Abstract
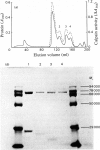
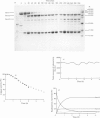
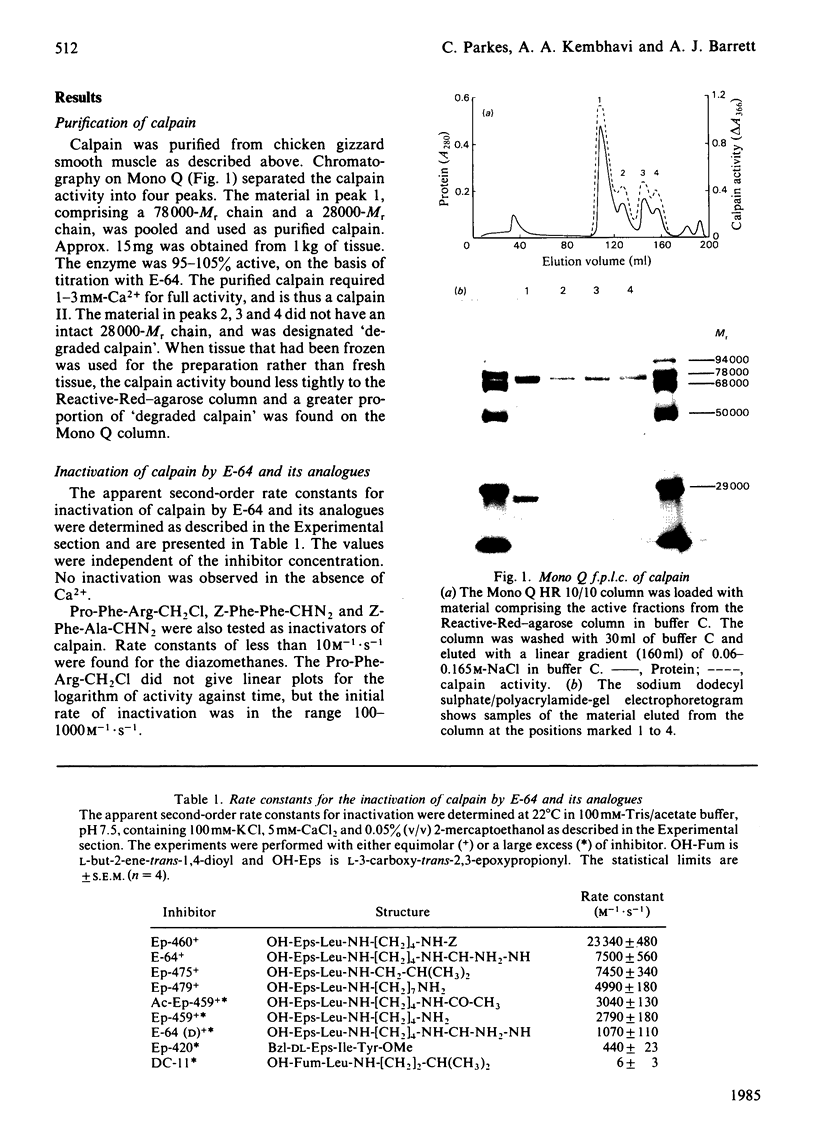
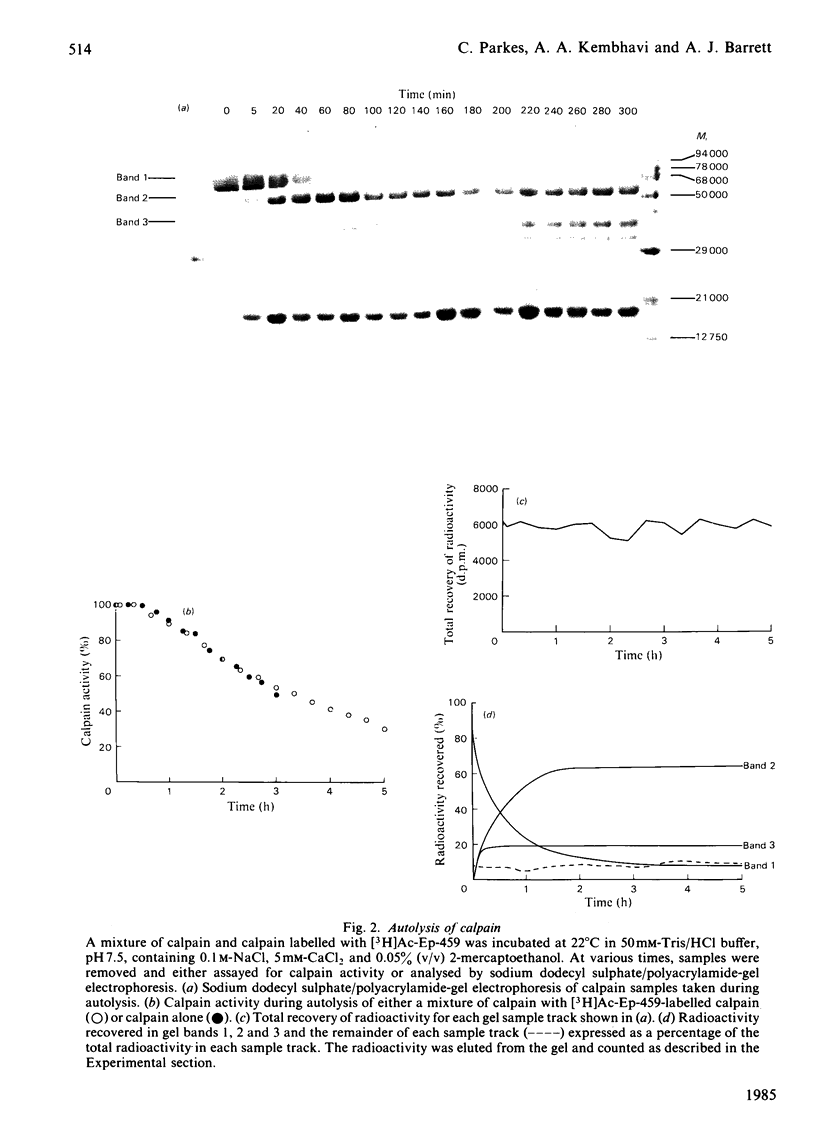
A Ca2+-activated cysteine proteinase (calpain II) was purified from chicken gizzard smooth muscle by use of isoelectric precipitation, (NH4)2SO4 fractionation, chromatography on DEAE-Sepharose CL-6B, Reactive-Red 120-agarose and Mono Q. The apparent second-order rate constants for the inactivation of calpain by a series of structural analogues of L-3-carboxy-trans-2, 3-epoxypropionyl-leucylamido-(4-guanidino)butane (E-64) were determined. The fastest rate of inactivation was observed with L-3-carboxy-trans-2, 3-epoxypropionyl-leucylamido-(4-benzyloxy-carbonylamino)buta ne. It was possible to determine the active-site molarity of solutions of calpain by titration with E-64. When incubated with Ca2+, calpain underwent several steps of intermolecular limited proteolysis, via multiple pathways, followed by a slower loss of enzymic activity. The proteolytic steps preceding the loss of activity did not affect the rates of reaction of calpain with E-64 analogues.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Dayton W. R. Comparison of low- and high-calcium-requiring forms of the calcium-activated protease with their autocatalytic breakdown products. Biochim Biophys Acta. 1982 Dec 20;709(2):166–172. doi: 10.1016/0167-4838(82)90457-5. [DOI] [PubMed] [Google Scholar]
- Dayton W. R., Reville W. J., Goll D. E., Stromer M. H. A Ca2+-activated protease possibly involved in myofibrillar protein turnover. Partial characterization of the purified enzyme. Biochemistry. 1976 May 18;15(10):2159–2167. doi: 10.1021/bi00655a020. [DOI] [PubMed] [Google Scholar]
- Hathaway D. R., Werth D. K., Haeberle J. R. Limited autolysis reduces the Ca2+ requirement of a smooth muscle Ca2+-activated protease. J Biol Chem. 1982 Aug 10;257(15):9072–9077. [PubMed] [Google Scholar]
- Hirao T., Takahashi K. Purification and characterization of a calcium-activated neutral protease from monkey brain and its action on neuropeptides. J Biochem. 1984 Sep;96(3):775–784. doi: 10.1093/oxfordjournals.jbchem.a134895. [DOI] [PubMed] [Google Scholar]
- Huston R. B., Krebs E. G. Activation of skeletal muscle phosphorylase kinase by Ca2+. II. Identification of the kinase activating factor as a proteolytic enzyme. Biochemistry. 1968 Jun;7(6):2116–2122. doi: 10.1021/bi00846a014. [DOI] [PubMed] [Google Scholar]
- Ishiura S., Sugita H., Suzuki K., Imahori K. Studies of a calcium-activated neutral protease from chicken skeletal muscle. II. Substrate specificity. J Biochem. 1979 Aug;86(2):579–581. doi: 10.1093/oxfordjournals.jbchem.a132558. [DOI] [PubMed] [Google Scholar]
- Kamakura K., Ishiura S., Sugita H., Toyokura Y. Identification of Ca2+-activated neutral protease in the peripheral nerve and its effects on neurofilament degeneration. J Neurochem. 1983 Apr;40(4):908–913. doi: 10.1111/j.1471-4159.1983.tb08072.x. [DOI] [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Shiota M., Nishizuka Y. Proteolytic activation of calcium-activated, phospholipid-dependent protein kinase by calcium-dependent neutral protease. J Biol Chem. 1983 Jan 25;258(2):1156–1164. [PubMed] [Google Scholar]
- Kishimoto A., Kajikawa N., Tabuchi H., Shiota M., Nishizuka Y. Calcium-dependent neural proteases, widespread occurrence of a species of protease active at lower concentrations of calcium. J Biochem. 1981 Sep;90(3):889–892. doi: 10.1093/oxfordjournals.jbchem.a133547. [DOI] [PubMed] [Google Scholar]
- Klein I., Lehotay D., Gondek M. Characterization of a calcium-activated protease that hydrolyzes a microtubule-associated protein. Arch Biochem Biophys. 1981 May;208(2):520–527. doi: 10.1016/0003-9861(81)90540-3. [DOI] [PubMed] [Google Scholar]
- McGowan E. B., Yeo K. T., Detwiler T. C. The action of calcium-dependent protease on platelet surface glycoproteins. Arch Biochem Biophys. 1983 Nov;227(1):287–301. doi: 10.1016/0003-9861(83)90373-9. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L. Canine cardiac calcium-dependent proteases: Resolution of two forms with different requirements for calcium. FEBS Lett. 1980 Jan 1;109(1):129–133. doi: 10.1016/0014-5793(80)81326-3. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L., Repetti A., Muck T. C., Easly J. Rabbit skeletal muscle calcium-dependent protease requiring millimolar CA2+. Purification, subunit structure, and Ca2+-dependent autoproteolysis. J Biol Chem. 1982 Jun 25;257(12):7203–7209. [PubMed] [Google Scholar]
- Murayama A., Fukai F., Murachi T. Action of calpain on the basic estrogen receptor molecule of porcine uterus. J Biochem. 1984 Jun;95(6):1697–1704. doi: 10.1093/oxfordjournals.jbchem.a134783. [DOI] [PubMed] [Google Scholar]
- Nelson W. J., Traub P. Purification and further characterization of the Ca2+-activated proteinase specific for the intermediate filament proteins vimentin and desmin. J Biol Chem. 1982 May 25;257(10):5544–5553. [PubMed] [Google Scholar]
- Ohno S., Emori Y., Imajoh S., Kawasaki H., Kisaragi M., Suzuki K. Evolutionary origin of a calcium-dependent protease by fusion of genes for a thiol protease and a calcium-binding protein? Nature. 1984 Dec 6;312(5994):566–570. doi: 10.1038/312566a0. [DOI] [PubMed] [Google Scholar]
- Pant H. C., Virmani M., Gallant P. E. Calcium-induced proteolysis of spectrin and band 3 protein in rat erythrocyte membranes. Biochem Biophys Res Commun. 1983 Dec 16;117(2):372–377. doi: 10.1016/0006-291x(83)91210-x. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Kikuchi T., Yumoto N., Yoshimura N., Murachi T. Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J Biol Chem. 1984 Oct 25;259(20):12489–12494. [PubMed] [Google Scholar]
- Sasaki T., Yoshimura N., Kikuchi T., Hatanaka M., Kitahara A., Sakihama T., Murachi T. Similarity and dissimilarity in subunit structures of calpains I and II from various sources as demonstrated by immunological cross-reactivity. J Biochem. 1983 Dec;94(6):2055–2061. doi: 10.1093/oxfordjournals.jbchem.a134561. [DOI] [PubMed] [Google Scholar]
- Sugita H., Ishiura S., Suzuki K., Imahori K. Inhibition of epoxide derivatives on chicken calcium-activated neutral protease (CANP) in vitro and in vivo. J Biochem. 1980 Jan;87(1):339–341. doi: 10.1093/oxfordjournals.jbchem.a132742. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Hayashi H., Hayashi T., Iwai K. Amino acid sequence around the active site cysteine residue of calcium-activated neutral protease (CANP). FEBS Lett. 1983 Feb 7;152(1):67–70. doi: 10.1016/0014-5793(83)80483-9. [DOI] [PubMed] [Google Scholar]
- Suzuki K. Reaction of calcium-activated neutral protease (CANP) with an epoxysuccinyl derivative (E64c) and iodoacetic acid. J Biochem. 1983 May;93(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a134264. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Tsuji S., Ishiura S. Effect of Ca2+ on the inhibition of calcium-activated neutral protease by leupeptin, antipain and epoxysuccinate derivatives. FEBS Lett. 1981 Dec 21;136(1):119–122. doi: 10.1016/0014-5793(81)81227-6. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Tsuji S., Ishiura S., Kimura Y., Kubota S., Imahori K. Autolysis of calcium-activated neutral protease of chicken skeletal muscle. J Biochem. 1981 Dec;90(6):1787–1793. doi: 10.1093/oxfordjournals.jbchem.a133656. [DOI] [PubMed] [Google Scholar]
- Takai Y., Yamamoto M., Inoue M., Kishimoto A., Nishizuka Y. A proenzyme of cyclic nucleotide-independent protein kinase and its activation by calcium-dependent neutral protease from rat liver. Biochem Biophys Res Commun. 1977 Jul 25;77(2):542–550. doi: 10.1016/s0006-291x(77)80013-2. [DOI] [PubMed] [Google Scholar]
- Tsuji S., Imahori K. Studies on the Ca2+-activated neutral proteinase of rabbit skeletal muscle. I. The characterization of the 80 K and the 30 K subunits. J Biochem. 1981 Jul;90(1):233–240. doi: 10.1093/oxfordjournals.jbchem.a133455. [DOI] [PubMed] [Google Scholar]
- Vedeckis W. V., Freeman M. R., Schrader W. T., O'Malley B. W. Progesterone-binding components of chick oviduct: partial purification and characterization of a calcium-activated protease which hydrolyzes the progesterone receptor. Biochemistry. 1980 Jan 22;19(2):335–343. doi: 10.1021/bi00543a014. [DOI] [PubMed] [Google Scholar]
- Waxman L. Calcium-activated proteases in mammalian tissues. Methods Enzymol. 1981;80(Pt 100):664–680. doi: 10.1016/s0076-6879(81)80051-1. [DOI] [PubMed] [Google Scholar]
- Wheelock M. J. Evidence for two structurally different forms of skeletal muscle Ca2+-activated protease. J Biol Chem. 1982 Nov 10;257(21):12471–12474. [PubMed] [Google Scholar]
- Yeaton R. W., Lipari M. T., Fox C. F. Calcium-mediated degradation of epidermal growth factor receptor in dislodged A431 cells and membrane preparations. J Biol Chem. 1983 Aug 10;258(15):9254–9261. [PubMed] [Google Scholar]
- Yoshida H., Murachi T., Tsukahara I. Limited proteolysis of bovine lens alpha-crystallin by calpain, a Ca2+-dependent cysteine proteinase, isolated from the same tissue. Biochim Biophys Acta. 1984 Apr 10;798(2):252–259. doi: 10.1016/0304-4165(84)90313-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman U. J., Schlaepfer W. W. Characterization of a brain calcium-activated protease that degrades neurofilament proteins. Biochemistry. 1982 Aug 17;21(17):3977–3982. doi: 10.1021/bi00260a012. [DOI] [PubMed] [Google Scholar]