Abstract
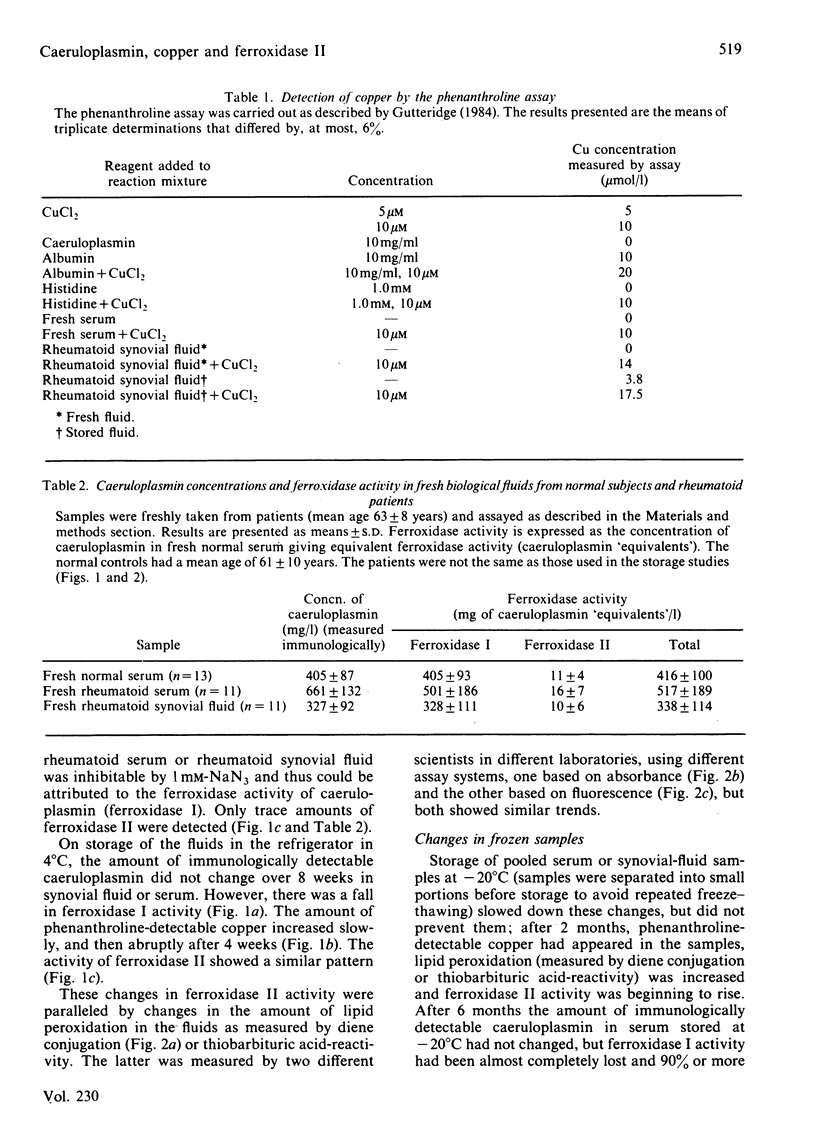
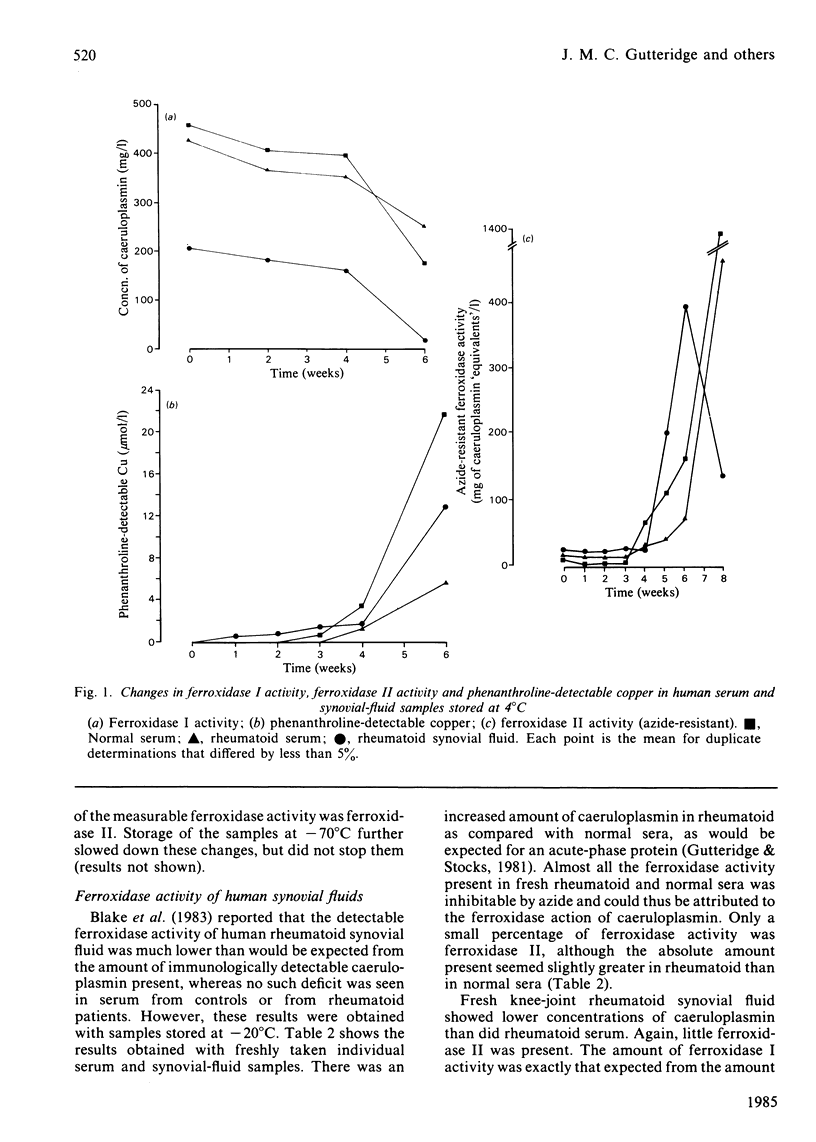
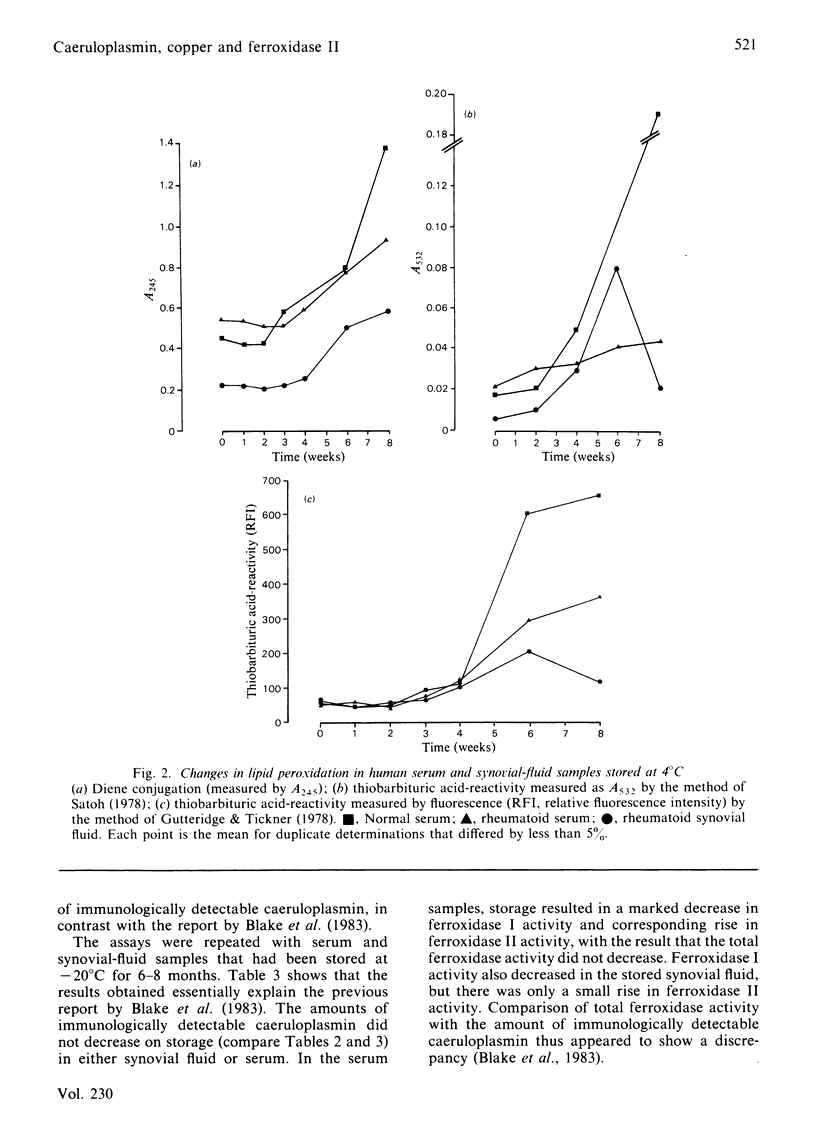
No Cu(II) ion is measurable in human serum or synovial fluid by the phenanthroline assay. On storage of human serum or synovial fluid at 4 degrees C, phenanthroline-detectable copper appears, lipid peroxidation occurs, ferroxidase I activity declines and ferroxidase II activity rises, yet there is no fall in immunologically detectable caeruloplasmin. Storage of body fluids at -20 degrees C or -70 degrees C slows, but does not prevent, these deteriorative changes. It is suggested that the presence of low-molecular-mass Cu(II) ion complexes, ferroxidase II activity, "cytotoxic factors' and "immunosuppressive factors' in body fluids may be, in part or in whole, an artifact of the storage and handling of the fluids. A report [Blake, Blann, Bacon, Farr, Gutteridge & Halliwell (1983) Clin. Sci. 64, 551-553] that the caeruloplasmin present in rheumatoid synovial fluid is deficient in ferroxidase activity is shown to be such an artifact. It is strongly recommended that all such experiments be performed upon freshly taken fluid samples.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. L., Tomasi T. B. Suppression of lymphocyte proliferation by copper-albumin chelates. J Biol Chem. 1984 Jun 25;259(12):7602–7606. [PubMed] [Google Scholar]
- Barrowcliffe T. W., Gray E., Kerry P. J., Gutteridge J. M. Triglyceride-rich lipoproteins are responsible for thrombin generation induced by lipid peroxides. Thromb Haemost. 1984 Aug 31;52(1):7–10. [PubMed] [Google Scholar]
- Blake D. R., Blann A., Bacon P. A., Farr M., Gutteridge J. M., Halliwell B. Ferroxidase and ascorbate oxidase activities of caeruloplasmin in synovial fluid from rheumatoid patients. Clin Sci (Lond) 1983 May;64(5):551–553. doi: 10.1042/cs0640551. [DOI] [PubMed] [Google Scholar]
- Blake D. R., Winyard P., Scott D. G., Brailsford S., Blann A., Lunec J. Endothelial cell cytotoxicity in inflammatory vascular diseases--the possible role of oxidised lipoproteins. Ann Rheum Dis. 1985 Mar;44(3):176–182. doi: 10.1136/ard.44.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chidambaram M. V., Barnes G., Frieden E. Ceruloplasmin and the reactions forming diferric transferrin. FEBS Lett. 1983 Aug 8;159(1-2):137–140. doi: 10.1016/0014-5793(83)80432-3. [DOI] [PubMed] [Google Scholar]
- Chin J. L., Calisch M. P., Topham R. W. Purification and characterization of the ferroxidase-II from rabbit (Oryctolagus cuniculus) serum. Int J Biochem. 1978;9(10):775–783. doi: 10.1016/0020-711x(78)90047-2. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Antioxidant properties of caeruloplasmin towards iron- and copper-dependent oxygen radical formation. FEBS Lett. 1983 Jun 27;157(1):37–40. doi: 10.1016/0014-5793(83)81111-9. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Copper-phenanthroline-induced site-specific oxygen-radical damage to DNA. Detection of loosely bound trace copper in biological fluids. Biochem J. 1984 Mar 15;218(3):983–985. doi: 10.1042/bj2180983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Stocks J. Caeruloplasmin: physiological and pathological perspectives. Crit Rev Clin Lab Sci. 1981;14(4):257–329. doi: 10.3109/10408368109105866. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. The protective action of superoxide dismutase on metal-ion catalysed peroxidation of phospholipids. Biochem Biophys Res Commun. 1977 Jul 11;77(1):379–386. doi: 10.1016/s0006-291x(77)80208-8. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Tickner T. R. The characterisation of thiobarbituric acid reactivity in human plasma and urine. Anal Biochem. 1978 Nov;91(1):250–257. doi: 10.1016/0003-2697(78)90838-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinecke J. W., Rosen H., Chait A. Iron and copper promote modification of low density lipoprotein by human arterial smooth muscle cells in culture. J Clin Invest. 1984 Nov;74(5):1890–1894. doi: 10.1172/JCI111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. A., Osaki S., Frieden E. A micromethod for the determination of ferroxidase (ceruloplasmin) in human serums. Clin Chem. 1967 Feb;13(2):142–150. [PubMed] [Google Scholar]
- Justement L. B., Patel S. T., Newman H. A., Zwilling B. S. Inhibition of macrophage-mediated tumor cell destruction by oxidized lipoproteins. J Natl Cancer Inst. 1984 Aug;73(2):469–474. doi: 10.1093/jnci/73.2.469. [DOI] [PubMed] [Google Scholar]
- Lando G., Berzofsky J. A., Reichlin M. Antigenic structure of sperm whale myoglobin. I. Partition of specificities between antibodies reactive with peptides and native protein. J Immunol. 1982 Jul;129(1):206–211. [PubMed] [Google Scholar]
- Lau S. J., Sarkar B. The interaction of copper(II) and glycyl-L-histidyl-L-lysine, a growth-modulating tripeptide from plasma. Biochem J. 1981 Dec 1;199(3):649–656. doi: 10.1042/bj1990649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. M. Malondialdehyde formation in stored plasma. Biochem Biophys Res Commun. 1980 Aug 29;95(4):1663–1672. doi: 10.1016/s0006-291x(80)80090-8. [DOI] [PubMed] [Google Scholar]
- Lunec J., Halloran S. P., White A. G., Dormandy T. L. Free-radical oxidation (peroxidation) products in serum and synovial fluid in rheumatoid arthritis. J Rheumatol. 1981 Mar-Apr;8(2):233–245. [PubMed] [Google Scholar]
- Lykins L. F., Akey C. W., Christian E. G., Duval G. E., Topham R. W. Dissociation and reconstitution of human ferroxidase II. Biochemistry. 1977 Feb 22;16(4):693–698. doi: 10.1021/bi00623a021. [DOI] [PubMed] [Google Scholar]
- Løvstad R. A. The protective action of ceruloplasmin on Fe2+ stimulated lysis of rat erythrocytes. Int J Biochem. 1981;13(2):221–224. doi: 10.1016/0020-711x(81)90159-2. [DOI] [PubMed] [Google Scholar]
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin Chim Acta. 1978 Nov 15;90(1):37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham R. W., Frieden E. Identification and purification of a non-ceruloplasmin ferroxidase of human serum. J Biol Chem. 1970 Dec 25;245(24):6698–6705. [PubMed] [Google Scholar]


