Embayment Approach to Determine Critical Nitrogen Loading Thresholds for the Waquoit Bay and Eel Pond Embayment Systems Towns of Falmouth and Mashpee, Massachusetts, Massachusetts Estuaries Project Report, School of Marine Science and Technology and MA Department of Environmental Protection, 2013. https://www.mashpeema.gov/sites/g/files/vyhlif3426/f/uploads/mep-waquoit-uc.pdf.
- Smithsonian Environmental Research Center . Mercenaria mercenaria. https://invasions.si.edu/nemesis/species_summary/-57 (accessed April 15, 2024).
- Hines A. H.Ecology of Juvenile and Adult Blue Crabs in Biology of the Blue Crab; Kenney V. S.; Cronin E., Eds.; Maryland Sea Grant College: College Park, MD, 2007; Chapter 14. [Google Scholar]
- Ebersole E.; Kennedy V. Prey Preferences of Blue Crabs Callinectes Sapidus Feeding on Three Bivalve Species. Mar. Ecol.: Prog. Ser. 1995, 118, 167–177. 10.3354/meps118167. [DOI] [Google Scholar]
- Smithsonian Environmental Research Center . Callinectes sapidus. https://invasions.si.edu/nemesis/species_summary/98696 (accessed April 15, 2024).
- Munoz G.; Budzinski H.; Babut M.; Drouineau H.; Lauzent M.; Menach K. L.; Lobry J.; Selleslagh J.; Simonnet-Laprade C.; Labadie P. Evidence for the Trophic Transfer of Perfluoroalkylated Substances in a Temperate Macrotidal Estuary. Environ. Sci. Technol. 2017, 51 (15), 8450–8459. 10.1021/acs.est.7b02399. [DOI] [PubMed] [Google Scholar]
- Barber L. B.; Pickard H. M.; Alvarez D. A.; Becanova J.; Keefe S. H.; LeBlanc D. R.; Lohmann R.; Steevens J. A.; Vajda A. M. Uptake of Per- and Polyfluoroalkyl Substances by Fish, Mussel, and Passive Samplers in Mobile-Laboratory Exposures Using Groundwater from a Contamination Plume at a Historical Fire Training Area, Cape Cod, Massachusetts. Environ. Sci. Technol. 2023, 57 (14), 5544–5557. 10.1021/acs.est.2c06500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun X.; Lewis A. J.; Stevens-King G.; Sales C. M.; Spooner D. E.; Kurz M. J.; Suri R.; McKenzie E. R. Bioaccumulation of Per- and Polyfluoroalkyl Substances by Freshwater Benthic Macroinvertebrates: Impact of Species and Sediment Organic Carbon Content. Sci. Total Environ. 2023, 866, 161208 10.1016/j.scitotenv.2022.161208. [DOI] [PubMed] [Google Scholar]
- Spaan K. M.; Seilitz F.; Plassmann M. M.; de Wit C. A.; Benskin J. P.. Pharmaceuticals Account for a Significant Proportion of the Extractable Organic Fluorine in Municipal Wastewater Treatment Plant Sludge. Environ. Sci. Technol. Lett. 202310328. 10.1021/acs.estlett.3c00108. [DOI] [Google Scholar]
- Bonvallot N.; Jamin E. L.; Regnaut L.; Chevrier C.; Martin J.-F.; Mercier F.; Cordier S.; Cravedi J.-P.; Debrauwer L.; Le Bot B. Suspect Screening and Targeted Analyses: Two Complementary Approaches to Characterize Human Exposure to Pesticides. Sci. Total Environ. 2021, 786, 147499 10.1016/j.scitotenv.2021.147499. [DOI] [Google Scholar]
- Baskakis C.; Magrioti V.; Cotton N.; Stephens D.; Constantinou-Kokotou V.; Dennis E. A.; Kokotos G. Synthesis of Polyfluoro Ketones for Selective Inhibition of Human Phospholipase A 2 Enzymes. J. Med. Chem. 2008, 51 (24), 8027–8037. 10.1021/jm800649q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.; Zhao Z.; Antalis C.; Zhao Z.; Emerson R.; Wei G.; Zhang S.; Zhang Z.-Y.; Xu Y. Combination Therapy of an Inhibitor of Group VIA Phospholipase A2 with Paclitaxel Is Highly Effective in Blocking Ovarian Cancer Development. Am. J. Pathol. 2011, 179 (1), 452–461. 10.1016/j.ajpath.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Xu C.; Johnson A. C.; Sun X.; Ding X.; Ding D.; Liu S.; Liang X. Source Apportionment and Crop Bioaccumulation of Perfluoroalkyl Acids and Novel Alternatives in an Industrial-Intensive Region with Fluorochemical Production, China: Health Implications for Human Exposure. J. Hazard. Mater. 2022, 423 (PA), 127019 10.1016/j.jhazmat.2021.127019. [DOI] [PubMed] [Google Scholar]
- Liddie J. M.; Schaider L. A.; Sunderland E. M. Sociodemographic Factors Are Associated with the Abundance of PFAS Sources and Detection in U.S. Community Water Systems. Environ. Sci. Technol. 2023, 57 (21), 7902–7912. 10.1021/acs.est.2c07255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis D. J.; Barton K. E.; Knappe D. R. U.; Kotlarz N.; McDonough C. A.; Higgins C. P.; Hoppin J. A.; Adgate J. L. Source Apportionment of Serum PFASs in Two Highly Exposed Communities. Sci. Total Environ. 2023, 855, 158842 10.1016/j.scitotenv.2022.158842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph N. T.; Schwichtenberg T.; Cao D.; Jones G. D.; Rodowa A. E.; Barlaz M. A.; Charbonnet J. A.; Higgins C. P.; Field J. A.; Helbling D. E. Target and Suspect Screening Integrated with Machine Learning to Discover Per- and Polyfluoroalkyl Substance Source Fingerprints. Environ. Sci. Technol. 2023, 57 (38), 14351–14362. 10.1021/acs.est.3c03770. [DOI] [PubMed] [Google Scholar]
- Chen Y.-J.; Wang R.-D.; Shih Y.-L.; Chin H.-Y.; Lin A. Y.-C. Emerging Perfluorobutane Sulfonamido Derivatives as a New Trend of Surfactants Used in the Semiconductor Industry. Environ. Sci. Technol. 2024, 58 (3), 1648–1658. 10.1021/acs.est.3c04435. [DOI] [PubMed] [Google Scholar]
- Kaboré H. A.; Vo Duy S.; Munoz G.; Méité L.; Desrosiers M.; Liu J.; Sory T. K.; Sauvé S. Worldwide Drinking Water Occurrence and Levels of Newly-Identified Perfluoroalkyl and Polyfluoroalkyl Substances. Sci. Total Environ. 2018, 616–617, 1089–1100. 10.1016/j.scitotenv.2017.10.210. [DOI] [PubMed] [Google Scholar]
- Eberhardt A. L.; Burdick D. M.; Dionne M.; Vincent R. E. Rethinking the Freshwater Eel: Salt Marsh Trophic Support of the American Eel, Anguilla Rostrata. Estuaries Coasts 2015, 38 (4), 1251–1261. 10.1007/s12237-015-9960-4. [DOI] [Google Scholar]
- Lookabaugh P. S.; Angermeier P. L. Diet Patterns of American Eel, Anguilla Rostrata, in the James River Drainage, Virginia. J. Freshwater Ecol. 1992, 7 (4), 425–431. 10.1080/02705060.1992.9664712. [DOI] [Google Scholar]
- Benskin J. P.; De Silva A. O.; Martin J. W.. Isomer Profiling of Perfluorinated Substances as a Tool for Source Tracking: A Review of Early Findings and Future Applications; Whitacre D. M., Ed.; , Ed.; Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, 2010; Vol. 208. [DOI] [PubMed] [Google Scholar]
- Buck R. C.; Franklin J.; Berger U.; Conder J. M.; Cousins I. T.; de Voogt P.; Jensen A. A.; Kannan K.; Mabury S. A.; van Leeuwen S. P. Perfluoroalkyl and Polyfluoroalkyl Substances in the Environment: Terminology, Classification, and Origins. Integr. Environ. Assess. Manage. 2011, 7 (4), 513–541. 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk S.; Failing K.; Georgii S.; Brunn H.; Stahl T. Tissue Specific Uptake and Elimination of Perfluoroalkyl Acids (PFAAs) in Adult Rainbow Trout (Oncorhynchus Mykiss) after Dietary Exposure. Chemosphere 2015, 129, 150–156. 10.1016/j.chemosphere.2014.06.061. [DOI] [PubMed] [Google Scholar]
- Chen M.; Qiang L.; Pan X.; Fang S.; Han Y.; Zhu L. In Vivo and in Vitro Isomer-Specific Biotransformation of Perfluorooctane Sulfonamide in Common Carp (Cyprinus Carpio). Environ. Sci. Technol. 2015, 49 (23), 13817–13824. 10.1021/acs.est.5b00488. [DOI] [PubMed] [Google Scholar]
- Zhong W.; Zhang L.; Cui Y.; Chen M.; Zhu L. Probing Mechanisms for Bioaccumulation of Perfluoroalkyl Acids in Carp (Cyprinus Carpio): Impacts of Protein Binding Affinities and Elimination Pathways. Sci. Total Environ. 2019, 647, 992–999. 10.1016/j.scitotenv.2018.08.099. [DOI] [PubMed] [Google Scholar]
- Martin J. W.; Mabury S. A.; Solomon K. R.; Muir D. C. G. Bioconcentration and Tissue Distribution of Perfluorinated Acids in Rainbow Trout (Oncorhynchus Mykiss). Environ. Toxicol. Chem. 2003, 22 (1), 196–204. 10.1002/etc.5620220126. [DOI] [PubMed] [Google Scholar]
- Andrews D. Q.; Hayes J.; Stoiber T.; Brewer B.; Campbell C.; Naidenko O. V. Identification of Point Source Dischargers of Per- and Polyfluoroalkyl Substances in the United States. AWWA Water Sci. 2021, 3 (5), e1252 10.1002/aws2.1252. [DOI] [Google Scholar]
- Hu X. C.; Ge B.; Ruyle B. J.; Sun J.; Sunderland E. M. A Statistical Approach for Identifying Private Wells Susceptible to Perfluoroalkyl Substances (PFAS) Contamination. Environ. Sci. Technol. Lett. 2021, 8 (7), 596–602. 10.1021/acs.estlett.1c00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. A.; Tokranov A. K.; Hull R. B.; LeBlanc D. R.; Haynes A. B.; Lane J. W. Hillslope Groundwater Discharges Provide Localized Stream Ecosystem Buffers from Regional Per- and Polyfluoroalkyl Substances Contamination. Hydrol. Processes 2020, 34 (10), 2281–2291. 10.1002/hyp.13752. [DOI] [Google Scholar]
- Walter D. A.; McCobb T. D.; Fienen M. N.. Use of a Numerical Model to Simulate the Hydrologic System and Transport of Contaminants Near Joint Base Cape Cod, Western Cape Cod, Massachusetts, Scientific Investigations Report; U.S. Geological Survey Scientific Investigations Report 2018-5139, 2019.
- Solomon C. T.; Carpenter S. R.; Rusak J. A.; Vander Zanden M. J. Long-Term Variation in Isotopic Baselines and Implications for Estimating Consumer Trophic Niches. Can. J. Fish. Aquat. Sci. 2008, 65 (10), 2191–2200. 10.1139/F08-125. [DOI] [Google Scholar]
- Vanderklift M. A.; Ponsard S. Sources of Variation in Consumer-Diet delta15N Enrichment: A Meta-Analysis. Oecologia 2003, 136 (2), 169–182. 10.1007/s00442-003-1270-z. [DOI] [PubMed] [Google Scholar]
- Macorps N.; Le Menach K.; Pardon P.; Guérin-Rechdaoui S.; Rocher V.; Budzinski H.; Labadie P. Bioaccumulation of Per- and Polyfluoroalkyl Substance in Fish from an Urban River: Occurrence, Patterns and Investigation of Potential Ecological Drivers. Environ. Pollut. 2022, 303, 119165 10.1016/j.envpol.2022.119165. [DOI] [PubMed] [Google Scholar]
- Ng C. A.; Hungerbühler K. Bioaccumulation of Perfluorinated Alkyl Acids: Observations and Models. Environ. Sci. Technol. 2014, 48 (9), 4637–4648. 10.1021/es404008g. [DOI] [PubMed] [Google Scholar]
- Sun J. M.; Kelly B. C.; Gobas F. A. P. C.; Sunderland E. M. A Food Web Bioaccumulation Model for the Accumulation of Per- and Polyfluoroalkyl Substances (PFAS) in Fish: How Important Is Renal Elimination?. Environ. Sci.: Processes Impacts 2022, 24 (8), 1152–1164. 10.1039/D2EM00047D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C. A.; Hungerbühler K. Bioconcentration of Perfluorinated Alkyl Acids: How Important Is Specific Binding?. Environ. Sci. Technol. 2013, 47 (13), 7214–7223. 10.1021/es400981a. [DOI] [PubMed] [Google Scholar]
- Wang Z.; MacLeod M.; Cousins I. T.; Scheringer M.; Hungerbühler K. Using COSMOtherm to Predict Physicochemical Properties of Poly- and Perfluorinated Alkyl Substances (PFASs). Environ. Chem. 2011, 8 (4), 389–398. 10.1071/EN10143. [DOI] [Google Scholar]
- Burkhard L. P.; Votava L. K. Biota-Sediment Accumulation Factors for Per- and Polyfluoroalkyl Substances. Environ. Toxicol. Chem. 2023, 42 (2), 277–295. 10.1002/etc.5526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz G.; Desrosiers M.; Duy S. V.; Labadie P.; Budzinski H.; Liu J.; Sauvé S. Environmental Occurrence of Perfluoroalkyl Acids and Novel Fluorotelomer Surfactants in the Freshwater Fish Catostomus Commersonii and Sediments Following Firefighting Foam Deployment at the Lac-Mégantic Railway Accident. Environ. Sci. Technol. 2017, 51 (3), 1231–1240. 10.1021/acs.est.6b05432. [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency . Fish and Shellfish Advisories and Safe Eating Guidelines. https://www.epa.gov/choose-fish-and-shellfish-wisely/fish-and-shellfish-advisories-and-safe-eating-guidelines.
- Lavoie R. A.; Jardine T. D.; Chumchal M. M.; Kidd K. A.; Campbell L. M. Biomagnification of Mercury in Aquatic Food Webs: A Worldwide Meta-Analysis. Environ. Sci. Technol. 2013, 47 (23), 13385–13394. 10.1021/es403103t. [DOI] [PubMed] [Google Scholar]
- Kelly B. C.; Ikonomou M. G.; Blair J. D.; Morin A. E.; Gobas F. A. P. C. Food Web-Specific Biomagnification of Persistent Organic Pollutants. Science 2007, 317 (5835), 236–239. 10.1126/science.1138275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.