Abstract
Orofacial clefts (OFCs) are one of the most common birth defects. The etiologies are complicated, with genetic, epigenetic, and environmental factors involved. Studies have found that maternal diabetes and metabolic syndrome are associated with a higher risk of OFCs in offspring. Metabolic syndrome is a clustering of several disease risk factors, including hyperglycemia, dyslipidemia, obesity, and hypertension. Metabolic disease during pregnancy can increase risk of adverse outcomes and significantly influence fetal development, including orofacial formation and fusion. An altered metabolic state may contribute to developmental disorders or congenital defects such as OFCs, potentially through epigenetic modulations, such as histone modulation, DNA methylation, and non-coding RNAs to alter activities of critical morphogenetic signaling or related developmental genes. This review summarizes the currently available evidence and underlying mechanisms of how the maternal metabolic syndrome is associated with OFCs in mostly human and some animal studies. It may provide a better understanding of the interactions between intrauterine metabolic status and fetal orofacial development which might be applied towards prevention and treatments of OFCs.
Keywords: orofacial clefts, maternal metabolic syndrome, diabetes, obesity, dyslipidemia, hypertension, epigenetics
Introduction
Orofacial clefts (OFCs) impact about 1 in 700 newborns globally (S. E. Watkins, Meyer, Strauss, & Aylsworth, 2014). The common types of OFCs are cleft lip with or without cleft palate (CL/P) and cleft palate only (CPO), and affected children experience significant difficulties in feeding, speech, and social integration. Although plastic and maxillofacial surgery, speech therapy, and psychosocial intervention are available, OFCs are associated with significant long-term health, life-style wellbeing, and socio-economic burdens for patients and their families (Dixon, Marazita, Beaty, & Murray, 2011). Facial primordia become evident by the fourth week of gestation in humans and embryonic day 9.5 in mice (Tarr, Lambi, Bradley, Barbe, & Popoff, 2018). It is a complicated and highly integrated process involving a precise series of morphological changes driven by extensive cell proliferation, differentiation, migration, and apoptosis (Y. Ji et al., 2020). Disruption of any of these processes may result in OFCs which may occur alone (nonsyndromic) or associated with other structural birth defects (syndromic OFCs) (Arias Urueña, Briceño Balcazar, Martinez Lozano, Collins, & Uricoechea Patiño, 2015; Mossey & Modell, 2012; Tolarová & Cervenka, 1998).
OFCs have complex and multifactorial etiology including genetic and epigenetic factors and can be influenced by gene-environment interactions (Garland, Reynolds, & Zhou, 2020; Garland, Sun, et al., 2020; Iwaya, Suzuki, & Iwata, 2023; Reynolds et al., 2020). Genome-wide association studies have identified loci (e.g. 8q24) or candidate genes (e.g., CREBBP, DICER1, FGFR1, GRHL3, IRF6, MSX1, MYC, NOG, PTCH1, SPRY2, TFAP2A, VAX1, WNT9B, and others) whose polymorphisms are associated with OFCs (Butali et al., 2018; Leslie et al., 2016; Mangold et al., 2010; Yu et al., 2017). Both human and animal studies demonstrate that environmental factors (e.g., folate deficiency, maternal diabetes, and smoking) increase the incidence of OFCs (reviews in (Garland, Reynolds, et al., 2020; Mossey & Modell, 2012; Waller et al., 2007)); for example, carriers of certain polymorphisms in transforming growth factor alpha (TGFA), whose mothers smoked during gestation, had significantly higher risks of developing cleft palate (Beaty et al., 1997; Hwang et al., 1995). Another example is that maternal supplementation with folic acid or vitamin A may reduce the occurrence rate of OFCs (Gildestad et al., 2015; Johansen, Lie, Wilcox, Andersen, & Drevon, 2008; Y. Zhou et al., 2020). Maternal health conditions, such as diabetes and metabolic syndrome, also contribute to OFCs. Metabolic syndrome is a clustering of several disease risk factors, including hyperglycemia, obesity, dyslipidemia, and hypertension (Huang, 2009). The purpose of this review is to explore the currently available evidence on how maternal metabolic syndrome is associated with OFCs and potential underlying epigenetic mechanisms. This may provide a better understanding of the interactions between intrauterine environment and orofacial development, which could help contribute to their future prevention.
Epigenetic mechanisms underlie orofacial clefts and related birth defects associated with maternal metabolic disorders
Birth defects may occur after fertilization due to teratogenic factors in the environment instead of an inherited genetic cause (Carstens, 2004; Cederberg, Picard, & Eriksson, 2003). A major link between the environment and the genome is the epigenome, such as DNA methylation and histone modification, which is influenced by the environment and regulates chromatin structure and genome functions without changing the nucleotide sequences (Messerschmidt, Knowles, & Solter, 2014; Xu, Li, Liu, & Gao, 2021). Two critical periods for epigenetic reprogramming in mammalian embryos occur shortly after fertilization and during the formation of the primordial germ cells (Kobayashi et al., 2013; Smith et al., 2012). Epigenetic reprogramming profoundly affects cell lineage and differentiation during embryonic development. Both human and animal studies have confirmed that epigenetic alterations contribute to OFCs. Rogers and colleagues found that administration of the DNA demethylating agent 5-aza-2’-deoxycytidine during pregnancy increased occurrence of cleft palate in mice (Rogers et al., 1994). The critical window for this effect is between embryonic days 11 and 14, a crucial period for palatal development in mice (Bulut, Ozdemir, Başimoglu-Koca, Korkmaz, & Atalay, 1999). Further, Kuriyama and colleagues confirmed that maternal exposure to all-trans retinoic acid, a teratogen known to induce cleft palate in mice, is associated with profound DNA methylation changes within CpG islands and globally reduced RNA expression on embryonic day 14.5 (Kuriyama et al., 2008). In addition to DNA methylation, microRNAs are also important effectors in regulating many key signaling mediators (e.g., transforming growth factor beta [TGFβ], bone morphogenic protein [BMP] and Wnt ligands) during orofacial development (Iwaya et al., 2023; Nakamura, Inloes, Katagiri, & Kobayashi, 2011; Seelan, Pisano, & Greene, 2022; Tomé et al., 2011; Yao et al., 2011). For example, microRNA-335 (miR-335) is known to regulate proliferation in human mesenchymal stem cells through the canonical Wnt signaling pathway, while Dnpep, as the target of miR-140, regulates BMP signaling to influence bone morphology and craniofacial development (Nakamura et al., 2011; Tomé et al., 2011). Another study identified 17 microRNAs as potential modifiers of human cleft palate-associated genes; for example, miR-133b overexpression downregulated target cleft palate genes, including FGFR1, GCH1, PAX7, SMC2, and SUMO1, and resulted in reduction of cell proliferation in human palatal mesenchymal cell cultures (Suzuki et al., 2019).
The prevalence of maternal metabolic syndrome is increased due to high-calorie/high-sugar diets and sedentary lifestyle. It has become one of the greatest health concerns for pregnant women since it increases the risk of congenital anomalies (e.g., cardiovascular, neural tube, and craniofacial defects) and gestational complications (e.g., preeclampsia, gestational diabetes, and preterm delivery) (Grieger et al., 2018; Krakowiak et al., 2012; Mossey & Modell, 2012). Studies have found that altered metabolic status affects epigenetic regulation and consequently influences fetal development as well as the offspring’s health in their adult life (review in (Lesseur & Chen, 2018)). For example, maternal obesity was associated with altered histone methylation during embryonic development and histone H3 Lysine 4 dimethylation (H3K4me2) was reduced in the embryos of obese mice (Pan et al., 2020). Epigenetic studies using human blood samples have demonstrated that both maternal diabetes and obesity can reprogram the DNA methylome of newborns or offspring beyond birth (Alba-Linares et al., 2023; Rizzo et al., 2020). Thus, while maternal metabolic disorders can negatively influence the fetal and offspring development acting through epigenetic alterations, it may be preventable or even reversible. Therefore, it is critical to understand the interactions between maternal risk factors and susceptible genes for specific types of congenital disorders. The associations and related mechanisms between specific metabolic disorders and orofacial clefts are discussed further in the following sections.
Maternal diabetes mellitus and orofacial clefts
Pregestational or preexisting diabetes mellitus (PGDM) contributes to an increased incidence and worsening of gestational glucose intolerance, which leads to the developing fetus being exposed to maternal hyperglycemic and/or hyperinsulin conditions in utero (Chung & Myrianthopoulos, 1975). Excessive glucose metabolism consumes more oxygen, exacerbating a hypoxic state which contributes to increased mitochondrial super-oxide production (R. Li, Chase, Jung, Smith, & Loeken, 2005). This hyperglycemia-induced hypoxia contributes to congenital abnormalities. For example, hypoxia in the developing fetus changes the DNA methylation status at Pax3, which plays a critical role in neural crest development, and may have detrimental effects neural tube closure and craniofacial development (Chang et al., 2003; R. Li et al., 2005; Wei & Loeken, 2014). A 2001 study found that infants of mothers with either PGDM or GDM had an increased prevalence of many different congenital malformations including OFCs. However, PGDM had an increased association with infants showing multiple defects, which was not observed in infants of mothers with gestational diabetes mellitus (GDM) (Aberg, Westbom, & Källén, 2001).
GDM is defined as a glucose tolerance disorder during pregnancy and that is initially diagnosed in the second or third trimester of pregnancy by a 75g oral glucose tolerance test (ADA, 2020; Schäfer-Graf et al., 2018). A population-based, nationwide analysis showed the overall prevalence of GDM in the USA is 13.2% (Melchior, Kurch-Bek, & Mund, 2017). GDM is associated with an increased rate of gestational complications (e.g., pre-eclampsia and C-section) and women with GDM showed significantly higher risks of developing type 2 diabetes in the years after the first diagnosis (Bellamy, Casas, Hingorani, & Williams, 2009; Rayanagoudar et al., 2016). GDM increases the risk of adverse effects on the heath of newborns, including macrosomia, shoulder dystocia, and hypoglycemia (Crowther et al., 2005; Landon et al., 2009; Metzger et al., 2008). Infants born to mothers with either GDM or PGDM have higher risks of having congenital anomalies, most frequently involving cardiovascular defects (Aberg et al., 2001; Moore, Singer, Bradlee, Rothman, & Milunsky, 2000; Spilson, Kim, & Chung, 2001). A recent meta-analysis of population-based studies confirmed that the relative risks (RRs) of overall congenital anomalies in offspring of women with PGDM were higher than those in offspring of women with GDM (T. N. Zhang et al., 2022).
While some early investigations had a difficult time directly connecting maternal diabetes with OFCs specifically (Correa et al., 2008b; Martinez-Frias et al., 2005; Stott-Miller, Heike, Kratz, & Starr, 2010), several more recent larger population-based association studies have shown an increased prevalence of OFCs in both babies born to mothers with PGDM and to mothers with GDM (Table 1). A report of live births in Canada found an increased overall prevalence of OFCs in infants born to mothers with PGDM with an odds ratio for type 1 DM of 2.48, and for type 2 DM of 2.77 (Liu et al., 2015). Analysis of USA births documented in the National Vital Statistics System found increased relative risk for both CL/P (p<0.001) and CPO (p<0.001) in babies born to mothers with PGDM. Mothers with GDM had an increased relative risk for babies with CL/P (p<0.001), and both PGDM and GDM showed increased association with all cleft subtypes combined (p<0.0001; p<0.0001) (Wu et al., 2020). Multiple reports from the National Birth Defect Prevention Study (NBDPS) also reported an increased prevalence of all types of clefts in infants born in the USA between 1997 and 2011 to mothers with PGDM and mothers with GDM (Correa et al., 2008a; Marchincin et al., 2023; Tinker et al., 2020). Another study specifically examining singletons born in upstate New York between 2004 and 2016 found an increased OFC risk to mothers with PGDM (p<0.001), but not GDM (Yang, Reece, Wang, & Gabbay-Benziv, 2015).
Table 1.
Representative association studies between maternal diabetes and orofacial clefts
| Study Description | Subject Population and Years | Maternal Condition | OFC Number total included in study (prevalence); RR/OR (95% CI) | OFC Subtype total included in study (prevalence); RR/OR (95% CI) | Reference |
|---|---|---|---|---|---|
| Population-based study of association between PGDM and GDM and birth defects in mother-infant pairs from birth registry data | 29,211,974 live births with maternal age ranging from 18–49 years old documented in the National Vital Statistics System of USA, 2011–2018 | PGDM 242,600 mothers (0.83%) | 412 (0.170%); RR1 2.31 (2.10–2.55), P<0.0001 |
CL/P 273 (0.113%); RR 2.06 (1.82–2.33) P<0.001 CPO 139 (0.057%); RR 2.35 (1.97–2.97), P<0.001 |
Wu et al 2020 |
| GDM 1,685,479 mothers (6.18%) | 1,688 (0.100%); RR 1.36 (1.30–1.43), P<0.0001 |
CL/P 1,145 (0.068%); RR 1.28 (1.20–1.36), P<0.001 CPO 543 (0.032%); RR 1.40 (1.28–1.53) |
|||
| Analysis of association between PGDM and GDM and birth defects using case-control data from National Birth Defects Prevention Study (NBDPS) | 13,030 infants with and 4,895 without birth defects in USA, 1997–2003 |
PGDM
All birth defects group: 1.7% of mothers |
33 OFC cases |
CL/P Isolated 14; OR 2.92 (1.45–5.87) Syndromic 8; OR 8.07 (3.05–21.39) CPO Isolated 5; OR 1.8 (0.67–4.87) Syndromic 6; OR 10.73 (3.99–28.86) |
Correa et al 2008 |
|
GDM
All birth defects group: 4.7% of mothers |
96 OFC cases |
CL/P Isolated 54; OR 1.45 (1.03–2.04) Syndromic 8; OR 1.22 (0.52–2.86) CPO Isolated 29; OR 1.54 (1.01–2.37) Syndromic 5; OR 1.26 (0.50–3.20) |
|||
| Expanded analysis of NBDPS data | 31,007 infants with and 11,477 without birth defects in USA, 1997–2011 |
PGDM All birth defects group: 775 mothers (2.5%), Control group: 71 mothers (0.6%) |
58 CL/P cases; OR 3.0 (2.1–4.3) 42 CPO cases; OR 4.3 (2.9–6.5) |
CL/P Isolated 37; OR 2.2 (1.4–3.3) Syndromic 21; OR 8.7 (5.0–15.0) CPO Isolated 19; OR 2.5 (1.4–4.2) Syndromic 23; OR 12.3 (7.3–20.7) |
Tinker et al 2020 |
|
GDM All birth defects group: 1,653 mothers (5.3%), Control group: 536 mothers (4.7%) |
161 CL/P cases; OR 1.1 (0.9–1.3) 96 CPO cases; OR 1.4 (1.1–1.8) |
CL/P Isolated 143; OR 1.1 (0.9–1.4) Syndromic 18; OR 1.0 (0.6–1.7) CPO Isolated 79; OR 1.4 (1.1–1.8) Syndromic 17; OR 1.4 (0.9–2.4) |
|||
| Analysis of NBDPS data for association between PGDM and birth defects stratified by type 1 and type 2 DM | 29,024 infants with and 10,898 infants without birth defects in USA, 1997–20112 |
PGDM Type 1 All birth defects group: 252 mothers (0.9%); Control group: 24 mothers (0.2%) |
27 OFC cases |
CLP 10; OR 2.3 (1.1–4.7) CLO 23 CPO 15; OR 4.1 (2.1–7.8) |
Marchincin et al 2022 |
|
PGDM Type 2 All birth defects group: 357 mothers (1.2%); Control group: 34 mothers (0.3%) |
55 OFC cases |
CLP 23; OR 4.1 (2.3–7.1) CLO 13; OR 4.8 (2.4–9.3) CPO 19; OR 4.4 (2.4–7.8) |
|||
| Population-based study of association between PGDM and GDM and birth defects in mother-infant pairs from birth registry data | 650,914 singletons born in Upstate New York, 2004–2016 | PGDM: 4,134 mothers (0.6%) | 12 OFC cases (0.290%); OR 2.9 (1.6–5.2), P<0.001 |
CL/P 7 (0.169%); OR 2.5 (1.2–5.3), P<0.05 CPO 5 (0.121%); OR 3.8 (1.6–9.3), P<0.01 |
Yang et al 2019 |
| GDM: 32,605 mothers (5.0%) | 32 OFC cases (0.098%); OR 1.0 (0.7–1.4), P>0.05 |
CL/P 22 (0.067%); OR 1.0 (0.6–1.5), P>0.05 CPO 10 (0.031%); OR 1.0 (0.5–1.8), P>0.05 |
|||
| Population-based study of birth defects assessing PGDM attributable risk percentage | 2,839,680 live births in Canada (except Quebec), 2002–2013 |
PGDM Type 1
0.27% (2002) – 0.28% (2012) of mothers |
0.36% OFC prevalence; OR 2.48 (1.70–3.63) |
(No OFC subtype available) | Liu et al. 2015 |
| PGDM Type 2 0.19% (2002) – 0.47% (2012) of mothers | 0.39% OFC prevalence; OR 2.77 (2.02–3.80) |
||||
| Population-based study of association between PGDM and GDM and birth defects in mother-infant pairs from birth registry data | 1,216,198 births in Sweden, 1987–1997 | PGDM 3,864 mothers (0.32%) | 19 OFC cases (0.492%); OR 2.33 (1.49–3.67) |
(No OFC subtype available) Isolated 11 (0.285%) Syndromic 8 (0.207%) |
Aberg et al 2001 |
| GDM 8,688 mothers (0.72%) | 18 OFC cases (0.207%); OR 0.98 (0.62–1.56) |
Isolated 14 (0.161%) Syndromic 4 (0.046%) |
RR adjusted for maternal age, race/ethnicity, education levels, marital status, parity, smoking before and during pregnancy, timing of initiation of prenatal care, pregnancy BMI, infant sex, and pregestational hypertension.
Mothers were excluded if they had GDM, if their first DM diagnosis was during any pregnancy, if they had an unknown DM type or diagnosis date, or if they had a type 1 PGDM diagnosis but did not use insulin during pregnancy.
OR not calculated if n<3
Studies in animal models suggest that maternal diabetes contributes to a teratogenic effect that results in craniofacial malformations. A hyperglycemic environment in diabetic rat pregnancies contributes to altered metabolism of inositol and prostaglandins, and increased presence of reactive oxygen species, all of which are likely contributing mechanisms (Eriksson et al., 2000). These changes particularly affect the development of neural crest-derived organs, especially the mandible and Meckel’s cartilage, causing a DiGeorge-like phenotype (Cederberg et al., 2003; Simán et al., 2000). DiGeorge Syndrome is a 22q11 deletion disorder which commonly includes micrognathia or retrognathia. This affects growth of maxillary structures, often resulting in OFCs (McDonald-McGinn et al., 2015). While clefts are most commonly observed in isolation without other defects, they often present as part of a broader syndrome. Given that altered metabolic states can affect many different craniofacial structures, it is possible that some OFC cases in diabetic pregnancy may be attributable to other primary defects resulting from altered maternal environment which affect development of the lip or palate resulting in a cleft as a secondary effect.
Maternal environment and metabolic status during pregnancy contribute to the offspring’s epigenetic profile, and embryonic exposure to maternal hyperglycemia may modify the chromatin, particularly since glucose metabolism regulates histone acetylation through the citrate lyase pathway. AATP citrate lyase (ACLY) converts glucose-derived citrate into acetyl-CoA which is necessary for histone acetylation (Wellen et al., 2009). ACLY dysregulation contributes to the etiology of type 2 diabetes, and Acly knockdown improves glucose tolerance in a diabetes mouse model (Q. Wang et al., 2009). Another study examined the deteriorated intrauterine environment in an intrauterine growth retardation rat model and found that the expression of Pdx1 was reduced due to the lower levels of histone acetylation markers. These altered epigenetic marks and reduced Pdx1 expression had adverse effects on fetal development, including pancreatic islets, lasting into adulthood with diabetes (Park, Stoffers, Nicholls, & Simmons, 2008). Overall, these studies indicate that the diabetic intrauterine environment could alter gene expression through influencing epigenetic modulators.
Wnt signaling is required to regulate patterning processes during craniofacial development by modulating cell proliferation, migration, and apoptosis, and mutations in more than 20 Wnt signaling genes are associated with OFCs in humans (see review summary in (Reynolds et al., 2019)). Wnt signaling is traditionally classified into the canonical and non-canonical pathways, the latter of which is further divided into the planar cell polarity and the Wnt/Ca2+ pathways (Gao & Chen, 2010; Komiya & Habas, 2008). Diabetes can cause dysregulation of Wnt signaling pathways which may contribute to the etiology of defects that present in infants born of mothers with PGDM or GDM. Interestingly, one study found that maternal diabetes-induced oxidative stress could lead to the impairment of the non-canonical Wnt5a-Ca2+ pathway (F. Wang, Fisher, Zhong, Wu, & Yang, 2015). Further, maternal diabetes induced the overexpression of Wnt antagonists Sfrp1 and Dkk1, which consequently suppressed canonical Wnt signaling by decreasing Dvl2 phosphorylation and increasing Gsk3β activity (F. Wang et al., 2015). Another study showed that maternal diabetes increases the risk of cleft palate and caudal regression induced by the vitamin A metabolite retinoic acid in mouse embryos, with diminished Wnt3a expression as a contributing mechanism (Chan et al., 2002). Further evidence for the role of epigenetic modifications in the presentation of congenital defects is the fact that genetically identical individuals often show variable penetrance of phenotypes. Variability of gene expression results from the combined changes in transcription factors and chromatin structure, as well as histone modification regulators (e.g., Ehmt2 and Kat2a) in diabetic pregnancy (Aberg et al., 2001; Lin et al., 2008; Wagschal et al., 2008). Differential DNA methylation may also contribute to the penetrance of OFCs, which is understudied in GDM and PGDM settings.
Maternal obesity and orofacial clefts
The consumption of highly processed food coupled with overall low physical activity both contribute to the increasing prevalence of obesity in women of reproductive age. Between 2011 and 2012, 31.8% of women aged 20–39 years were obese in the USA while the proportion was less than 10% in the 1970s (Ogden, Carroll, Kit, & Flegal, 2014; K. Rasmussen, 2012). Maternal obesity is strongly associated with pregnancy complications such as maternal diabetes, pre-eclampsia, and early pregnancy loss, as well as with stillbirth and congenital anomalies (review in (Poston et al., 2016)). Maternal obesity significantly increases the occurrence of neural tube defects, cardiovascular, orofacial, and limb anomalies (Mikhail, Walker, & Mittendorf, 2002; Moore et al., 2000; Ray, Wyatt, Vermeulen, Meier, & Cole, 2005).
Differences in sample size, different criteria for obesity, ethnicity, socioeconomic and geographical differences may contribute to variability in the reported association between maternal obesity and OFCs. While several early studies into the association between obesity and OFCs reported no association (Shaw, Todoroff, Schaffer, & Selvin, 2000; M. L. Watkins, Rasmussen, Honein, Botto, & Moore, 2003), a number of more recent larger meta-analyses have shown an increased prevalence of cleft presentation in infants born to obese or overweight mothers (Table 2). One meta-analysis of 2 studies from the USA and one from Sweden showed a significant association between obesity in mothers with a BMI above 30 and cleft lip and palate (CLP) (p=0.02) as well as CPO (p=0.02) in infants (Stothard, Tennant, Bell, & Rankin, 2009). Another meta-analysis of data from USA patients including the two aforementioned studies with one other found an odds ratio for CL/P of 1.16 (CI = 1.0–1.34) and for CPO of 1.14 (CI = 0.95–1.37) in obese mothers. A study including data from 8 groups in the USA, Australia, and Sweden found an increased odds ratio in obese mothers (BMI > 30) for either CL/P (OR = 1.13/CI = 1.04–1.23) or CPO (OR = 1.22/CI = 1.09–1.35) (Blanco, Colombo, & Suazo, 2015). Another more recent analysis of 6 cohorts from Northern Europe and the USA found the risk of any form of cleft palate increased in babies with overweight mothers, and increase correlated with the degree of obesity. Mothers with a BMI between 30 and 35 showed an odds ratio of 1.09 (CI = 0.95–1.25) for a baby born with cleft palate, but mothers with a BMI over 35 had an odds ratio 1.36 (CI = 1.16–1.58) (Kutbi et al., 2017).
Table 2.
Meta-analyses of association studies between maternal obesity and orofacial clefts
| Study Description | Pregestational maternal condition BMI Characterization | OFC subtype OR (95% CI) | References |
|---|---|---|---|
| Analysis of 6 pooled case-control studies (1987–2008) from Northern Europe and USA for association between maternal weight and orofacial clefts | Obese I BMI: ≥ 30, < 35 | All cleft palate; OR 1.09 (0.95–1.25) | Kutbi et al 2017 |
| Obese II & III BMI: > 35 | All cleft palate; OR 1.36 (1.16–1.58); | ||
| Overweight BMI: 25–29.9 | All cleft palate; OR 1.02 (0.92–1.13) | ||
| Normal BMI: 18.5–24.9 | N/A | ||
| Underweight BMI: <18.5 | CL/P; OR 1.16 (0.98–1.36) | ||
| Meta-analysis of 8 studies (5 from USA, 1 from Australia, and 2 from Sweden) to assess increased orofacial cleft risk with maternal obesity | Obese BMI: ≥ 30 (except one cohort study > 29) |
CL/P; OR 1.13 (1.04–1.23) CPO; OR 1.22 (1.09–1.35) |
Meta-analysis: Blanco et al 2015 Selected studies: Watkins 2003 Cedergren 2005 Honein 2007 Waller 2007 Oddy 2009 Blomberg 2010 Stott-Millier 2010 Carmichael 2012 |
| Normal BMI: 18.5–24.9 (except one cohort study 19.8–26) | N/A | ||
| Review and meta-analysis of 3 studies from USA for association between maternal weight and orofacial clefts | Obese BMI: ≥ 30 |
CL/P; OR 1.16 (1.0–1.34) CPO; OR 1.14 (0.95–1.37) |
Meta-analysis: Izedonmwen et al 2015 Selected studies: Watkins 2003 Waller 2007 Stott-Millier 2010 |
| Overweight BMI: 25–29.9 | CL/P; OR 1.06 (0.93–1.21) | ||
| Normal BMI: 18.5–24.9 | N/A | ||
| Meta-analysis of several studies for association between maternal obesity and congenital anomalies | Obese BMI: ≥ 30 or > 29 |
CLP; OR 1.20 (1.03–1.40), P=0.02 CPO; OR 1.23 (1.03–1.47), P=0.02 |
Meta-analysis: Stothard et al 2009 Selected studies: Watkins 2003 Cedergren & Kallen 2005 Waller 2007 |
| Normal BMI: 18.5–24.9 or 19.8–26 | N/A |
Additional differences in the linkage between obesity and cleft risk might also depend on whether the studies analyzed specific cleft types. In addition to CPO vs cleft lip forms, the difference in etiologies between CLO versus CLP as distinct forms of CL/P should also be considered. Studies have found different distributions and patterns of the risks of recurrence between CLO and CL/P or CPO (Harville, Wilcox, Lie, Vindenes, & Abyholm, 2005; Kutbi et al., 2017; Stoll, Alembik, Dott, & Roth, 2000; Tolarová & Cervenka, 1998). Specifically, there are a few distribution characteristics that separates CL/P from CLO in which 1) CL/P more frequently co-exists with other congenital defects compared with CLO (Harville et al., 2005; Stoll et al., 2000; Tolarová & Cervenka, 1998); 2) males have higher risks of developing CL/P than CLO, while females have higher frequencies of developing CPO, and twins have CLO more often than CL/P (Harville et al., 2005; Mossey & Modell, 2012; Shapira, Lubit, Kuftinec, & Borell, 1999). Different cleft forms similarly show differential association with maternal weight as well. For example, Villamor et al. (2008) conducted a large population-based cohort study in Sweden and found that higher maternal weight gain and longer interpregnancy intervals positively were associated with the occurrence of CPO, but not CL/P (Villamor, Sparén, & Cnattingius, 2008). Besides maternal weight gain, another study found that maternal obesity is associated with a mild increase in risk of CPO, while underweight maternity may be associated with CL/P (Waller et al., 2007). A meta-analysis concluded that maternal obesity is significantly associated with increased risk of CPO and CL/P, but not associated with cleft lip only (CLO) (Stothard et al., 2009).
The precise mechanisms underlying obesity-associated OFCs remain unclear but undiagnosed type 2 diabetes, hyperlipidemia, or maternal dietary changes in conjunction with maternal obesity could contribute. Maternal obesity is also associated with epigenetic changes. Histone H3 Lysine 4 dimethylation (H3K4me2) was reduced in the embryos of obese mice (Pan et al., 2020), which may alter signaling gene activities critical for orofacial development. A more recent animal study demonstrates that maternal high-fat diet changes DNA methylation in early embryos by disrupting the TCA cycle intermediary α-ketoglutarate, which is necessary for DNA demethylation by TET enzymes (Penn, McPherson, Fullston, Arman, & Zander-Fox, 2023). These studies suggest that maternal obesity may alter DNA and histone methylation at OFC-associated genes, and provide new directions for related mechanistic studies.
Maternal dyslipidemia and orofacial clefts
During pregnancy, maternal physical adaptations such as fat accumulation and hormonal changes are necessary for fetal development. The accumulation of fat during the first two-thirds of gestation are mainly attributed to hyperphagia and increased lipid synthesis (Murphy & Abrams, 1993; Palacín, Lasunción, Asunción, & Herrera, 1991). The levels of blood triglycerides are markedly increased (100–200%) during pregnancy, while a moderate increase (40–50%) in cholesterol levels is also observed (Basaran, 2009; Palacín et al., 1991). The presence of lipoprotein receptors in the placenta allows uptake of maternal polyunsaturated fatty acids and release into the fetal plasma (Herrera, Amusquivar, López-Soldado, & Ortega, 2006). Maternal non-esterified fatty acids and cholesterol are also able to be transferred through the placenta and be used by the fetus. In the early stage of gestation, maternal cholesterol and cholesterol synthesis is the major source for fetal cholesterol which will further be utilized for cell proliferation, synthesis of steroid hormones, and fetal growth (Herrera et al., 2006).
Dyslipidemia is characterized by elevated levels of triglycerides and total blood cholesterol, including increased low-density lipoprotein (LDL) and reduced high-density lipoprotein (HDL) (NCEP, 2002). Studies have found that maternal obesity and the western dietary pattern, which is rich in fat and poor in fruits, are significantly associated with prevalence of CL/P (Blanco et al., 2015; Vujkovic et al., 2007). Zhou et. al. reported that a high fat maternal diet significantly increased the prevalence and severity of triamcinolone-induced cleft palate in mice (M. Zhou & Walker, 1993). One mechanism by which a maternal diet affects development is through alterations in the epigenome, and high-fat diet contributes to alterations in DNA methylation, histone methylation, and non-coding RNA expression (C. C. Li et al., 2013; Vucetic, Kimmel, Totoki, Hollenbeck, & Reyes, 2010). Another study using human palatal mesenchyme cells found that microRNAs are significantly involved in regulating cleft palate-associated genes, and KEGG pathway analysis showed several of these target genes (e.g., DHCR7, DHCR24 and PAFAH1B1) are enriched in cholesterol and steroid metabolic processes (Suzuki et al., 2019). Maternal variants in genes associated with lipid metabolism, including DHCR7, which functions in cholesterol biosynthesis, contribute to Smith-Lemli-Opitz syndrome (SLOS) in which the affected infants sometimes present with CL/P. Further, variants in two other genes involved in lipid transport or uptake, ABCA1 and APOE, contribute to the severity of defects observed in SLOS patients (Lanthaler, Steichen-Gersdorf, Kollerits, Zschocke, & Witsch-Baumgartner, 2013). Lipid metabolism defects are associated with reduced cell proliferation during mouse palatogenesis. Tgfbr2 mutant mouse embryos showed cleft palate in which the ablation of Tgfbr2 reduced Adcy2 and Pde4b expression and consequently led to the accumulation of lipid droplets and a reduction in proliferation of palatal mesenchymal cells (Iwata et al., 2014). Lipid droplet accumulation in palatal tissue also occurs in RA-induced cleft palate models, and mass spectrometry showed that the lipid droplet content is altered as well, with increased triacylglycerides but reduced fatty acids and ceramides (W. Zhang et al., 2020).
Maternal hypertension and orofacial clefts
Hypertension (blood pressure > 140 mmHg systolic or > 90 mmHg diastolic) in pregnancy includes chronic hypertension, gestational hypertension, pre-eclampsia and eclampsia, which are observed in 5 to 10% of all pregnancies (Hutcheon, Lisonkova, & Joseph, 2011; Lo, Mission, & Caughey, 2013). Chronic hypertension is characterized as hypertension diagnosed before pregnancy or before 20 weeks of gestation; up to 1.5% of pregnancies are complicated with chronic hypertension (Croke, 2019; NHBPEP, 2000). Gestational hypertension is initially diagnosed during pregnancy after 20 weeks of gestation and returns to normal within 12 weeks postpartum in the absence of proteinuria (urinary excretion of ≥ 300mg of protein in 24 h. Pre-eclampsia is defined as a systemic syndrome originating in the placenta with hypertension and proteinuria during pregnancy (NHBPEP, 2000). Different from the chronic hypertension, the insufficient placental cytotrophoblast invasion is thought to be mainly attributed to pre-eclampsia, which usually accompanies maternal endothelial dysfunction, placental ischemia, and hypoxia; consequently, this causes a reduced blood supply for the developing fetus (L. Ji et al., 2013; Sircar, Thadhani, & Karumanchi, 2015). A subset of pre-eclamptic pregnancies result in eclampsia, characterized by convulsions and possibly coma during pregnancy or in the immediate postpartum period, frequently accompanied by blurred vision, nausea, and headaches (Wallace, Harris, & Bean, 2019).
Maternal hypertension is associated with increased risk of adverse outcomes for the mother, fetus, or infant. For example, pre-eclampsia and placental insufficiency are associated with mental disorders such as autism spectrum disorder, attention-deficit-hyperactivity-disorder, and schizophrenia (Dachew, Mamun, Maravilla, & Alati, 2018; Dachew, Scott, Betts, Mamun, & Alati, 2020; Dachew, Scott, Mamun, & Alati, 2019; Maher et al., 2018). Maternal pre-eclampsia specifically has been shown to positively correlate with fetal growth restriction (S. Rasmussen & Irgens, 2003).
Several studies have demonstrated an association between maternal-related hypertension and OFCs. Uruena et. al. found a positive association between maternal hypertension and syndromic forms of OFCs, and suggested potential mechanisms may include mutations of essential genes with roles in both involved in angiogenesis and palatogenesis (Arias Urueña et al., 2015). One study of births in California showed relative risk of CL/P and CPO in births to mothers with pre-existing hypertension (1.74 and 1.15, respectively), gestational hypertension (1.07 and 1.14), mild pre-eclampsia (1.52 and 0.96), and severe pre-eclampsia (1.18 and 1.44), and found a more drastically increased risk for mothers with pre-existing hypertension with pre-eclampsia (1.68 and 1.95). This study also examined the joint effects of diabetic and hypertensive disorders, and showed a relative risk of 1.99 for CL/P and of 1.94 for CPO in mothers with any hypertensive disorder and any diabetic disorder (Weber et al., 2018). A study of Chinese live births did not find any increased risk of clefts with gestational hypertension, but showed that pre-eclampsia generated an increased relative risk for all types of nonsyndromic OFC of 2.02 (CI = 1.27 – 3.20) (An et al., 2022). Another analysis of birth defect data from 29 countries showed an adjusted odds ratio of OFC defects with maternal chronic hypertension of 4.2 (CI = 1.5–11.6), while in mothers with chronic hypertension in addition to pre-eclampsia, the adjusted odds ratio was increased to 8.2 (CI = 2.0–34.3) (Bellizzi et al., 2016). While pregnancy-related hypertensive disorders are typically diagnosed around gestational week 20, there is evidence to suggest that associated pathological changes may occur earlier. The pathogenic process of pre-eclampsia begins in the first trimester, well prior to clinical presentation (Gathiram and Moodley, 2016). Although the etiology of pre-eclampsia and eclampsia are incompletely understood, improper trophoblast differentiation and placental development could affect the formation of facial structures before significantly altering blood pressure or protein content of urine.
Although lacking direct study, maternal hypertension disorder may influence fetal palatal development through epigenomic modulations. Studies have found that the maternal preeclampsia is associated with altered methylation at the IGF2 and HSD11B2 loci in fetal cord blood, two genes which are essential for regulating embryonic development and metabolism, respectively (Ching et al., 2015; He et al., 2013; Hu et al., 2014). A meta-analysis study including 10 cohort studies further validated that preeclampsia alone is associated with epigenome profile alterations in offspring cord blood (Kazmi et al., 2019). Another study of a US cohort of patients with maternal hypertension found significant association with alterations in DNA methylation at 24 genomic sites in placenta samples (Workalemahu et al., 2020). A follow up study specifically looking at methylation changes in specific cell types was able to replicate significance with 5 of the original 24 sites (Broséus et al., 2022), further strengthening the evidence that maternal hypertensive disorders can affect gene expression through epigenomic alterations, as a possible mechanism for congenital defects.
Conclusions
While genomic variants play a key role in the development of craniofacial abnormalities, increasing evidence in recent years has strengthened the association between OFCs and environmental factors, including maternal risk factors. One important contribution is maternal metabolic disorders, including diabetes (both pre-existing diabetes and GDM), obesity, dyslipidemia, and hypertension, which are frequently co-morbid and exacerbated by each other. With increased accumulation of clinical data along with advancing research technologies, we are becoming better able to determine not only the contributions of maternal metabolic disorders to OFCs and related birth defects, but also specific underlying mechanisms, mainly epigenetic alterations that may disrupt critical target genes (Figure 1). As our understanding of the epigenetic and metabolic contribution of uterine environment on embryonic development advances, our ability to use that knowledge for the betterment of outcomes will continue to improve. A better understanding of the role of maternal metabolism in influencing orofacial development will be helpful for the prevention of not just OFCs, but diverse congenital disorders that are influenced by external factors.
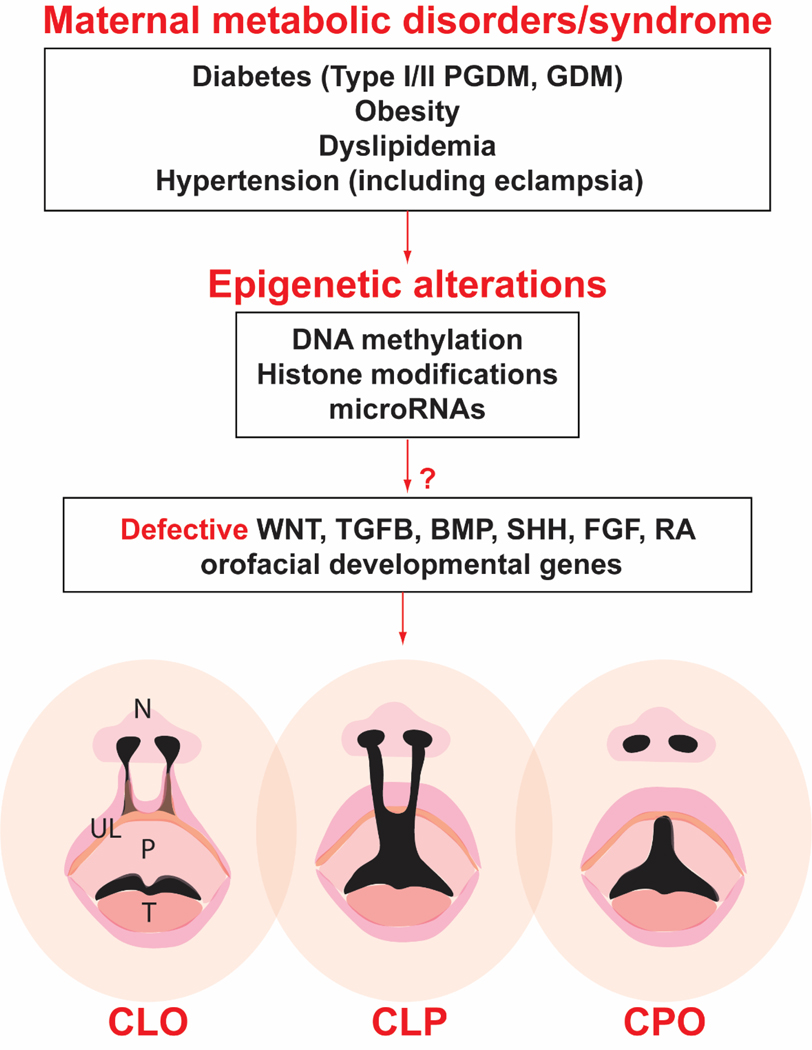
Figure 1. Epigenetic mechanisms implicated in orofacial clefts associated with maternal metabolic disorders.
Maternal metabolic disorders or syndromes can alter fetal epigenome, including DNA methylation, histone modifications, and microRNA productions which may further affect expression activities of critical signaling genes, such as Wnt and other morphogenetic signaling pathways that are required for orofacial development, leading to orofacial clefts. CLO, cleft lip only; CLP, cleft lip with cleft palate; CPO, cleft palate only; GDM, gestational diabetes; N, nose; P, palate; PGDM, pregestational diabetes; T, tongue; UL, upper lip. Cleft lip (CLO or CLP) can be unilateral or bilateral, only the latter is shown in the diagram.
Acknowledgments
This work is supported by grants from the NIH (R01DE026737 & R01NS102261 to C.J.Z.) and the Shriners Hospitals for Children (71039 to B.S., and 85105 to C.J.Z.). We are grateful to the rest of Zhou lab members for their general supports during the manuscript preparation. We apologize to colleagues whose important work we were unable to cite due to space constraints or inadvertently overlooking.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
DATA SHARING AND DATA ACCESSIBILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- Aberg A, Westbom L, & Källén B. (2001). Congenital malformations among infants whose mothers had gestational diabetes or preexisting diabetes. Early Hum Dev, 61(2), 85–95. doi: 10.1016/s0378-3782(00)00125-0 [DOI] [PubMed] [Google Scholar]
- ADA. (2020). 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2020. Diabetes Care, 43(Suppl 1), S14–s31. doi: 10.2337/dc20-S002 [DOI] [PubMed] [Google Scholar]
- Alba-Linares JJ, Perez RF, Tejedor JR, Bastante-Rodriguez D, Ponce F, Carbonell NG, . . . Lurbe E. (2023). Maternal obesity and gestational diabetes reprogram the methylome of offspring beyond birth by inducing epigenetic signatures in metabolic and developmental pathways. Cardiovasc Diabetol, 22(1), 44. doi: 10.1186/s12933-023-01774-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- An H, Jin M, Li Z, Zhang L, Zhang Y, Li H, . . . Li N. (2022). Association of gestational hypertension and preeclampsia with nonsyndromic orofacial clefts in China: a large prospective cohort study. Journal of Hypertension, 40(7), 1352–1358. doi: 10.1097/hjh.0000000000003150 [DOI] [PubMed] [Google Scholar]
- Arias Urueña L, Briceño Balcazar I, Martinez Lozano J, Collins A, & Uricoechea Patiño DA (2015). Clinical Aspects associated with Syndromic forms of Orofacial Clefts in a Colombian population. Colomb Med (Cali), 46(4), 162–167. [PMC free article] [PubMed] [Google Scholar]
- Basaran A. (2009). Pregnancy-induced hyperlipoproteinemia: review of the literature. Reprod Sci, 16(5), 431–437. doi: 10.1177/1933719108330569 [DOI] [PubMed] [Google Scholar]
- Beaty TH, Maestri NE, Hetmanski JB, Wyszynski DF, Vanderkolk CA, Simpson JC, . . . Wulfsberg EA (1997). Testing for interaction between maternal smoking and TGFA genotype among oral cleft cases born in Maryland 1992–1996. Cleft Palate Craniofac J, 34(5), 447–454. doi: 10.1597/1545-1569_1997_034_0447_tfibms_2.3.co_2 [DOI] [PubMed] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, & Williams D. (2009). Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet, 373(9677), 1773–1779. doi: 10.1016/s0140-6736(09)60731-5 [DOI] [PubMed] [Google Scholar]
- Bellizzi S, Ali MM, Abalos E, Betran AP, Kapila J, Pileggi-Castro C, . . . Merialdi M. (2016). Are hypertensive disorders in pregnancy associated with congenital malformations in offspring? Evidence from the WHO Multicountry cross sectional survey on maternal and newborn health. BMC Pregnancy and Childbirth, 16(1), 198. doi: 10.1186/s12884-016-0987-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco R, Colombo A, & Suazo J. (2015). Maternal obesity is a risk factor for orofacial clefts: a meta-analysis. Br J Oral Maxillofac Surg, 53(8), 699–704. doi: 10.1016/j.bjoms.2015.05.017 [DOI] [PubMed] [Google Scholar]
- Broséus L, Vaiman D, Tost J, Martin CRS, Jacobi M, Schwartz JD, . . . Lepeule J. (2022). Maternal blood pressure associates with placental DNA methylation both directly and through alterations in cell-type composition. BMC Medicine, 20(1), 397. doi: 10.1186/s12916-022-02610-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulut HE, Ozdemir O, Başimoglu-Koca Y, Korkmaz M, & Atalay A. (1999). Effects of a DNA demethylating agent−-5-azacytidine--on testicular morphology during mouse embryo development. Okajimas Folia Anat Jpn, 76(1), 47–53. doi: 10.2535/ofaj1936.76.1_47 [DOI] [PubMed] [Google Scholar]
- Butali A, Mossey PA, Adeyemo WL, Eshete MA, Gowans LJJ, Busch TD, . . . Adeyemo AA (2018). Genomic analyses in African populations identify novel risk loci for cleft palate. Human Molecular Genetics, 28(6), 1038–1051. doi: 10.1093/hmg/ddy402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens MH (2004). Neural tube programming and craniofacial cleft formation. I. The neuromeric organization of the head and neck. Eur J Paediatr Neurol, 8(4), 181–210; discussion 179–180. doi: 10.1016/j.ejpn.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Cederberg J, Picard JJ, & Eriksson UJ (2003). Maternal diabetes in the rat impairs the formation of neural-crest derived cranial nerve ganglia in the offspring. Diabetologia, 46(9), 1245–1251. doi: 10.1007/s00125-003-1100-1 [DOI] [PubMed] [Google Scholar]
- Chan BW, Chan KS, Koide T, Yeung SM, Leung MB, Copp AJ, . . . Shum AS (2002). Maternal diabetes increases the risk of caudal regression caused by retinoic acid. Diabetes, 51(9), 2811–2816. doi: 10.2337/diabetes.51.9.2811 [DOI] [PubMed] [Google Scholar]
- Chang TI, Horal M, Jain SK, Wang F, Patel R, & Loeken MR (2003). Oxidant regulation of gene expression and neural tube development: Insights gained from diabetic pregnancy on molecular causes of neural tube defects. Diabetologia, 46(4), 538–545. doi: 10.1007/s00125-003-1063-2 [DOI] [PubMed] [Google Scholar]
- Ching T, Ha J, Song MA, Tiirikainen M, Molnar J, Berry MJ, . . . Garmire LX (2015). Genome-scale hypomethylation in the cord blood DNAs associated with early onset preeclampsia. Clin Epigenetics, 7(1), 21. doi: 10.1186/s13148-015-0052-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CS, & Myrianthopoulos NC (1975). Factors affecting risks of congenital malformations. II. Effect of maternal diabetes on congenital malformations. Birth Defects Orig Artic Ser, 11(10), 23–38. [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, . . . Reece EA (2008a). Diabetes mellitus and birth defects. Am J Obstet Gynecol, 199(3), 237.e231–239. doi: 10.1016/j.ajog.2008.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, . . . Reece EA (2008b). Diabetes mellitus and birth defects. Am J Obstet Gynecol, 199(3), 237 e231–239. doi: 10.1016/j.ajog.2008.06.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croke L. (2019). Managing Chronic Hypertension in Pregnant Women: ACOG Releases Updated Practice Bulletin. Am Fam Physician, 100(12), 782–783. [PubMed] [Google Scholar]
- Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, & Robinson JS (2005). Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med, 352(24), 2477–2486. doi: 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- Dachew BA, Mamun A, Maravilla JC, & Alati R. (2018). Association between hypertensive disorders of pregnancy and the development of offspring mental and behavioural problems: A systematic review and meta-analysis. Psychiatry Res, 260, 458–467. doi: 10.1016/j.psychres.2017.12.027 [DOI] [PubMed] [Google Scholar]
- Dachew BA, Scott JG, Betts K, Mamun A, & Alati R. (2020). Hypertensive disorders of pregnancy and the risk of offspring depression in childhood: Findings from the Avon Longitudinal Study of Parents and Children. Dev Psychopathol, 32(3), 845–851. doi: 10.1017/s0954579419000944 [DOI] [PubMed] [Google Scholar]
- Dachew BA, Scott JG, Mamun A, & Alati R. (2019). Pre-eclampsia and the risk of attention-deficit/hyperactivity disorder in offspring: Findings from the ALSPAC birth cohort study. Psychiatry Res, 272, 392–397. doi: 10.1016/j.psychres.2018.12.123 [DOI] [PubMed] [Google Scholar]
- Dixon MJ, Marazita ML, Beaty TH, & Murray JC (2011). Cleft lip and palate: understanding genetic and environmental influences. Nature Reviews Genetics, 12(3), 167–178. doi: 10.1038/nrg2933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson UJ, Håkan Borg LA, Cederberg J, Nordstrand H, Simán CM, Wentzel C, Wentzel P, 2000. Pathogenesis of Diabetes-Induced Congenital Malformations. Upsala Journal of Medical Sciences 105, 53–84. [DOI] [PubMed] [Google Scholar]
- Gao C, & Chen YG (2010). Dishevelled: The hub of Wnt signaling. Cell Signal, 22(5), 717–727. doi: 10.1016/j.cellsig.2009.11.021 [DOI] [PubMed] [Google Scholar]
- Garland MA, Reynolds K, & Zhou CJ (2020). Environmental mechanisms of orofacial clefts. Birth Defects Res, 112(19), 1660–1698. doi: 10.1002/bdr2.1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland MA, Sun B, Zhang S, Reynolds K, Ji Y, & Zhou CJ (2020). Role of epigenetics and miRNAs in orofacial clefts. Birth Defects Res, 112(19), 1635–1659. doi: 10.1002/bdr2.1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gathiram P, Moodley J, 2016. Pre-eclampsia: its pathogenesis and pathophysiolgy. Cardiovasc J Afr 27, 71–78. 10.5830/CVJA-2016-009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildestad T, Bjørge T, Vollset SE, Klungsøyr K, Nilsen RM, Haaland Ø A, & Øyen N. (2015). Folic acid supplements and risk for oral clefts in the newborn: a population-based study. Br J Nutr, 114(9), 1456–1463. doi: 10.1017/s0007114515003013 [DOI] [PubMed] [Google Scholar]
- Grieger JA, Bianco-Miotto T, Grzeskowiak LE, Leemaqz SY, Poston L, McCowan LM, . . . Roberts CT (2018). Metabolic syndrome in pregnancy and risk for adverse pregnancy outcomes: A prospective cohort of nulliparous women. PLoS Med, 15(12), e1002710. doi: 10.1371/journal.pmed.1002710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harville EW, Wilcox AJ, Lie RT, Vindenes H, & Abyholm F. (2005). Cleft lip and palate versus cleft lip only: are they distinct defects? Am J Epidemiol, 162(5), 448–453. doi: 10.1093/aje/kwi214 [DOI] [PubMed] [Google Scholar]
- He J, Zhang A, Fang M, Fang R, Ge J, Jiang Y, . . . Dong M. (2013). Methylation levels at IGF2 and GNAS DMRs in infants born to preeclamptic pregnancies. BMC Genomics, 14, 472. doi: 10.1186/1471-2164-14-472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera E, Amusquivar E, López-Soldado I, & Ortega H. (2006). Maternal lipid metabolism and placental lipid transfer. Horm Res, 65 Suppl 3, 59–64. doi: 10.1159/000091507 [DOI] [PubMed] [Google Scholar]
- Hu W, Weng X, Dong M, Liu Y, Li W, & Huang H. (2014). Alteration in methylation level at 11β-hydroxysteroid dehydrogenase type 2 gene promoter in infants born to preeclamptic women. BMC Genet, 15, 96. doi: 10.1186/s12863-014-0096-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL (2009). A comprehensive definition for metabolic syndrome. Disease models & mechanisms, 2(5–6), 231–237. doi: 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheon JA, Lisonkova S, & Joseph KS (2011). Epidemiology of pre-eclampsia and the other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol, 25(4), 391–403. doi: 10.1016/j.bpobgyn.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Beaty TH, Panny SR, Street NA, Joseph JM, Gordon S, . . . Francomano CA (1995). Association study of transforming growth factor alpha (TGF alpha) TaqI polymorphism and oral clefts: indication of gene-environment interaction in a population-based sample of infants with birth defects. Am J Epidemiol, 141(7), 629–636. doi: 10.1093/oxfordjournals.aje.a117478 [DOI] [PubMed] [Google Scholar]
- Iwata J, Suzuki A, Pelikan RC, Ho TV, Sanchez-Lara PA, & Chai Y. (2014). Modulation of lipid metabolic defects rescues cleft palate in Tgfbr2 mutant mice. Hum Mol Genet, 23(1), 182–193. doi: 10.1093/hmg/ddt410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaya C, Suzuki A, & Iwata J. (2023). MicroRNAs and Gene Regulatory Networks Related to Cleft Lip and Palate. Int J Mol Sci, 24(4). doi: 10.3390/ijms24043552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L, Brkić J, Liu M, Fu G, Peng C, & Wang YL (2013). Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Mol Aspects Med, 34(5), 981–1023. doi: 10.1016/j.mam.2012.12.008 [DOI] [PubMed] [Google Scholar]
- Ji Y, Garland MA, Sun B, Zhang S, Reynolds K, McMahon M, . . . Zhou CJ (2020). Cellular and developmental basis of orofacial clefts. Birth Defects Res, 112(19), 1558–1587. doi: 10.1002/bdr2.1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen AMW, Lie RT, Wilcox AJ, Andersen LF, & Drevon CA (2008). Maternal Dietary Intake of Vitamin A and Risk of Orofacial Clefts: A Population-based Case-Control Study in Norway. American Journal of Epidemiology, 167(10), 1164–1170. doi: 10.1093/aje/kwn035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmi N, Sharp GC, Reese SE, Vehmeijer FO, Lahti J, Page CM, . . . Relton CL (2019). Hypertensive Disorders of Pregnancy and DNA Methylation in Newborns. Hypertension, 74(2), 375–383. doi: 10.1161/hypertensionaha.119.12634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Sakurai T, Miura F, Imai M, Mochiduki K, Yanagisawa E, . . . Kono T. (2013). High-resolution DNA methylome analysis of primordial germ cells identifies gender-specific reprogramming in mice. Genome Res, 23(4), 616–627. doi: 10.1101/gr.148023.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komiya Y, & Habas R. (2008). Wnt signal transduction pathways. Organogenesis, 4(2), 68–75. doi: 10.4161/org.4.2.5851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krakowiak P, Walker CK, Bremer AA, Baker AS, Ozonoff S, Hansen RL, & Hertz-Picciotto I. (2012). Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics, 129(5), e1121–1128. doi: 10.1542/peds.2011-2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama M, Udagawa A, Yoshimoto S, Ichinose M, Sato K, Yamazaki K, . . . Mori C. (2008). DNA methylation changes during cleft palate formation induced by retinoic acid in mice. Cleft Palate Craniofac J, 45(5), 545–551. doi: 10.1597/07-134.1 [DOI] [PubMed] [Google Scholar]
- Kutbi H, Wehby GL, Moreno Uribe LM, Romitti PA, Carmichael S, Shaw GM, . . . Munger RG (2017). Maternal underweight and obesity and risk of orofacial clefts in a large international consortium of population-based studies. Int J Epidemiol, 46(1), 190–199. doi: 10.1093/ije/dyw035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, . . . Anderson GB (2009). A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med, 361(14), 1339–1348. doi: 10.1056/NEJMoa0902430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanthaler B, Steichen-Gersdorf E, Kollerits B, Zschocke J, & Witsch-Baumgartner M. (2013). Maternal ABCA1 genotype is associated with severity of Smith-Lemli-Opitz syndrome and with viability of patients homozygous for null mutations. Eur J Hum Genet, 21(3), 286–293. doi: 10.1038/ejhg.2012.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie EJ, Liu H, Carlson JC, Shaffer JR, Feingold E, Wehby G, . . . Marazita ML (2016). A Genome-wide Association Study of Nonsyndromic Cleft Palate Identifies an Etiologic Missense Variant in GRHL3. Am J Hum Genet, 98(4), 744–754. doi: 10.1016/j.ajhg.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesseur C, & Chen J. (2018). Adverse Maternal Metabolic Intrauterine Environment and Placental Epigenetics: Implications for Fetal Metabolic Programming. Curr Environ Health Rep, 5(4), 531–543. doi: 10.1007/s40572-018-0217-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CC, Young PE, Maloney CA, Eaton SA, Cowley MJ, Buckland ME, . . . Suter CM (2013). Maternal obesity and diabetes induces latent metabolic defects and widespread epigenetic changes in isogenic mice. Epigenetics, 8(6), 602–611. doi: 10.4161/epi.24656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Chase M, Jung SK, Smith PJ, & Loeken MR (2005). Hypoxic stress in diabetic pregnancy contributes to impaired embryo gene expression and defective development by inducing oxidative stress. Am J Physiol Endocrinol Metab, 289(4), E591–599. doi: 10.1152/ajpendo.00441.2004 [DOI] [PubMed] [Google Scholar]
- Lin W, Zhang Z, Srajer G, Chen YC, Huang M, Phan HM, & Dent SY (2008). Proper expression of the Gcn5 histone acetyltransferase is required for neural tube closure in mouse embryos. Dev Dyn, 237(4), 928–940. doi: 10.1002/dvdy.21479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Rouleau J, León JA, Sauve R, Joseph KS, & Ray JG (2015). Impact of pre-pregnancy diabetes mellitus on congenital anomalies, Canada, 2002–2012. Health Promot Chronic Dis Prev Can, 35(5), 79–84. doi: 10.24095/hpcdp.35.5.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo JO, Mission JF, & Caughey AB (2013). Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gynecol, 25(2), 124–132. doi: 10.1097/GCO.0b013e32835e0ef5 [DOI] [PubMed] [Google Scholar]
- Maher GM, O’Keeffe GW, Kearney PM, Kenny LC, Dinan TG, Mattsson M, & Khashan AS (2018). Association of Hypertensive Disorders of Pregnancy With Risk of Neurodevelopmental Disorders in Offspring: A Systematic Review and Meta-analysis. JAMA Psychiatry, 75(8), 809–819. doi: 10.1001/jamapsychiatry.2018.0854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold E, Ludwig KU, Birnbaum S, Baluardo C, Ferrian M, Herms S, . . . Nöthen MM (2010). Genome-wide association study identifies two susceptibility loci for nonsyndromic cleft lip with or without cleft palate. Nature Genetics, 42(1), 24–26. doi: 10.1038/ng.506 [DOI] [PubMed] [Google Scholar]
- Marchincin SL, Howley MM, Van Zutphen AR, Fisher SC, Nestoridi E, Tinker SC, & Browne ML (2023). Risk of birth defects by pregestational type 1 or type 2 diabetes: National Birth Defects Prevention Study, 1997–2011. Birth Defects Res, 115(1), 56–66. doi: 10.1002/bdr2.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Frias ML, Frias JP, Bermejo E, Rodriguez-Pinilla E, Prieto L, & Frias JL (2005). Pre-gestational maternal body mass index predicts an increased risk of congenital malformations in infants of mothers with gestational diabetes. Diabet Med, 22(6), 775–781. doi: 10.1111/j.1464-5491.2005.01492.x [DOI] [PubMed] [Google Scholar]
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE, Scambler PJ, Bassett AS, 2015. 22q11.2 deletion syndrome. Nature Reviews Disease Primers 1, 15071. 10.1038/nrdp.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior H, Kurch-Bek D, & Mund M. (2017). The Prevalence of Gestational Diabetes. Deutsches Arzteblatt international, 114(24), 412–418. doi: 10.3238/arztebl.2017.0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmidt DM, Knowles BB, & Solter D. (2014). DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev, 28(8), 812–828. doi: 10.1101/gad.234294.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, . . . Sacks DA (2008). Hyperglycemia and adverse pregnancy outcomes. N Engl J Med, 358(19), 1991–2002. doi: 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- Mikhail LN, Walker CK, & Mittendorf R. (2002). Association between maternal obesity and fetal cardiac malformations in African Americans. J Natl Med Assoc, 94(8), 695–700. [PMC free article] [PubMed] [Google Scholar]
- Moore LL, Singer MR, Bradlee ML, Rothman KJ, & Milunsky A. (2000). A prospective study of the risk of congenital defects associated with maternal obesity and diabetes mellitus. Epidemiology, 11(6), 689–694. doi: 10.1097/00001648-200011000-00013 [DOI] [PubMed] [Google Scholar]
- Mossey PA, & Modell B. (2012). Epidemiology of oral clefts 2012: an international perspective. Front Oral Biol, 16, 1–18. doi: 10.1159/000337464 [DOI] [PubMed] [Google Scholar]
- Murphy SP, & Abrams BF (1993). Changes in energy intakes during pregnancy and lactation in a national sample of US women. Am J Public Health, 83(8), 1161–1163. doi: 10.2105/ajph.83.8.1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Inloes JB, Katagiri T, & Kobayashi T. (2011). Chondrocyte-specific microRNA-140 regulates endochondral bone development and targets Dnpep to modulate bone morphogenetic protein signaling. Mol Cell Biol, 31(14), 3019–3028. doi: 10.1128/mcb.05178-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCEP. (2002). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation, 106(25), 3143–3421. [PubMed] [Google Scholar]
- NHBPEP. (2000). Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol, 183(1), S1–s22. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, & Flegal KM (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. Jama, 311(8), 806–814. doi: 10.1001/jama.2014.732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacín M, Lasunción MA, Asunción M, & Herrera E. (1991). Circulating metabolite utilization by periuterine adipose tissue in situ in the pregnant rat. Metabolism, 40(5), 534–539. doi: 10.1016/0026-0495(91)90237-q [DOI] [PubMed] [Google Scholar]
- Pan MH, Zhu CC, Ju JQ, Xu Y, Luo SM, Sun SC, & Ou XH (2020). Single-cell transcriptome analysis reveals that maternal obesity affects DNA repair, histone methylation, and autophagy level in mouse embryos. J Cell Physiol. doi: 10.1002/jcp.30201 [DOI] [PubMed] [Google Scholar]
- Park JH, Stoffers DA, Nicholls RD, & Simmons RA (2008). Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest, 118(6), 2316–2324. doi: 10.1172/jci33655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn A, McPherson N, Fullston T, Arman B, & Zander-Fox D. (2023). Maternal high-fat diet changes DNA methylation in the early embryo by disrupting the TCA cycle intermediary alpha ketoglutarate. Reproduction, 165(4), 347–362. doi: 10.1530/REP-22-0302 [DOI] [PubMed] [Google Scholar]
- Poston L, Caleyachetty R, Cnattingius S, Corvalán C, Uauy R, Herring S, & Gillman MW (2016). Preconceptional and maternal obesity: epidemiology and health consequences. Lancet Diabetes Endocrinol, 4(12), 1025–1036. doi: 10.1016/s2213-8587(16)30217-0 [DOI] [PubMed] [Google Scholar]
- Rasmussen K. (2012). Public health policies relating to obesity in childbearing women. In (pp. 237–244). [Google Scholar]
- Rasmussen S, & Irgens LM (2003). Fetal growth and body proportion in preeclampsia. Obstet Gynecol, 101(3), 575–583. doi: 10.1016/s0029-7844(02)03071-5 [DOI] [PubMed] [Google Scholar]
- Ray JG, Wyatt PR, Vermeulen MJ, Meier C, & Cole DE (2005). Greater maternal weight and the ongoing risk of neural tube defects after folic acid flour fortification. Obstet Gynecol, 105(2), 261–265. doi: 10.1097/01.AOG.0000151988.84346.3e [DOI] [PubMed] [Google Scholar]
- Rayanagoudar G, Hashi AA, Zamora J, Khan KS, Hitman GA, & Thangaratinam S. (2016). Quantification of the type 2 diabetes risk in women with gestational diabetes: a systematic review and meta-analysis of 95,750 women. Diabetologia, 59(7), 1403–1411. doi: 10.1007/s00125-016-3927-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Kumari P, Sepulveda Rincon L, Gu R, Ji Y, Kumar S, & Zhou CJ (2019). Wnt signaling in orofacial clefts: crosstalk, pathogenesis and models. Disease models & mechanisms, 12(2). doi: 10.1242/dmm.037051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Zhang S, Sun B, Garland MA, Ji Y, & Zhou CJ (2020). Genetics and signaling mechanisms of orofacial clefts. Birth Defects Res, 112(19), 1588–1634. doi: 10.1002/bdr2.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo HE, Escaname EN, Alana NB, Lavender E, Gelfond J, Fernandez R, . . . Blanco CL (2020). Maternal diabetes and obesity influence the fetal epigenome in a largely Hispanic population. Clin Epigenetics, 12(1), 34. doi: 10.1186/s13148-020-0824-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JM, Francis BM, Sulik KK, Alles AJ, Massaro EJ, Zucker RM, . . . Chernoff N. (1994). Cell death and cell cycle perturbation in the developmental toxicity of the demethylating agent, 5-aza-2’-deoxycytidine. Teratology, 50(5), 332–339. doi: 10.1002/tera.1420500504 [DOI] [PubMed] [Google Scholar]
- Schäfer-Graf UM, Gembruch U, Kainer F, Groten T, Hummel S, Hösli I, . . . Bancher-Todesca D. (2018). Gestational Diabetes Mellitus (GDM) - Diagnosis, Treatment and Follow-Up. Guideline of the DDG and DGGG (S3 Level, AWMF Registry Number 057/008, February 2018). Geburtshilfe und Frauenheilkunde, 78(12), 1219–1231. doi: 10.1055/a-0659-2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelan RS, Pisano MM, & Greene RM (2022). MicroRNAs as epigenetic regulators of orofacial development. Differentiation, 124, 1–16. doi: 10.1016/j.diff.2022.01.002 [DOI] [PubMed] [Google Scholar]
- Shapira Y, Lubit E, Kuftinec MM, & Borell G. (1999). The distribution of clefts of the primary and secondary palates by sex, type, and location. Angle Orthod, 69(6), 523–528. doi: [DOI] [PubMed] [Google Scholar]
- Shaw GM, Todoroff K, Schaffer DM, & Selvin S. (2000). Maternal height and prepregnancy body mass index as risk factors for selected congenital anomalies. Paediatr Perinat Epidemiol, 14(3), 234–239. doi: 10.1046/j.1365-3016.2000.00274.x [DOI] [PubMed] [Google Scholar]
- Simán CM, Gittenberger-De Groot AC, Wisse B, Eriksson UJ, 2000. Malformations in offspring of diabetic rats: Morphometric analysis of neural crest-derived organs and effects of maternal vitamin E treatment. Teratology 61, 355–367. [DOI] [PubMed] [Google Scholar]
- Sircar M, Thadhani R, & Karumanchi SA (2015). Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens, 24(2), 131–138. doi: 10.1097/mnh.0000000000000105 [DOI] [PubMed] [Google Scholar]
- Smith ZD, Chan MM, Mikkelsen TS, Gu H, Gnirke A, Regev A, & Meissner A. (2012). A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature, 484(7394), 339–344. doi: 10.1038/nature10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilson SV, Kim HJ, & Chung KC (2001). Association between maternal diabetes mellitus and newborn oral cleft. Ann Plast Surg, 47(5), 477–481. doi: 10.1097/00000637-200111000-00001 [DOI] [PubMed] [Google Scholar]
- Stoll C, Alembik Y, Dott B, & Roth MP (2000). Associated malformations in cases with oral clefts. Cleft Palate Craniofac J, 37(1), 41–47. doi: 10.1597/1545-1569_2000_037_0041_amicwo_2.3.co_2 [DOI] [PubMed] [Google Scholar]
- Stothard KJ, Tennant PW, Bell R, & Rankin J. (2009). Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. Jama, 301(6), 636–650. doi: 10.1001/jama.2009.113 [DOI] [PubMed] [Google Scholar]
- Stott-Miller M, Heike CL, Kratz M, & Starr JR (2010). Increased risk of orofacial clefts associated with maternal obesity: case-control study and Monte Carlo-based bias analysis. Paediatr Perinat Epidemiol, 24(5), 502–512. doi: 10.1111/j.1365-3016.2010.01142.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Li A, Gajera M, Abdallah N, Zhang M, Zhao Z, & Iwata J. (2019). MicroRNA-374a, −4680, and −133b suppress cell proliferation through the regulation of genes associated with human cleft palate in cultured human palate cells. BMC Med Genomics, 12(1), 93. doi: 10.1186/s12920-019-0546-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarr JT, Lambi AG, Bradley JP, Barbe MF, & Popoff SN (2018). Development of Normal and Cleft Palate: A Central Role for Connective Tissue Growth Factor (CTGF)/CCN2. J Dev Biol, 6(3). doi: 10.3390/jdb6030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker SC, Gilboa SM, Moore CA, Waller DK, Simeone RM, Kim SY, . . . Reefhuis J. (2020). Specific birth defects in pregnancies of women with diabetes: National Birth Defects Prevention Study, 1997–2011. Am J Obstet Gynecol, 222(2), 176.e171–176.e111. doi: 10.1016/j.ajog.2019.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolarová MM, & Cervenka J. (1998). Classification and birth prevalence of orofacial clefts. Am J Med Genet, 75(2), 126–137. [PubMed] [Google Scholar]
- Tomé M, López-Romero P, Albo C, Sepúlveda JC, Fernández-Gutiérrez B, Dopazo A, . . . González MA (2011). miR-335 orchestrates cell proliferation, migration and differentiation in human mesenchymal stem cells. Cell Death Differ, 18(6), 985–995. doi: 10.1038/cdd.2010.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villamor E, Sparén P, & Cnattingius S. (2008). Risk of oral clefts in relation to prepregnancy weight change and interpregnancy interval. Am J Epidemiol, 167(11), 1305–1311. doi: 10.1093/aje/kwn065 [DOI] [PubMed] [Google Scholar]
- Vucetic Z, Kimmel J, Totoki K, Hollenbeck E, & Reyes TM (2010). Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology, 151(10), 4756–4764. doi: 10.1210/en.2010-0505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vujkovic M, Ocke MC, van der Spek PJ, Yazdanpanah N, Steegers EA, & Steegers-Theunissen RP (2007). Maternal Western dietary patterns and the risk of developing a cleft lip with or without a cleft palate. Obstet Gynecol, 110(2 Pt 1), 378–384. doi: 10.1097/01.AOG.0000268799.37044.c3 [DOI] [PubMed] [Google Scholar]
- Wagschal A, Sutherland HG, Woodfine K, Henckel A, Chebli K, Schulz R, . . . Feil R. (2008). G9a histone methyltransferase contributes to imprinting in the mouse placenta. Mol Cell Biol, 28(3), 1104–1113. doi: 10.1128/mcb.01111-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace K, Harris S, & Bean C. (2019). Chapter 10 - The Cerebral Circulation During Pregnancy and Preeclampsia. In LaMarca B. & Alexander BT (Eds.), Sex Differences in Cardiovascular Physiology and Pathophysiology (pp. 149–163): Academic Press. [Google Scholar]
- Waller DK, Shaw GM, Rasmussen SA, Hobbs CA, Canfield MA, Siega-Riz AM, . . . Correa A. (2007). Prepregnancy obesity as a risk factor for structural birth defects. Arch Pediatr Adolesc Med, 161(8), 745–750. doi: 10.1001/archpedi.161.8.745 [DOI] [PubMed] [Google Scholar]
- Wang F, Fisher SA, Zhong J, Wu Y, & Yang P. (2015). Superoxide Dismutase 1 In Vivo Ameliorates Maternal Diabetes Mellitus-Induced Apoptosis and Heart Defects Through Restoration of Impaired Wnt Signaling. Circulation. Cardiovascular genetics, 8(5), 665–676. doi: 10.1161/CIRCGENETICS.115.001138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Jiang L, Wang J, Li S, Yu Y, You J, . . . Liu Y. (2009). Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology, 49(4), 1166–1175. doi: 10.1002/hep.22774 [DOI] [PubMed] [Google Scholar]
- Watkins ML, Rasmussen SA, Honein MA, Botto LD, & Moore CA (2003). Maternal obesity and risk for birth defects. Pediatrics, 111(5 Pt 2), 1152–1158. [PubMed] [Google Scholar]
- Watkins SE, Meyer RE, Strauss RP, & Aylsworth AS (2014). Classification, epidemiology, and genetics of orofacial clefts. Clin Plast Surg, 41(2), 149–163. doi: 10.1016/j.cps.2013.12.003 [DOI] [PubMed] [Google Scholar]
- Weber KA, Mayo JA, Carmichael SL, Stevenson DK, Winn VD, & Shaw GM (2018). Occurrence of Selected Structural Birth Defects Among Women With Preeclampsia and Other Hypertensive Disorders. American Journal of Epidemiology, 187(4), 668–676. doi: 10.1093/aje/kwx269 [DOI] [PubMed] [Google Scholar]
- Wei D, & Loeken MR (2014). Increased DNA methyltransferase 3b (Dnmt3b)-mediated CpG island methylation stimulated by oxidative stress inhibits expression of a gene required for neural tube and neural crest development in diabetic pregnancy. Diabetes, 63(10), 3512–3522. doi: 10.2337/db14-0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, & Thompson CB (2009). ATP-citrate lyase links cellular metabolism to histone acetylation. Science, 324(5930), 1076–1080. doi: 10.1126/science.1164097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workalemahu T, Ouidir M, Shrestha D, Wu J, Grantz KL, & Tekola-Ayele F. (2020). Differential DNA Methylation in Placenta Associated With Maternal Blood Pressure During Pregnancy. Hypertension, 75(4), 1117–1124. doi:doi: 10.1161/HYPERTENSIONAHA.119.14509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Liu B, Sun Y, Du Y, Santillan MK, Santillan DA, . . . Bao W. (2020). Association of Maternal Prepregnancy Diabetes and Gestational Diabetes Mellitus With Congenital Anomalies of the Newborn. Diabetes Care, 43(12), 2983–2990. doi: 10.2337/dc20-0261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Li C, Liu X, & Gao S. (2021). Insights into epigenetic patterns in mammalian early embryos. Protein Cell, 12(1), 7–28. doi: 10.1007/s13238-020-00757-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Reece EA, Wang F, & Gabbay-Benziv R. (2015). Decoding the oxidative stress hypothesis in diabetic embryopathy through proapoptotic kinase signaling. Am J Obstet Gynecol, 212(5), 569–579. doi: 10.1016/j.ajog.2014.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Q, Cao S, Li C, Mengesha A, Kong B, & Wei M. (2011). Micro-RNA-21 regulates TGF-β-induced myofibroblast differentiation by targeting PDCD4 in tumor-stroma interaction. Int J Cancer, 128(8), 1783–1792. doi: 10.1002/ijc.25506 [DOI] [PubMed] [Google Scholar]
- Yu Y, Zuo X, He M, Gao J, Fu Y, Qin C, . . . Bian Z. (2017). Genome-wide analyses of non-syndromic cleft lip with palate identify 14 novel loci and genetic heterogeneity. Nature communications, 8, 14364–14364. doi: 10.1038/ncomms14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang TN, Huang XM, Zhao XY, Wang W, Wen R, & Gao SY (2022). Risks of specific congenital anomalies in offspring of women with diabetes: A systematic review and meta-analysis of population-based studies including over 80 million births. PLoS Med, 19(2), e1003900. doi: 10.1371/journal.pmed.1003900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhao H, Chen J, Zhong X, Zeng W, Zhang B, . . . Tang S. (2020). A LCMS-based untargeted lipidomics analysis of cleft palate in mouse. Mechanisms of Development, 162, 103609. doi: 10.1016/j.mod.2020.103609 [DOI] [PubMed] [Google Scholar]
- Zhou M, & Walker BE (1993). Potentiation of triamcinolone-induced cleft palate in mice by maternal high dietary fat. Teratology, 48(1), 53–57. doi: 10.1002/tera.1420480109 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Sinnathamby V, Yu Y, Sikora L, Johnson CY, Mossey P, & Little J. (2020). Folate intake, markers of folate status and oral clefts: An updated set of systematic reviews and meta-analyses. Birth Defects Res, 112(19), 1699–1719. doi: 10.1002/bdr2.1827 [DOI] [PubMed] [Google Scholar]