Abstract
目的
探究阿美替尼联合安罗替尼抑制非小细胞肺癌的作用。
方法
CCK-8法、集落克隆法和流式细胞术检测不同浓度阿美替尼和安罗替尼对PC-9细胞和HCC827细胞增殖、细胞存活和细胞凋亡的影响;SynergyFinder模型评价阿美替尼与安罗替尼的协同作用;Transwell小室实验检测阿美替尼联合安罗替尼对PC-9细胞和HCC827细胞侵袭及迁移的作用;Western blotting实验检测阿美替尼联合安罗替尼对PC-9细胞和HCC827细胞凋亡和侵袭迁移相关蛋白Bax、Bcl-2、E-Cadherin、Vimentin、MMP2和MMP9及PI3K-Akt通路关键蛋白表达的影响。
结果
阿美替尼作用于PC-9细胞的IC50为1.701 μmol/L,安罗替尼作用于PC-9细胞的IC50=4.979 μmol/L,协同得分(ZIP)为19.112;阿美替尼对HCC827细胞IC50=2.961 μmol/L,安罗替尼对HCC827细胞IC50=7.934 μmol/L,协同得分(ZIP)为12.325,阿美替尼联合安罗替尼能够显著抑制PC-9细胞和HCC827细胞增殖(P<0.05)。相比于单药组,集落克隆结果表明阿美替尼联合安罗替尼能够显著抑制PC-9细胞和HCC827细胞存活(P<0.01);流式细胞术结果表明,阿美替尼联合安罗替尼能够促进PC-9细胞和HCC827细胞发生凋亡(P<0.05);Transwell实验结果表明,阿美替尼联合安罗替尼能够显著抑制PC-9细胞和HCC827细胞的侵袭迁移能力(P<0.01);Western blotting实验结果表明,阿美替尼联合安罗替尼能够显著促进PC-9细胞和HCC827细胞中E-Cadherin和Bax蛋白的表达,抑制Bcl-2、Vimentin、MMP2和MMP9蛋白的表达(P<0.01),同时显著降低了PI3K-Akt通路中关键蛋白PI3K、Akt的磷酸化蛋白水平(P<0.01)。
结论
阿美替尼联合安罗替尼通过下调PI3K-Akt通路抑制NSCLC肿瘤细胞,这种联合治疗方式可能成为临床上治疗 NSCLC患者的一种潜在治疗策略。
Keywords: 非小细胞肺癌, 阿美替尼, 安罗替尼, 凋亡, 侵袭迁移, PI3K-Akt
Abstract
Objective
To investigate the inhibitory effect of aumolertinib combined with anlotinib on proliferation of non-small cell lung cancer (NSCLC) cells.
Methods
CCK-8 assay, colony formation assay, and flow cytometry were used to assess the effect of different concentrations of aumolertinib or anlotinib on proliferation, survival, and apoptosis of PC-9 and HCC827 cells, and their synergistic effect was evaluated using the SynergyFinder model. In PC-9 and HCC827 cells treated with aumolertinib combined with anlotinib, the changes in cell invasion and migration abilities were assessed with Transwell assay, and the expressions of apoptosis- and invasion/migration-related proteins (Bax, Bcl-2, E-cadherin, vimentin, MMP2, and MMP9) and the key PI3K-Akt pathway proteins were detected using Western blotting.
Results
In PC-9 cells, the IC50 of aumolertinib and anlotinib was 1.701 μmol/L and 4.979 μmol/L, respectively, with a synergy score (ZIP) of 19.112; in HCC827 cells, their IC50 was 2.961 μmol/L and 7.934 μmol/L, respectively, with a ZIP of 12.325. Compared with aumolertinib and anlotinib used alone, their combined treatment more strongly inhibited the proliferation and survival, enhanced apoptosis and suppressed invasion and migration abilities of PC-9 and HCC827 cells. Western blotting showed that in both PC-9 and HCC827 cells, the combined treatment significantly upregulated the expressions of E-cadherin and Bax proteins, downregulated the expressions of Bcl-2, vimentin, MMP2, and MMP9 proteins, and reduced phosphorylation levels of PI3K and Akt.
Conclusion
Aumolertinib combined with anlotinib can effectively inhibit NSCLC cell proliferation by downregulating the PI3K-Akt pathway, suggesting a potentially new option for NSCLC treatment.
Keywords: non-small cell lung cancer, aumolertinib, anlotinib, apoptosis, invasion and migration, PI3K-Akt pathway
最新癌症统计报告显示,肺癌新增病例和死亡人数均位于世界第一,严重威胁人类健康[1]。临床上非小细胞肺癌(NSCLC)发病率通常占肺癌的80%~85%[2, 3]。虽然临床上积极采用各种方法用于非小细胞肺癌治疗,但其治疗依然是临床瓶颈问题[4-6]。因此,积极探索防治非小细胞肺癌的策略势在必行。
阿美替尼(Aum)是一种不可逆的第3代表皮生长因子受体酪氨酸激酶抑制剂,用于治疗表皮生长因子受体(EGFR)突变型NSCLC[7]。安罗替尼(An)是一种口服小分子多受体酪氨酸激酶抑制剂,对肿瘤血管生成和生长具有广谱抑制作用,An已被批准用于治疗局部晚期或转移性NSCLC患者[8-10]。Aum和An联合使用治疗伴有EGFR突变的胶质母细胞瘤患者,患者受益于Aum联合An和替莫唑胺[11]。一项Ⅱ期临床研究在未经治疗的EGFR突变和晚期NSCLC患者中,进行了一项非随机II期试验(ChiCTR2000035140),口服多靶点抗血管生成TKI与第3代EGFR-TKI(osimertinib或aumolertinib)。结果表明:在未经治疗的EGFR突变型晚期NSCLC患者中,联合应用An和第3代EGFR- TKIs的毒性显著增加,表明联合治疗策略在这种情况下是不恰当的治疗选择[12]。而在陈华林教授团队Aum联合An一线治疗EGFR突变型NSCLC患者的II期临床研究ALWAYS的最新数据中,3代EGFR-TKI联合抗血管生成药物的联合方案使患者客观缓解率(ORR)达到96.15%,疾病控制率(DCR)则为100%[13]。但目前尚无相关基础研究,基于此,我们进行相关基础研究,观察Aum联合An对NSCLC细胞是否具有更好的抑制作用并探索其作用机制。
磷脂酰肌醇3-激酶蛋白激酶B(PI3K/Akt)信号通路的失调与肺癌的肿瘤发生、转移、凋亡和耐药密切相关[14-16]。PI3K/Akt信号通路的激活与NSCLC恶性进展密切相关,可能是抑制非小细胞肺癌的重要靶点[17, 18]。有研究表明,PI3K抑制剂改善An耐药,且PI3K抑制剂与An联合治疗通过降低PI3K/Akt/mTOR抑制An耐药骨肉瘤细胞增殖、迁移、侵袭和细胞骨架形成,并诱导凋亡[19-22]。Aum联合An对PI3K/Akt通路的作用还有待研究。
本研究以PC-9和HCC827细胞作为研究对象,旨在探索Aum联合An对NSCLC细胞增殖、凋亡、侵袭、迁移以及PI3K-Akt通路的作用,使用SynergyFinder模型进行两药协同评分,为临床寻找合适的联用剂量提供了基础研究数据,证明了Aum联合An通过下调PI3K-Akt通路抑制NSCLC细胞,为Aum联合An用于临床治疗NSCLC提供理论和实验依据。
1. 材料和方法
1.1. 主要实验材料
细胞株:非小细胞肺癌PC-9细胞和HCC827细胞购自武汉普诺赛生命科技有限公司。试剂:Aum(江苏豪森药业有限公司,)、An(正大天晴药业有限公司)、1640培养基(Gibco)、胎牛血清(ExCellBio)、CCK-8试剂(APEBIO)、胰酶消化液(Biosharp)、青霉素-链霉素溶液(Biosharp)、RIPA裂解液(碧云天)、AnnexinⅤ-FITC/PI试剂盒(贝博生物)、Transwell小室(Corning公司)、BCA蛋白浓度测定试剂盒(Biosharp)。抗体信息(表1)。
表1.
抗体信息
Tab.1 Antibodies used in this study
| Antibody name | Company | Dilution rate |
|---|---|---|
| Anti-beta actin | Affinity biosciences | 1:5000 |
| Anti-E-cadherin | Proteintech | 1:5000 |
| Anti-Vimentin | Proteintech | 1:1000 |
| Anti-Bax | Proteintech | 1:2000 |
| Anti-Bcl-2 | Proteintech | 1:2000 |
| Anti-MMP2 | Affinity biosciences | 1:1000 |
| Anti-MMP9 | Affinity biosciences | 1:1000 |
| Anti-PI3K | Proteintech | 1:1000 |
| Anti-AKT | Proteintech | 1:5000 |
| Anti-p-PI3K | Cell signaling technology | 1:1000 |
| Anti-p-AKT | Cell signaling technology | 1:2000 |
1.2. 方法
1.2.1. 细胞培养
PC-9细胞和HCC827细胞培养于含有10%胎牛血清和1%青霉素-链霉素的1640培养液中,置于37 ℃、5% CO2、饱和湿度的恒温培养箱中。
1.2.2. CCK-8法检测细胞存活率
消化处理PC-9、HCC827细胞,牛鲍计数板计数,使细胞悬液浓度为5×104 /mL,充分混悬后接种于96孔板中,每孔加100 μL细胞悬液,放于37 ℃、5% CO2培养箱培养。待细胞密度达到60%~80%时,吸除培养液,加入含有不同浓度Aum和An(0、0.25、0.5、1、2、4、8、16、32 μmol/L)的培养基,每组设置6个复孔,孵育24 h后,每孔加入10 μL的CCK-8溶液(避光),37 ℃培养箱中孵育30 min,酶标仪在450 nm处测定吸光度(A)值;PC-9细胞联合给药浓度为Aum:0、0.25、0.5、1、2 μmol/L;An:0、0.5、1、2、4 μmol/L,HCC827细胞联合给药浓度为Aum:0、0.25、0.5、1、2 μmol/L;An:0、1、2、4、8 μmol/L。配置含药培养基时,使用含不同浓度Aum(0、0.25、0.5、1、2 μmol/L)的培养基作为母液,使用倍比稀释法配置含不同浓度An(0、0.5、1、2、4 μmol/L)的培养基对PC-9细胞给药,24 h后,每孔加入10 μLCCK-8溶液,培养箱中孵育30 min左右,测定吸光度A值。HCC827同PC-9细胞操作。细胞存活率=(实验组A值-空白组A值)/(对照组A值-空白组A值)×100%。
1.2.3. 流式细胞仪检测细胞凋亡
将PC-9和HCC827细胞接种于6孔板中,2×105/孔,完全培养液培养,待细胞密度达到60%~80%时,吸去孔内培养液,PC-9细胞每孔分别加入空白培养液、含Aum (1 μmol/L)的培养液、含An(4 μmol/L)的培养液、含Aum(1 μmol/L)和An(4 μmol/L)的培养液各2 mL,HCC827细胞每孔分别加入空白培养液、含Aum(3 μmol/L)的培养液、含An(8 μmol/L)的培养液、含Aum(3 μmol/L)和An(8 μmol/L)的培养液各2 mL,置于37 ℃、5% CO2培养箱中培养24 h。收集细胞于10 mLEP管中,1500 r/min离心10 min,弃上清,每管加入200 μL AnnexinⅤ结合液重悬细胞,每管3 μL Annexin Ⅴ-FITC,冰上孵育15 min,每管加入5 μL PI染色液,避光混匀、冰上孵育10 min。每管再加入200 μL Annexin-Ⅴ结合液,混匀后用纱布过滤,加入流式管中,样品在1 h内用流式细胞仪检测。
1.2.4. Transwell侵袭及迁移实验
基质胶与无血清培养液按照1∶8比例稀释,在Transwell小室上室内加入60 μL基质胶,置于37 ℃恒温培养箱中40 min,待基质胶凝固。消化处理PC-9细胞,分别使用无血清1640培养液调整为含Aum(1 μmol/L)、含An(4 μmol/L)、含Aum(1 μmol/L)和An(4 μmol/L)的2×105 /mL细胞悬液;消化处理HCC827细胞,分别使用无血清1640培养液调整为含Aum(3 μmol/L)、含An(8 μmol/L)、含Aum(3 μmol/L)和An(8 μmol/L)的2×105/mL细胞悬液。取100 μL细胞悬液加入上室,下室加入700 μL含10%胎牛血清的培养液,每组设置2个复孔,实验重复3次;培养24 h后取出小室,用PBS轻轻冲洗,用4%多聚甲醛室温固定15 min,用结晶紫染液室温染色20 min。光学倒置显微镜下计数侵袭至微孔膜下层的细胞,每个样本选取3个视野,取平均值。迁移实验无铺基质胶步骤,其余步骤同侵袭实验。
1.2.5. 集落克隆实验
通过集落形成实验探究Aum联合放射治疗对细胞存活的影响。将PC-9 和HCC827细胞以2×103 /孔种于6孔板中,将细胞置于37 ℃、含5% CO2的培养箱中培养。待细胞完全贴壁后,用Aum(0.1 μmol/L)和An(0.4 μmol/L)分别单药处理或联合处理PC-9细胞,Aum(0.3 μmol/L)和An(0.8 μmol/L)分别单药处理或联合处理HCC827细胞。细胞在6孔板中培养约10 d,约每3 d更换1次细胞培养基。10 d后,吸除培养基,多聚甲醛固定形成的集落,然后用0.5%结晶紫对集落进行染色,风干后计数细胞集落的数量,采用Image J软件分析计算集落数量。
1.2.6. Western blotting实验检测蛋白表达
将PC-9和HCC827细胞接种于100 mm大皿中,当细胞密度达到60%~80%时,PC-9细胞中分别加入1640培养液、含Aum(1 μmol/L)、含An(4 μmol/L)、含Aum(1 μmol/L)和An(4 μmol/L)的培养液处理,HCC827细胞中分别加入1640培养液、含Aum(3 μmol/L)、含An(8 μmol/L)、含Aum(3 μmol/L)和An(8 μmol/L)的培养液处理。给药24 h后刮取蛋白,BCA蛋白浓度试剂盒测定蛋白浓度。根据公式计算加入上样缓冲液和裂解液量,充分混匀,使终浓度相同,蛋白在95 ℃条件下加热5 min。20 μg蛋白上样电泳(上层胶电泳70 V 30 min,下层胶电泳120 V 90 min),半干转法转至PVDF膜上(恒压24 V 30 min),快速封闭液封闭20 min。根据目标蛋白分子量剪切PVDF膜,孵育相应一抗,4 ℃孵育过夜。次日用TPBS室温清洗3次,放入对应二抗中,室温孵育1.5 h,曝光机显影曝光,采用Image J软件分析条带灰度值。
1.2.7. 协同得分的测定
通过 CCK-8 实验探究Aum联合An对细胞增殖的影响并计算细胞存活率,步骤同上述CCK-8实验方法。选用Aum或An单独给药PC-9和HCC827细胞时,细胞存活率为80%的浓度为起始浓度,包括IC50值在内,共设置5个联合给药浓度点。其中,PC-9细胞联合给药浓度为Aum:0、0.25、0.5、1、2 μmol/L;An:0、0.5、1、2、4 μmol/L。HCC827细胞联合给药浓度为Aum:0、0.25、0.5、1、2 μmol/L;An:0、1、2、4、8 μmol/L。配置含药培养基时,使用含不同浓度Aum(0、0.25、0.5、1、2 μmol/L)的培养基作为母液,使用倍比稀释法配置含不同浓度An(0、0.5、1、2、4 μmol/L)的培养基对PC-9细胞给药。细胞存活率=(实验组OD值-空白组OD值)/(对照组OD值-空白组OD值)×100%。同理,使用Aum联合An对HCC827细胞给药。CCK-8实验检测给药24 h后两种细胞的存活率。使用SynergyFinder程序进行分析,将Aum和An联合用药处理PC-9和HCC827细胞的细胞存活率以矩阵格式输入SynergyFinder进行分析,ZIP synergy score若大于10,两种药物之间的相互作用是协同的;ZIP synergy score小于-10,两种药物之间的相互作用是拮抗的;ZIP synergy score从 -10 到 10:两种药物之间的相互作用是相加的。
1.2.8. 统计学方法
使用GraphpadPrism8.0对数据进行统计学分析并作图,计量资料以均数±标准差示,组间均数比较采用LSD-t检验,以P<0.05为差异具有统计学意义。
2. 结果
2.1. Aum和An抑制NSCLC细胞增殖与存活
CCK-8实验测得Aum作用于PC-9细胞的IC50为1.701 μmol/L(图1A),An作用于PC-9细胞的IC50为4.979 μmol/L(图1B);Aum对HCC827细胞IC50为2.961 μmol/L(图1C),An对HCC827细胞IC50为7.934 μmol/L(图1D)。
图1.
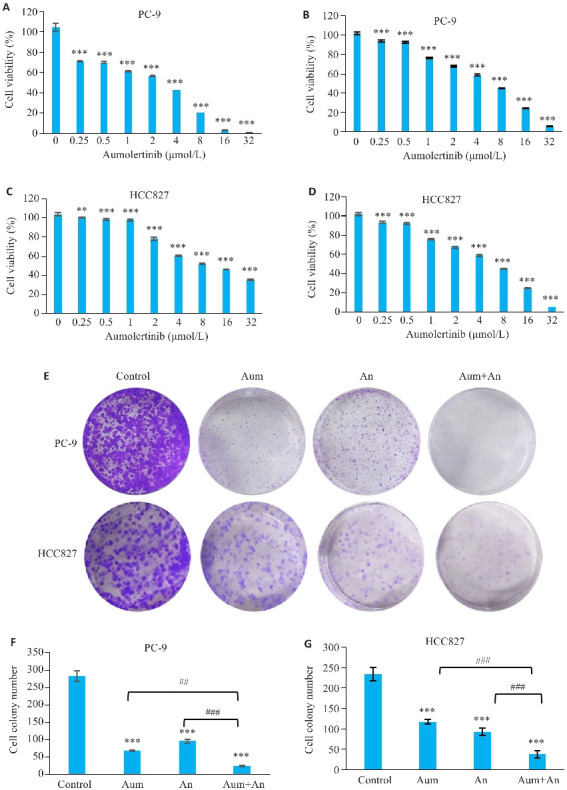
Aum和An抑制NSCLC细胞增殖与存活
Fig.1 Aumolertinib (Aum), anlotinib (An) and their combination significantly inhibits NSCLC cell proliferation and survival. A: Effect of Aum on PC-9 cells. B: Inhibitory effect of An in PC-9 cells. C: Inhibitory effect of Aum in HCC827 cells. D. Inhibitory effect of An in HCC827 cells. E: Colony cloning. F, G: Statistics of colony formation in HCC827 cells, **P<0.01, ***P<0.001 vs 0 μmol/L Control, ## P<0.01, ### P<0.001.
集落克隆结果显示:与空白组相比,Aum组、An组以及两药联合组抑制PC-9及HCC827的细胞活力,其中Aum联合An组作用优于单独使用Aum或An组,且差异具有统计学意义(P<0.01,图1E~G)。
2.2. Aum联合An对PC-9及HCC827细胞的抑制具有协同作用
CCK-8实验检测给药24 h后PC-9和HCC827细胞活力,相较于单独处理组,Aum联合An能够进一步抑制细胞增殖和存活(图2A、B)。得到两药联合处理PC-9细胞的ZIP synergy score为19.112(图2C、D),两药联合处理HCC827细胞的ZIP synergy score为12.325(图2E、F)。
图2.
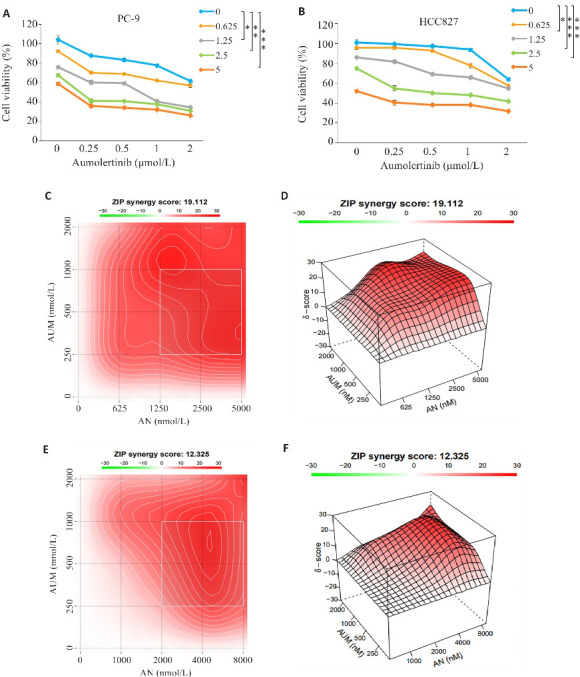
Aum联合An对PC-9及HCC827细胞的抑制具有协同作用
Fig.2 Aumolertinib combined with anlotinib synergistically inhibit viability of PC-9 and HCC827 cells. A, B: CCK-8 assay. C, D: Synergy scores of Aumolertinib combined with anlotinib in PC-9 cells (ZIP<0 indicates an antagonistic effect of the two drugs, 0<ZIP<10 indicates that the two drugs have antagonistic effects, and 0<ZIP<10 indicates that the two drugs have antagonistic effect, and 0<ZIP<10 indicates that the two drugs have synergy effect). ZIP<10 indicates that the two drugs have additive effect, and 10<ZIP indicates that the two drugs have synergistic effect. E,F: Synergy scores of aumolertinib combined with anlotinib in HCC827 cells. *P<0.05, **P<0.01, ***P<0.001.
2.3. Aum联合An诱导PC-9细胞及HCC827细胞凋亡
流式细胞仪检测细胞凋亡情况,与对照组相比,Aum和An处理PC-9和HCC827细胞凋亡率明显增加,差异具有显著性(P<0.05,图3A~C),与单药组相比,联合组中PC-9细胞的凋亡率具有显著性(P<0.01,图3A、B),HCC827细胞的凋亡率也具有显著性(P<0.05,图3A、C);Western blotting实验检测凋亡相关蛋白Bax和Bcl-2的表达,结果表明,与对照组相比,不同浓度Aum或An处理组PC-9和HCC827细胞中Bax蛋白表达显著升高(P<0.01,图3E),Bcl-2蛋白表达显著降低(P<0.05,图3E),与单用Aum组相比,两药联合时两种蛋白表达也具有显著性(P<0.01,图3D~F)。
图 3.
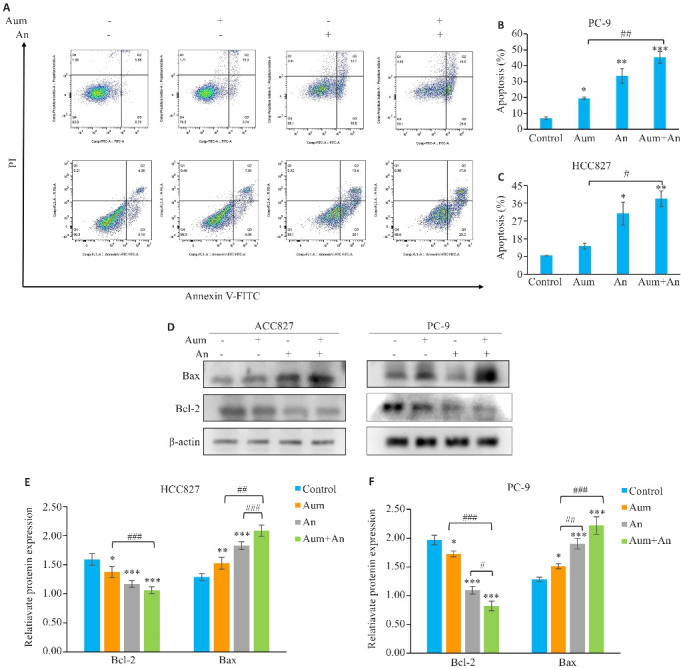
Aum联合An明显促进PC-9细胞及HCC827细胞的凋亡
Fig.3 Aumolertinib combined with anlotinib promotes apoptosis of PC-9 cells and HCC827 cells. A: Flow cytometric analysis of apoptosis in different treatment groups. B, C: Apoptosis rates in the 4 groups. D: Apoptosis-related proteins analyzed using protein immunoblotting; E,F: Quantitative analysis of apoptotic protein expression levels. *P<0.05, **P<0.01, ***P<0.001 vs control; # P<0.05, ## P<0.01, ### P<0.001.
2.4. Aum联合An抑制PC-9细胞及HCC827细胞的侵袭及迁移
与对照组和单药组相比,Aum和An联合处理组中PC-9和HCC827细胞的侵袭迁移数目明显减少,且细胞迁移速度明显减慢(P<0.05,图4)。Western blotting实验检测侵袭迁移相关蛋白E-Cadherin、Vimentin、MMP2和MMP9的表达,结果表明,与对照组相比,Aum和An单药及联合处理组中,PC-9和HCC827细胞的E-Cadherin蛋白表达显著升高(P<0.01),Vimentin、MMP2蛋白表达显著下降(P<0.01,图5)。
图4.
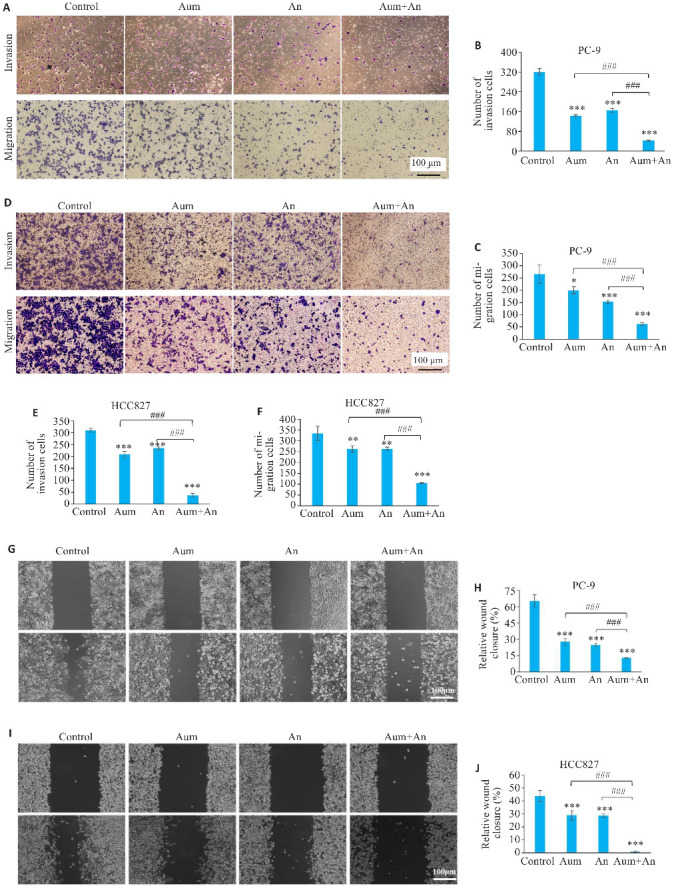
Aum联合An抑制PC-9细胞及HCC827细胞的侵袭和迁移
Fig.4 Aumolertinib combined with anlotinib significantly inhibits invasion and migration of PC-9 cells and HCC827 cells. A,D: Transwell assay for assessing cell invasion and migration in different groups (crystalline violet staining, original magnification: ×200). G, I: Scratch assay for assessing migration ability of the cells in different groups (×200). H,J: Quantitative analysis of PC-9 and HCC827 cell migration. *P<0.05, **P<0.01, ***P<0.001 vs control; ### P<0.001.
图 5.
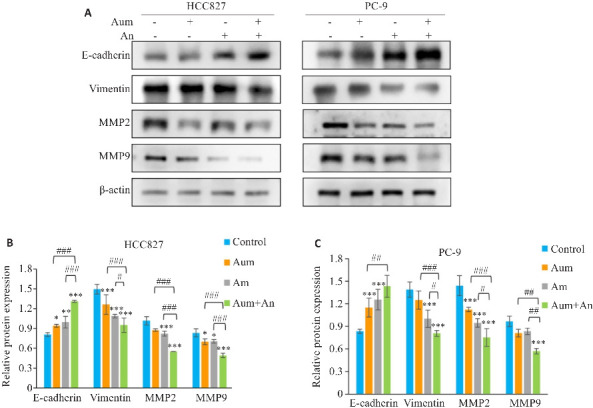
Aum联合An调控PC-9细胞及HCC827细胞的侵袭迁移相关蛋白的表达
Fig.5 Western blotting for the expression of E-cadherin, vimentin, MMP2 and MMP9 in PC-9 cells or HCC827 cells (A) and their relative of protein expression levels (B, C). *P<0.05, **P<0.05,***P<0.001 vs control; # P<0.05,## P<0.01, ### P<0.001.
与单药组相比,Aum联合An时MMP9蛋白表达显著下降(P<0.01,图5C)。
2.5. Aum联合An下调NSCLC中PI3K-Akt通路蛋白
Western blottimg结果显示,与对照组相比,Aum和An单药及联合处理组中,PC-9和HCC827细胞的P-PI3K、P-Akt蛋白表达。显著下降 (P<0.01,图6)。与单药组相比,Aum联合An显著降低了PI3K-Akt通路中关键蛋白PI3K、Akt的磷酸化蛋白水平(P<0.01,图6)。
图6.
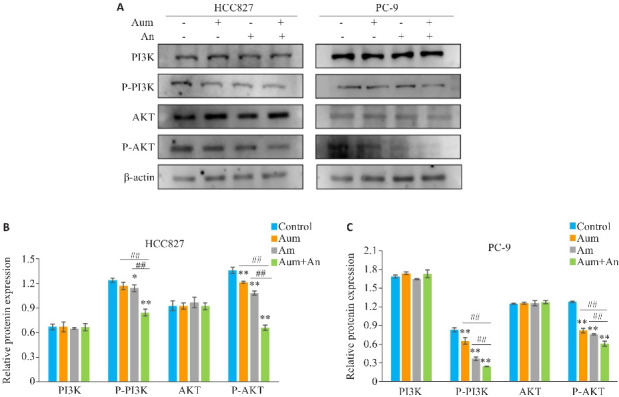
Aum联合An通过阻断PI3K及Akt的磷酸化抑制PC-9细胞及HCC827细胞的侵袭和迁移
Fig.6 Aumolertinib combined with anlotinib inhibits invasion and migration of PC-9 cells and HCC827 cells by blocking phosphorylation of PI3K and Akt. A: Protein immunoblotting of PI3K-Akt pathway related proteins. B, C: Quantitative analysis of expression levels of PI3K-Akt pathway-related proteins. *P<0.05,**P<0.01 vs control,## P<0.01.
3. 讨论
Aum是第3代EGFR-TKI,通过在EGFR酪氨酸激酶结构域的ATP结合位点与Cys797不可逆地结合抑制EGFR激活,抑制肿瘤细胞的生长,加速细胞凋亡[23]。An是一种小分子多靶点酪氨酸激酶抑制剂,其通过靶向血管内皮生长因子受体(VEFGR)、血小板衍生生长因子受体(PDGF)、干细胞因子受体(c-Kit)以及成纤维细胞生长因子受体(FGFR)等[24-26],对肿瘤血管生成和NSCLC细胞生长具有广泛的抑制作用[27]。临床上被批准为晚期肺癌患者三线治疗药物[28, 29]。研究发现,An可以通过抑制PI3K/Akt/mTOR等通路,抑制NSCLC细胞的生长及侵袭迁移[30],从而抑制NSCLC的恶性发展[31], 已有研究发现An可以通过PI3K/AKT/mTOR通路抑制套细胞淋巴瘤增殖和诱导凋亡 [32]。目前Aum联合An的研究较少,缺乏相关基础研究[11],本实验研究了Aum联合An对NSCLC细胞的的作用,发现Aum联合An相比于单药显著抑制PC-9和HCC827细胞增殖同时促进凋亡。
虽然有II期临床试验对An联合三代EGFR-TKI(osimertinib或aumolertinib)对晚期NSCLC的治疗策略进行研究,表明在未经治疗的EGFR突变型晚期NSCLC患者中,联合应用An和第3代EGFR-TKIs是不恰当的治疗选择[12],但其样本量较少(仅11例),使用Aum联合An的患者仅2例(1例19DEL突变,1例L858R突变),其中L858R突变患者在经历两个月联合治疗后病情趋于稳定,19DEL突变患者虽因为肺栓塞第1次成像评估之前停止试验,因而联合用药临床疗效有待扩大样本量进行验证[12]。相反,近期陈华林教授团队最新的临床研究报告了II期临床研究ALWAYS的初步数据,总计纳入26例晚期EGFR突变型NSCLC患者的完整数据,显示Aum联合An一线治疗晚期EGFR突变型NSCLC患者取得了较好的疗效[13];有正在进行的临床研究表明An联合EGFR-TKIs有较好的效果:ORR达95.45%,DCR达100%。中位缓解深度(DpR)为42.0%,且安全性可控[33, 34]。一项临床回顾性分析也评价了阿美替尼联合安罗替尼对于治疗肺癌脑转移患者有更好的疗效[35]。以上数据表明Aum联合An在EGFR突变的脑转移NSCLC患者的一线治疗中显示出初步疗效。有相关临床研究表明已有EGFR p.L858R突变胶质瘤患者受益于Aum联合An和替莫唑胺[11],Aum联合An是一种具有潜力的联合用药策略。我们通过一系列基础实验研究,发现Aum联合An的效果优于单药,并且通过不同剂量的设置,使用SynergyFinder模型进行两药协同评分,为临床寻找合适的联用剂量提供了基础研究数据。
肿瘤细胞侵袭迁移是肿瘤恶化的主要驱动因素,是影响NSCLC患者预后及导致患者死亡的主要原因[36]。上皮间质转化(EMT)是一个关键的细胞程序,使极化上皮细胞向间充质表型过渡,增加细胞运动性[37, 38]。为了获得癌症转移进展的侵袭性表型,癌细胞利用EMT促进其与原发肿瘤分离并传播到血液循环中[39, 40]。EMT在肿瘤细胞侵袭转移中起着关键作用,其分子生物学特征在于上皮细胞标志物(如E-cadherin表达下调)和间质细胞标志物(如Vimentin、MMP2及MMP9表达上调)表达的变化[41-43]。基质金属蛋白(MMPs)是与肿瘤发生相关的最重要的蛋白酶家族,细胞外基质和基底是癌细胞转移过程中的一道屏障,其降解是肿瘤侵袭迁移的重要环节 [44]。MMPs是锌依赖性内肽酶,降解细胞外基质(ECM)中的蛋白质,作为多种疾病的潜在治疗靶点,MMPs引起了科学家们的极大关注[1, 2]。例如,MMP2和MMP9在促进癌症转移和侵袭方面具有重要作用[47]。MMP2、MMP9能够降解IV型胶原蛋白,促进肿瘤细胞转移[44]。Transwell 实验和划痕实验结果表明相比于单药组,Aum联合An能够显著抑制PC-9和HCC827细胞的侵袭和迁移能力,Western blotting实验结果表明相比于单药组,Aum联合An处理后,PC-9和HCC827细胞中E-cadherin 蛋白表达显著上调,Vimentin 、MMP2和MMP9蛋白表达显著下调,。以上结果表明Aum联合An可能通过逆转上皮间质转化,抑制PC-9细胞和HCC827细胞侵袭和迁移。
PI3K/Akt/mTOR信号传导通路活化可以抑制多种刺激诱发的细胞凋亡,促进细胞周期进展,从而促进细胞的生存和增殖,同时参与血管形成,在肿瘤的形成中扮演重要角色[40, 48, 49]。有研究表明,在非小细胞肺癌中,PI3K/Akt通路在肿瘤发生和疾病进展中都有重要作用[17, 50, 51];PI3K/Akt信号失活有助于非小细胞肺癌的细胞凋亡[52, 53],PI3K/Akt信号通路可以调控NSCLC细胞的增殖[21]、侵袭迁移[7, 54-56]。研究发现,EGFR可以激活PI3K/Akt/mTOR途径介导细胞存活[57, 58]。Aum通过抑制EGFR激活从而抑制NSCLC细胞的生长[59],An可以通过负调控PI3K/Akt信号通路抑制多种肿瘤细胞的生长和转移[60-62]。为进一步探究Aum联合An抑制NSCLC的作用机制,本研究通过Western blot实验检测PI3K-Akt通路关键蛋白表达,结果表明相比于单药组,Aum联合An处理后,PC-9和HCC827细胞中PI3K、AKT磷酸化蛋白表达显著降低。以上结果表明Aum联合An能够阻断NSCLC细胞PI3K/Akt通路关键蛋白的磷酸化。
综上所述,本研究发现Aum联合An具有较好的抗NSCLC的作用,两者联合可能通过抑制PI3K-Akt通路协同抑制NSCLC增殖、侵袭、迁移,诱导细胞凋亡。Aum联合An有望提高NSCLC临床治疗效果,为临床联合用药提供理论依据。
基金资助
安徽省重点研究与开发计划项目(202104g01020017);安徽省重点生化工程中心科研平台开放课题(2023SYKFD05);国家级大学生创新创业训练计划项目(202210367064);安徽省大学生创新创业训练计划项目(S202310367111);安徽省优秀科研创新团队项目(2022AH010084);安徽省自然科学基金(1908085QH373);安徽高校自然科学研究项目(KJ2020A0565);安徽高校自然科学研究项目(KJ2021A0702)
参考文献
- 1. Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2024, 74(3): 229-63. [DOI] [PubMed] [Google Scholar]
- 2. Liang J, Guan XJ, Bao GY, et al. Molecular subtyping of small cell lung cancer[J]. Semin Cancer Biol, 2022, 86(Pt 2): 450-62. [DOI] [PubMed] [Google Scholar]
- 3. Succony L, Rassl DM, Barker AP, et al. Adenocarcinoma spectrum lesions of the lung: detection, pathology and treatment strategies[J]. Cancer Treat Rev, 2021, 99: 102237. [DOI] [PubMed] [Google Scholar]
- 4. Reck M, Remon J, Hellmann MD. First-line immunotherapy for non-small-cell lung cancer[J]. J Clin Oncol, 2022, 40(6): 586-97. [DOI] [PubMed] [Google Scholar]
- 5. Zugazagoitia J, Paz-Ares L. Extensive-Stage Small-Cell Lung Cancer: First-Line and Second-Line Treatment Options [J]. J Clin Oncol, 2022, 40(6): 671-80. [DOI] [PubMed] [Google Scholar]
- 6. Chaft JE, Shyr Y, Sepesi B, et al. Preoperative and postoperative systemic therapy for operable non-small-cell lung cancer[J]. J Clin Oncol, 2022, 40(6): 546-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ge X, Zhang Y, Huang F, et al. EGFR tyrosine kinase inhibitor Almonertinib induces apoptosis and autophagy mediated by reactive oxygen species in non-small cell lung cancer cells[J]. Hum Exp Toxicol, 2021, 40(12_suppl): S49-S62. [DOI] [PubMed] [Google Scholar]
- 8. Han BH, Li K, Wang QM, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: the ALTER 0303 phase 3 randomized clinical trial[J]. JAMA Oncol, 2018, 4(11): 1569-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shen GS, Zheng FC, Ren DF, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development[J]. J Hematol Oncol, 2018, 11(1): 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Syed YY. Anlotinib: first global approval[J]. Drugs, 2018, 78(10): 1057-62. [DOI] [PubMed] [Google Scholar]
- 11. Hou ZW, Wu HG, Luo NN, et al. Almonertinib combined with anlotinib and temozolomide in a patient with recurrent glioblastoma with EGFR L858R mutation[J]. Oncologist, 2023, 28(5): 449-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li T, Chang KJ, Qiu X, et al. A pilot study of anlotinib with third-generation epidermal growth factor receptor tyrosine kinase inhibitors in untreated EGFR-mutant patients with advanced non-small cell lung cancer[J]. Transl Lung Cancer Res, 2023, 12(6): 1256-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen LK, Chen J, Li MC, et al. OA03.03 aumolertinib plus anlotinib in advanced NSCLC with brain metastasis: a single-arm, phase II study[J]. J Thorac Oncol, 2023, 18(11): S48-9. [Google Scholar]
- 14. Yang Q, Jiang W, Hou P. Emerging role of PI3K/AKT in tumor-related epigenetic regulation[J]. Semin Cancer Biol, 2019, 59: 112-24. [DOI] [PubMed] [Google Scholar]
- 15. Xu ZR, Han X, Ou DM, et al. Targeting PI3K/AKT/mTOR-mediated autophagy for tumor therapy[J]. Appl Microbiol Biotechnol, 2020, 104(2): 575-87. [DOI] [PubMed] [Google Scholar]
- 16. Kim SH, Seung BJ, Cho SH, et al. Dysregulation of PI3K/akt/PTEN pathway in canine mammary tumor[J]. Animals, 2021, 11(7): 2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu T, Wang Y, Liu F, et al. Synergistic Inhibitory Effect of Berberine and Low-Temperature Plasma on Non-Small-Cell Lung Cancer Cells via PI3K-AKT-Driven Signaling Axis [J]. Molecules, 2023, 28(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moghbeli M. PI3K/AKT pathway as a pivotal regulator of epithelial-mesenchymal transition in lung tumor cells[J]. Cancer Cell Int, 2024, 24(1): 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen CL, Guo Y, Huang QS, et al. PI3K inhibitor impairs tumor progression and enhances sensitivity to anlotinib in anlotinib-resistant osteosarcoma[J]. Cancer Lett, 2022, 536: 215660. [DOI] [PubMed] [Google Scholar]
- 20. Zhang ZR, Zhang MX, Liu H, et al. AZD9291 promotes autophagy and inhibits PI3K/Akt pathway in NSCLC cancer cells[J]. J Cell Biochem, 2019, 120(1): 756-67. [DOI] [PubMed] [Google Scholar]
- 21. Lv YH, Du TT, Ji M, et al. A novel PI3K/mTOR dual inhibitor XH002 exhibited robust antitumor activity in NSCLC[J]. J Drug Target, 2019, 27(4): 451-9. [DOI] [PubMed] [Google Scholar]
- 22. Ciuffreda L, Incani UC, Steelman LS, et al. Signaling intermediates (MAPK and PI3K) as therapeutic targets in NSCLC[J]. Curr Pharm Des, 2014, 20(24): 3944-57. [DOI] [PubMed] [Google Scholar]
- 23. Erira A, Velandia F, Penagos J, et al. Differential regulation of the EGFR/PI3K/AKT/PTEN pathway between low- and high-grade gliomas[J]. Brain Sci, 2021, 11(12): 1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin BY, Song XM, Yang DW, et al. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1[J]. Gene, 2018, 654: 77-86. [DOI] [PubMed] [Google Scholar]
- 25. Qin TT, Liu ZJ, Wang J, et al. Anlotinib suppresses lymphangiogenesis and lymphatic metastasis in lung adeno-carcinoma through a process potentially involving VEGFR-3 signaling[J]. Cancer Biol Med, 2020, 17(3): 753-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hong C, Wei JP, Zhou T, et al. FGFR2-ERC1: a subtype of FGFR2 oncogenic fusion variant in lung adenocarcinoma and the response to anlotinib[J]. Onco Targets Ther, 2022, 15: 651-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liang LJ, Hui KY, Hu CX, et al. Autophagy inhibition potentiates the anti-angiogenic property of multikinase inhibitor anlotinib through JAK2/STAT3/VEGFA signaling in non-small cell lung cancer cells[J]. J Exp Clin Cancer Res, 2019, 38(1): 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Carlo E, Bertoli E, del Conte A, et al. Brain metastases management in oncogene-addicted non-small cell lung cancer in the targeted therapies era[J]. Int J Mol Sci, 2022, 23(12): 6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sun JJ, Wu SK, Jin ZX, et al. Lymph node micrometastasis in non-small cell lung cancer[J]. Biomedecine Pharmacother, 2022, 149: 112817. [DOI] [PubMed] [Google Scholar]
- 30. Tan AC. Targeting the PI3K/Akt/mTOR pathway in non-small cell lung cancer (NSCLC)[J]. Thorac Cancer, 2020, 11(3): 511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Han D, Zhang JJ, Bao YW, et al. Anlotinib enhances the antitumor immunity of radiotherapy by activating cGAS/STING in non-small cell lung cancer[J]. Cell Death Discov, 2022, 8(1): 468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang J, Xu Z, Lai Y, et al. Anlotinib Inhibiting Mantle Cell Lymphoma Proliferation and Inducing Apoptosis through PI3K/AKT/mTOR Pathway [J]. Current Molecular Medicine, 2024, 24. [DOI] [PubMed] [Google Scholar]
- 33. Zhang LL, Wang LC, Wang JY, et al. Anlotinib plus icotinib as a potential treatment option for EGFR-mutated advanced non-squamous non-small cell lung cancer with concurrent mutations: final analysis of the prospective phase 2, multicenter ALTER-L004 study[J]. Mol Cancer, 2023, 22(1): 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao HY, Yao WX, Min XH, et al. Apatinib plus gefitinib as first-line treatment in advanced EGFR-mutant NSCLC: the phase III ACTIVE study (CTONG1706)[J]. J Thorac Oncol, 2021, 16(9): 1533-46. [DOI] [PubMed] [Google Scholar]
- 35. 杜宝萍, 梁诗欣, 余 辉, 等. EGFR T790M抑制剂联合安罗替尼治疗非小细胞肺癌疗效的回顾分析[J]. 今日药学, 2023, 33(12): 922-7. [Google Scholar]
- 36. Zhao Y, Li SY, Yang X, et al. Overall survival benefit of osimertinib and clinical value of upfront cranial local therapy in untreated EGFR-mutant nonsmall cell lung cancer with brain metastasis[J]. Int J Cancer, 2022, 150(8): 1318-28. [DOI] [PubMed] [Google Scholar]
- 37. Lu W, Kang YB. Epithelial-mesenchymal plasticity in cancer progression and metastasis[J]. Dev Cell, 2019, 49(3): 361-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition[J]. Nat Rev Mol Cell Biol, 2014, 15(3): 178-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jiang Y, Han QJ, Zhao HJ, et al. Promotion of epithelial-mesenchymal transformation by hepatocellular carcinoma-educated macrophages through Wnt2b/β‑catenin/c-Myc signaling and reprogramming glycolysis[J]. J Exp Clin Cancer Res, 2021, 40(1): 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang GF, Zheng G, Zhang HP, et al. MUC1 induces the accumulation of Foxp3+ Treg cells in the tumor microenvironment to promote the growth and metastasis of cholangiocarcinoma through the EGFR/PI3K/Akt signaling pathway[J]. Int Immunopharmacol, 2023, 118: 110091. [DOI] [PubMed] [Google Scholar]
- 41. Yang SC, Liu Y, Li MY, et al. FOXP3 promotes tumor growth and metastasis by activating Wnt/β-catenin signaling pathway and EMT in non-small cell lung cancer[J]. Mol Cancer, 2017, 16(1): 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feng X, Xu ES. Alectinib and lorlatinib function by modulating EMT-related proteins and MMPs in NSCLC metastasis[J]. Bosn J Basic Med Sci, 2021, 21(3): 331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Emery S, Fieux S, Vidal B, et al. Preclinical validation of [(18)F]2FNQ1P as a specific PET radiotracer of 5-HT(6) receptors in rat, pig, non-human primate and human brain tissue[J]. Nucl Med Biol, 2020, 82/83: 57-63. [DOI] [PubMed] [Google Scholar]
- 44. Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment[J]. Cell, 2010, 141(1): 52-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Scheau C, Badarau I A, Costache R, et al. The Role of Matrix Metalloproteinases in the Epithelial-Mesenchymal Transition of Hepatocellular Carcinoma [J]. Analytical Cellular Pathology, 2019, 2019: 1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Scheau C, Badarau IA, Costache R, et al. The role of matrix metalloproteinases in the epithelial-mesenchymal transition of hepatocellular carcinoma[J]. Anal Cell Pathol, 2019, 2019: 9423907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rashid ZA, Bardaweel SK. Novel matrix metalloproteinase-9 (MMP-9) inhibitors in cancer treatment[J]. Int J Mol Sci, 2023, 24(15): 12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Miao Y, Bi XY, Zhao M, et al. Acetylcholine inhibits tumor necrosis factor α activated endoplasmic reticulum apoptotic pathway via EGFR-PI3K signaling in cardiomyocytes[J]. J Cell Physiol, 2015, 230(4): 767-74. [DOI] [PubMed] [Google Scholar]
- 49. Tang M, Deng HD, Zheng KL, et al. Ginsenoside 3β‑O-Glc-DM (C3DM) suppressed glioma tumor growth by downregulating the EGFR/PI3K/AKT/mTOR signaling pathway and modulating the tumor microenvironment[J]. Toxicol Appl Pharmacol, 2023, 460: 116378. [DOI] [PubMed] [Google Scholar]
- 50. Shirley M, Keam SJ. Aumolertinib: a review in non-small cell lung cancer[J]. Drugs, 2022, 82(5): 577-84. [DOI] [PubMed] [Google Scholar]
- 51. He JY, Huang ZR, Han LZ, et al. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer (Review)[J]. Int J Oncol, 2021, 59(5): 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Qu QH, Jiang SZ, Li XY. LncRNA TBX5-AS1 regulates the tumor progression through the PI3K/AKT pathway in non-small cell lung cancer[J]. Onco Targets Ther, 2020, 13: 7949-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang D, Deng T, Yuan W, et al. Glaucocalyxin A induces apoptosis of non-small cell lung carcinoma cells by inhibiting the PI3K/Akt/GSK3β pathway[J]. Clin Exp Pharmacol Physiol, 2022, 49(8): 797-804. [DOI] [PubMed] [Google Scholar]
- 54. Zhou J, Peng Y, Gao YC, et al. Targeting DNAJC19 overcomes tumor growth and lung metastasis in NSCLC by regulating PI3K/AKT signaling[J]. Cancer Cell Int, 2021, 21(1): 338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cao SY, Li LF, Li J, et al. MiR-1299 impedes the progression of non-small-cell lung cancer through EGFR/PI3K/AKT signaling pathway[J]. Onco Targets Ther, 2020, 13: 7493-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang F, Zheng YF, Luo Q, et al. Knockdown of NCAPD3 inhibits the tumorigenesis of non-small cell lung cancer by regulation of the PI3K/Akt pathway[J]. BMC Cancer, 2024, 24(1): 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Guan JK, Borenäs M, Xiong JF, et al. IGF1R contributes to cell proliferation in ALK-mutated neuroblastoma with preference for activating the PI3K-AKT signaling pathway[J]. Cancers, 2023, 15(17): 4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bang J, Jun M, Lee S, et al. Targeting EGFR/PI3K/AKT/mTOR signaling in hepatocellular carcinoma[J]. Pharmaceutics, 2023, 15(8): 2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shi C, Zhang C, Fu ZW, et al. Antitumor activity of aumolertinib, a third-generation EGFR tyrosine kinase inhibitor, in non-small-cell lung cancer harboring uncommon EGFR mutations[J]. Acta Pharm Sin B, 2023, 13(6): 2613-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu YL, Li F, Wang QY, et al. Anlotinib inhibits growth of human esophageal cancer TE-1 cells by negative regulating PI3K/Akt signaling pathway[J]. Discov Oncol, 2024, 15(1): 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lu YF, Lin J, Duan M, et al. Anlotinib suppresses oral squamous cell carcinoma growth and metastasis by targeting the RAS protein to inhibit the PI3K/akt signalling pathway[J]. Anal Cell Pathol, 2021, 2021: 5228713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen QL, Lai Q, Jiang YL, et al. Anlotinib exerts potent antileukemic activities in Ph chromosome negative and positive B-cell acute lymphoblastic leukemia via perturbation of PI3K/AKT/mTOR pathway[J]. Transl Oncol, 2022, 25: 101516. [DOI] [PMC free article] [PubMed] [Google Scholar]


