Abstract
Background
Limited published data exist on the association between parity (number of deliveries) and diabetes mellitus (DM). This study was conducted to evaluate the association between parity and type 2 DM (T2DM) among Sudanese women.
Method
A multistage sampling survey was conducted in four villages in the River Nile State, Sudan, between July and September 2022. The World Health Organization’s three-level stepwise questionnaire was adopted to collect women’s sociodemographic characteristics (age, sex, height, weight, marital status, parity, education level, occupation, detailed obstetric history, and family history of T2DM). Multivariate analyses were performed.
Results
A total of 397 women were recruited. Their median (interquartile range) age was 45.0 (33.0‒55.7) years. A total of 154 women (38.8%) were nulliparous, whereas 93 (23.4%), 70 (17.6%), and 80 (20.2%) had para 1‒3, 4 or 5, and more than 5, respectively. A total of 112 (28.2%) women had T2DM. In multivariate analysis, older age (adjusted odds ratio, AOR, 1.04, 95%, confidence interval, CI, 1.02‒1.06), high parity (AOR, 1.1, 95% C, 1.01‒1.20), and a family history of DM (AOR, 3.26, 95% CI, 1.98‒5.38) were associated with T2DM. Compared with the nulliparity, para 1‒3 (AOR, 2.33; 95% CI, 1.17‒4.61), para 4 or 5 (AOR, 2.12; 95% CI, 1.04‒4.30), and para > 5 (AOR, 2.16; 95% CI, 1.09‒4.27) were at higher risk of T2DM. In women aged < 50 years, high parity (AOR, 1.22; 95% CI, 1.06‒1.44) was associated with T2DM. Compared with the nulliparous women, para 4 or 5 (AOR, 3.47; 95% CI, 1.16‒10.34) and para > 5 (AOR, 4.63; 95% CI, 1.54‒13.87) were associated with T2DM, whereas para 1‒3 was not associated with T2DM. In the women aged ≥ 50 years, parity and parity groups were not associated with T2DM.
Conclusion
There is a high prevalence of T2DM among Sudanese women. Parity and high parity are significant predictors of T2DM among these women in this part of Sudan.
Keywords: Parity, Age, Diabetes mellitus, Associated factors, Sudan
Introduction
Diabetes mellitus (DM) is a chronic medical condition that affects 10.5% of the world’s population (537 million) aged 20–79 years, and it is expected to rise to 783 million (12.2%) by 2045 [1, 2]. Middle-income countries are expected to have the greatest relative increase in the prevalence of DM between 2021 and 2045 (21.1% ) [2]. The Middle East and North Africa (MENA) region had the highest prevalence of DM (18.1%) in people aged 20–79 years in 2021; low-income countries reported the highest proportion of undiagnosed DM (50.5%) [1]. Type 2 DM (T2DM) accounts for approximately 90% of all cases of DM and is a leading cause of long-term complications, such as cardiovascular disease, blindness, kidney failure, neuropathy, lower limb amputation, and cognitive diseases [1, 3]. Furthermore, DM-related mortality corresponds to 12.2% of global deaths from all causes in people aged 20–79 years, and the second highest region for mortality is the MENA region, with 20.2% of all deaths related to DM [1]. The financial burden of DM continues to rise, with the disease-causing at least USD 966 billion in health expenditures, a 316% increase over the last 15 years [1].
Pregnancy is associated with marked alterations in metabolic parameters, such as increased insulin resistance, hyperinsulinemia, and the accumulation and redistribution of body fat [4]. Previous studies have shown that high fasting plasma glucose levels [5] and elevated glycated hemoglobin [6] were associated with the number of live births. The data on the association between parity and T2DM remains inconclusive. While some studies have shown an association between parity and DM [5–11], others have reported no association between parity and DM [12–14] or parity was associated with reduced risk of DM [12].
The International Diabetes Federation (IDF) identified Sudan among countries that have a prevalence of DM of more than 12% and an estimated undiagnosed DM of less than 34% [1]. A community-based study showed that 20.8% of the adults in eastern Sudan had T2DM [15]. Moreover, a previous study in Northern Sudan has shown that females had a higher prevalence of T2DM (19.9%) compared with males (18.1%) [16]. High parity was reported among Sudanese women, as most of them had five deliveries or more (multiparity) at a younger age, before 35 years [17]. Given the importance of the two clinical entities and the limited published clinical data in Sudan, the MENA region, and Africa, the current study aims to investigate the influence of parity and high parity on the development of T2DM among Sudanese women.
Methods
This multistage sampling study recruited adult women from River Nile State, northern Sudan, during the period of July–September 2022. River Nile State, one of the 18 Sudanese states, has a population of 1,120,441 [18]. Almatamah, one of seven localities (the lowest administrative units in Sudan) in the River Nile state, was initially selected by simple random sampling. Adult women in households of four villages (i.e., Hajer Alteer, Athawra Kabota, Alkoumer, and Wadi Alshohda) were chosen randomly within the Almatamah locality based on the population size. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement standard checklists were adopted for the selection [19].
Inclusion and exclusion criteria
All the Sudanese women (> 18 years of age) from the selected household who agreed to participate were recruited for the study. Exclusion criteria included non-Sudanese nationalities, current pregnancy during the study, current use of contraceptive pills, current use of steroid medications for any medical problem, antiviral medication for chronic hepatitis infections, substance abuse, mental illness, disabilities, congenital deformities, and a competing chronic disease or malignancy. Further, those with known hemoglobinopathy (sickle cell disease, thalassemia, and glucose-6-phosphate dehydrogenase deficiency), patients with type 1 DM, severe anemia, renal failure, and liver failure, and those who refused to participate in the study were also excluded. Two general practitioners conducted interviews with the participants recruited for the study. Each participant signed informed consent after a proper explanation of their role and the aim of the study. A questionnaire was then used to collect the sociodemographic, clinical, and physical measurements, weight and height, family history and current history of DM, and blood pressure and glycated hemoglobin data. The World Health Organization’s (WHO) three-level stepwise questionnaire was adopted for data collection [20]. The questionnaire contained questions regarding the sociodemographic characteristics of the participants, including their age, marital status (married, widow, or divorced), education level (≤ secondary level or > secondary level); family history and medical history of DM, current history of hypertension, and drug history (steroid therapy). Moreover, a detailed history was obtained regarding their menopausal status, miscarriage, and the live birth/parity number. According to tradition, smoking and alcohol are not female’ habits; hence, we intentionally avoided including them in the questionnaire to encourage participants’ cooperation.
Definition of variables
Parity
Our main variable of interest was parity, which was obtained from the participants’ responses to the following question in the interview: “How many live births have you had?” The response was modeled as a categorical variable: nulliparous (0 births), 1–2 livebirths, 3–4 livebirths, and grand multiparity (≥ 5 live births).
Diabetes mellitus
A diagnosis of DM was considered for those who had documentation of T2DM and whether they were on diet control or glucose-lowering drugs during the study period or met the American Diabetes Association’s diagnosis of DM for nonpregnant adults and definition by the International Diabetes Federation [1, 21]. Random plasma glucose levels of ≥ 200 mg/dl (11.1 mmol/L) in patients with classic symptoms of hyperglycemia (polydipsia, polyuria, and polyphagia) or hyperglycaemic crisis or a glycated hemoglobin level of ≥ 6.5% were the primary diagnostic criterion.
Hypertension
Participants were diagnosed with hypertension and received treatment during the study period or fit the criteria for hypertension diagnosis based on blood pressure measurement, as described below.
Procedure for measuring blood pressure
An OMRON 3 (with an appropriate cuff size) automated blood pressure measuring device was used to obtain at least two blood pressure readings. Each participant was offered rest for at least 10 min before checking their blood pressure. The participant’s arms were adjusted to the level of their hearts. The mean of two blood pressure readings (at an interval of 1–2 min) was obtained and registered for each female. If there was a significant difference of more than 5 mmHg between the two readings, new measurements were recommended until a stable reading was obtained. The participants were considered hypertensive if systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or both criteria were recorded in both of the repeated measurements [22].
Body mass index
Body mass index (BMI) was computed from the patient’s weight and height and classified based on the WHO classification for females: underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 (kg/m2), or obese (≥ 30.0 kg/m2) [23].
Sample size
The sample size of 397 women was calculated using the Open Epi Menu [24] with an assumption of a type I error of 5% and an adequate power of 80% (β = 0.2). The estimated sample size of 397 was calculated by assuming the prevalence of T2DM (30.0%). This assumption of the prevalence of T2DM was based on our previous observations in eastern Sudan [15]. Thus, a ratio of 2:1 was expected between women without T2DM and women with T2DM. We assumed that 35.5% of women with T2DM and 20.0% without T2DM would have parity ≥ 5. This assumption was based on a study that evaluated the multiparity and risk of DM [7].
Statistics
The Statistical Package for the Social Sciences (SPSS) for Windows (IBM SPSS v.25) was employed to analyze the data. The chi-square test was used to compare the proportions between women with T2DM and those without T2DM. Continuous data were evaluated for normality using the Shapiro–Wilk test. A t-test and Mann–Whitney test were performed to assess the normally distributed and non-normally distributed data between the two groups of women (T2DM and non-T2DM), respectively. Univariate binary analysis was conducted by entering the dependent (T2DM) and independent variables (age, BMI, education, occupation, family history of DM, past medical history of hypertension, and live birth/parity number). The independent variables with a univariate P value < 0.20 were entered into the model of multivariate binary analysis. Backward likelihood ratio adjustments were processed in the different models. AORs and 95% CIs were calculated, with P < 0.05 considered statistically significant.
Results
This study enrolled 397 women. Their median (interquartile range [IQR]) age was 45.0 (33.0‒55.7) years. The parity of these 397 women ranged from 0 to 10, with a median of 2. A total of 154 women (38.8%) were nulliparous, whereas 93 (23.4%), 70 (17.6%), and 80 (20.2%) had para 1‒3, 4 or 5, and more than 5, respectively. Of the 397 women, 267 (67.3%) had an educational level ≥ secondary education, and 218 (54.9%) were housewives. Age increased with parity, and women who had a para > 5 had the highest median (IQR) age (53.0 [42.0‒60.0] years). Of the 397 enrolled women, 124 (31.2%), 39 (9.8%), 120 (30.2%), and 114 (28.7%) were normal weight, underweight, overweight, and obese, respectively. The median (IQR) BMI of 27.6 (23.7‒32.9) kg/m2 was highest in women with para 4 or 5. A borderline correlation existed between age (r = 0.135), parity (r = 0.210), and BMI. A significantly higher number of housewives was found in women with para > 5. Educational level was not statistically different among women in the parity groups (Table 1).
Table 1.
Comparison of the factors between the parity groups in women in Sudan, 2022
| Characteristics | Total (number = 397) | Nulliparity (number = 154) |
Para 1‒3 (number = 93) | Para 4 and 5(number = 70) | Para > 5(number = 80) | P | |
|---|---|---|---|---|---|---|---|
| Age | 45.0(33.0‒55.7) | 41.4(26.0‒55.0) | 40.0(32.0‒50.0) | 50.0(35.0‒57.0) | 53.0(42.0‒60.0) | < 0.001 | |
| Body mass index, kg/m2 | 26.4(22.5‒30.5) | 24.5(19.9‒28.6) | 27.1(23.5‒30.8) | 27.6 (23.7‒32.9) | 27.0 (23.7‒31.3) | < 0.001 | |
| Education level | ≥ secondary | 267(67.3) | 103(66.9) | 59 (63.4) | 48(68.6) | 57(71.3) | 0739 |
| < secondary | 130(32.7) | 51(33.1) | 34(36.6) | 22(31.4) | 23(28.7) | ||
| Occupation | Housewives | 218(54.9) | 69 (44.8) | 57(61.3) | 41 (58.6) | 51(63.7) | 0.013 |
| Employed | 179(45.1) | 85(55.2) | 36(38.7) | 29(41.4) | 29 (36.3) | ||
| Type 2 diabetes mellitus | No | 285(71.8) | 126(81.8) | 64(68.8) | 46(65.7) | 49(61.3) | |
| Yes | 112(28.2) | 28(18.2) | 29(31.2) | 24(34.3) | 31(38.8) | 0.003 |
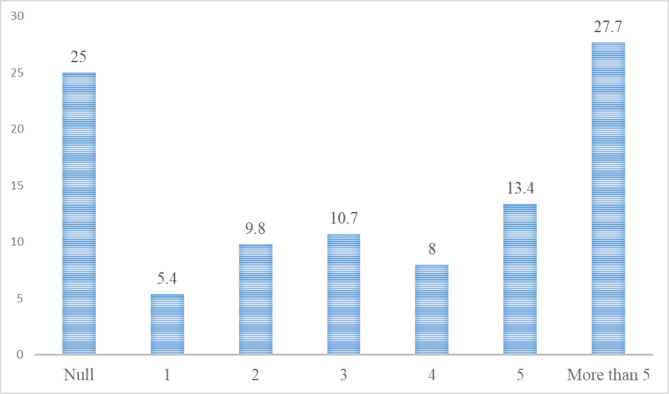
A total of 112 (28.2%) women had T2DM, 45 (11.3%) women had known T2DM and 67 (16.9%) women had newly discovered T2DM. The prevalence of T2DM increased with parity and ranged from 18.2 to 38.8%. Women who had para > 5 had the highest prevalence (38.8%) of T2DM (Table 1; Fig. 1). In the univariate analysis, older age, high parity, a family history of DM, and a higher BMI were associated with T2DM. Educational level and occupation were not associated with T2DM (Table 2). However, after adjustment in the multivariate analysis, older age (AOR, 1.04, 95% CI, 1.02‒1.06), high parity (AOR, 1.1, 95% C, 1.01‒1.20), and a family history of DM (AOR, 3.26, 95% CI, 1.98‒5.38) were associated with T2DM. Moreover, we removed parity as a continuous variable from the model and entered the parity groups into the model. In this case, compared with the nulliparity, para 1‒3 (AOR, 2.33; 95% CI, 1.17‒4.61), para 4 or 5 (AOR, 2.12; 95% CI, 1.04‒4.30), and para > 5 (AOR, 2.16; 95% CI, 1.09‒4.27) were at higher risk of T2DM (Table 3). Thereafter, we divided the women into two age groups (≥ 50 and < 50 years). In women aged < 50 years, high parity (AOR, 1.22; 95% CI, 1.06‒1.44) was associated with T2DM. Compared with the nulliparous women, although para 4 or 5 (AOR, 3.47; 95% CI, 1.16‒10.34) and para > 5 (AOR, 4.63; 95% CI, 1.54‒13.87) were associated with T2DM, para 1‒3 was not associated with T2DM. In the women aged ≥ 50 years, parity and parity groups were not associated with T2DM (Table 3).
Fig. 1.
Shows the prevalence of type 2 diabetes mellitus stratified by parity among Sudanese women in 2022
Table 2.
Univariate analysis of the factors (unadjusted) associated with hypertension among women in Sudan, 2022
| Women with type 2 diabetes mellites (number = 112) | Women without type 2 diabetes mellites (number = 285) | OR (95% CI) | P | ||
|---|---|---|---|---|---|
| Median (interquartile range) | |||||
| Age, years | 50.0(38.0‒60.0) | 38.0(28.0‒50.0) | 1.04(1.02‒1.05) | < 0.001 | |
| Para | 3(0‒6) | 1(0‒4) | 1.16(1.07‒1.25) | < 0.001 | |
| Body mass index, kg/m2 | 27.6(24.0‒31.3) | 24.3(19.5‒28.3) | 1.02 (0.99‒1.06) | 0.156 | |
| Frequency (proportion) | |||||
| Education level | ≥ secondary | 154(67.5) | 122(67.8) | Reference | 0.753 |
| < secondary | 74(32.5) | 58(32.2) | 1.07 (0.67‒1.72) | ||
| Occupation | Housewives | 132(57.9) | 91(50.6) | Reference | 0.218 |
| Employed | 96(42.1) | 89(49.4) | 1.32 (0.84‒2.05) | ||
| Family history of diabetes mellites | No | 100(43.9) | 110(61.1) | Reference | < 0.001 |
| Yes | 128(56.1) | 70(38.9) | 3.29 (2.07‒5.23) | ||
| Para | Null | 69(30.3) | 89(49.4) | Reference | |
| 1‒3 | 53(23.2) | 42(23.3) | 2.03 (1.11‒3.71) | 0.020 | |
| 4 and 5 | 45(19.7) | 28(15.6) | 2.34 (1.23‒4.45) | 0.009 | |
| More than 5 | 61(26.8) | 21(11.7) | 2.84 (1.55‒5.23) | 0.001 |
Table 3.
Multivariate analysis of the adjusted factors associated with type 2 diabetes mellitus among women in Sudan, 2022
| All women (397) | Women with age ≥ 50 years | Women with age < 50 years | |||||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | OR (95% CI) | P | ||
| Age, years | 1.04(1.02‒1.06) | < 0.001 | ‒ | ‒ | |||
| Parity | 1.11(1.01‒1.20) | 0.017 | 1.04 (0.93 ‒1.16) | 0.448 | 1.22 (1.06‒1.44) | 0.004 | |
| Body mass index | 0.98 (0.94‒1.03) | 0.552 | 0.99 (0.94‒1.05) | 0. 981 | 0.97 (0.91‒1.04) | 0.425 | |
| Family history of hypertension | No | Reference | < 0.00 | Reference | Reference | 0.007 | |
| Yes | 3.26 (1.98‒5.38) | 3.42(1.75‒6.69) | < 0.001 | 2.88 (1.34‒6.22) | |||
| Para | Nulli | Reference | Reference | Reference | |||
| 1‒3 | 2.33 (1.17‒4.61) | 0.015 | 2.87 (1.01‒8.16) | 0.048 | 2.40 (0.89‒6.46) | 0.082 | |
| 4 and 5 | 2.12 (1.04‒4.30) | 0.037 | 1.33 (0. 52‒3.93) | 0.551 | 3.47 (1.16‒10.34) | 0.025 | |
| > 5 | 2.16 (1.09‒4.27) | 0.027 | 1.29 (0.54‒3.07) | 0.557 | 4.63 (1.54‒13.87) | 0.006 | |
Discussion
The main finding of this study was that high parity was associated with T2DM (compared with the nulliparity, para 1‒3, para 4 or 5, and para > were at higher risk of T2DM). This is consistent with similar results of a population-based, prospective cohort study of 7,024 Caucasian and African-American women (45–64 years) with nine years of follow-up, which documented that grand multiparity (five or more live births) was associated with a higher prevalence of T2DM (44%) and predictive of future risk (27%) [6]. Another longitudinal cohort study of adult American women aged ≥ 65 years (3,211 participants) found that women with grand multiparity (≥ five live births) had a higher prevalence of T2DM (25.0%) at baseline compared with those with fewer births or nulliparous women [5]. Similarly, a prospective cohort study that evaluated a total of 25,021 Singaporean Chinese women aged 45–74 years for about 5.7 years of follow-up demonstrated a significant association between high parity, particularly for women who had five or more live births, and a higher risk of developing T2DM (74%) [11]. Further, a significant association was observed between grand multiparity (6 or more births) and increased prevalence of T2DM (10.3%) among postmenopausal Hispanic women compared to those with nulliparity [25], and 50.0% of Filipino American women had T2DM compared with low parity women [7]. Similarly, one study from China that recruited a total of 14,196 women (aged ≥ 45 years) showed a higher prevalence of T2DM (26.2%) and a significant association of a higher risk of T2DM among multiparous women (four or more live births) compared with women who had one live birth [10]. The results of two meta-analysis studies strengthened this. The first study (286,840 female participants) showed a linear relationship between high parity and the risk of T2DM, which increased by 42% [8]. The second study (296,923 participants) revealed that high parity (at least three live births) was associated with a significantly increased risk of T2DM (54%) [9]. Similarly, a Danish study (100669 participants) reported a T2DM rate of 3.69% among women with parity ≥ 4, and parity was associated with a significantly higher risk of T2DM compared with those with one child [26]. Further, Iranian women with parity ≥ 4 were shown to have more than 60% higher risk of developing T2DM compared to those with one parity during a median follow-up of 15.4 years (2552 participants, aged 30–65 years) [27]. A similar significant association between the risk of T2DM and parity was found among nulliparous women and those with 2 or ≥ 3 births compared to those with one birth in an observational cohort study that included 131,174 Chinese females aged ≥ 40 years [28].
In the current study, while in women aged < 50 years, high parity was associated with T2DM, in the women aged ≥ 50 years, parity and parity groups were not associated with T2DM. In a prospective cohort study (113,606 participants, aged 30 to 55 years) among United States registered nurses with follow-up for 12 years, parity was not found to be associated with a significantly increased risk of subsequent clinical T2DM among women with six or more births compared with nulliparous women [13]. This was strengthened by the non-significance association among women with parity ≥ 4 children and age-matched low-parity women (1 and 2 children) in terms of insulin resistance and β-pancreatic cell function [14]. Moreover, high parity was associated with higher fasting insulin levels and reduced insulin sensitivity, which increases the risk of subsequent clinical insulin resistance [29]. This indicates that reproductive-age hires have a higher risk for developing T2DM compared to post-menopausal women, as pregnancy is associated with milder degrees of dysglycemia [30]. This may be linked to weight retention and increased insulin resistance associated with pregnancy [31]. Additionally, pregnancy is associated with reduced physical activity and some cardiometabolic changes (weight gain and dyslipidemia) that can exacerbate insulin resistance [31]. Moreover, repeated pregnancy is considered another contributing factor [32]. Reproductive age increases the chance of using contraceptive methods, in particular, pills for family planning; long-term use of contraceptive methods is associated with a significant risk of T2DM among this age group [33]. This higher prevalence of T2DM, associated with higher parity compared to nulliparity, may reflect the influence of parity as a significant risk factor for developing T2DM.
On the other hand, previous studies showed that parity was not associated with an increased risk of subsequent DM [13, 14]. Moreover, there were no differences observed in insulin sensitivity or insulin secretion among women with high parity when compared with age-matched and BMI-matched women in a lower parity group [14]. On the other hand, data from a population-based study that recruited 383 females (aged 12–79 years) in a Native Canadian community documented an association between parity and a significantly reduced risk of T2DM (nulliparous vs. ≥1 birth) [12].
The outcomes of these studies must be compared cautiously with our results. It is obvious that there were discrepancies in the methods adopted in these studies, and there were modeling differences. While some studies compared women with high parity (≥ 5 births) to low-to-moderate parity and nulliparous as references, other studies did not include nulliparous women in the studies as references. Moreover, different cutoffs of age or range of participants were chosen for each study. The difference in lifestyle, cultural, and genetic influences and the difference in the prevalence of T2DM may influence the results of these studies, which were conducted in different populations [34]. Women with a certain genetic heritage exhibit insulin resistance during pregnancy, a phenomenon also reflected in the occurrence of polycystic ovarian syndrome, gestational diabetes, and/or T2DM, as more than 250 loci are linked with T2DM [35]. Compared with nulliparity, metabolic syndrome was significantly associated with parity, and for each increment of one live birth, the risk of metabolic syndrome was positively and directly related [36]. In addition, more women are overweight or obese after the age of 45, indicating the influence of aging [37]. The diagnosis of metabolic syndrome is associated with a potential risk of hyperglycaemia and developing T2DM among this group of subjects [36]. The significant association of T2DM among women over 50 years of age may be explained by aging, as it is associated with central obesity, which is responsible for insulin resistance and future T2DM [38]. Further, reduced muscle tissue (30–40%) favoring adipose tissue, reduced physical activity, and usage of potentially diabetogenic medications, such as diuretics, beta-adrenolytics, corticosteroids, and psychotropic medications [39]. Moreover, the sensitivity of pancreatic β cells for incretins declines with aging, leading to lower postprandial insulin levels weaker suppression of glucagon secretion [40], and growing insulin resistance with increased age [41]. Further, low estrogen after the menopausal period negatively affects body fat distribution, and fat accumulation is an important contributor to T2DM risk [42]. Moreover, evidence suggests that the female hormone, 17β-oestradiol protects insulin production and prevents T2DM because it acts primarily via two distinct estrogen receptors, alpha (ERα) and beta (ERβ ): while ERα promotes β-cell survival, ERβ reduces ERα function and enhances β-cell apoptosis [42]. Alterations in cytokines during pregnancy are a potential pathophysiological mechanism behind the increase in insulin resistance, as white adipose tissue and the placenta represent endocrine organs, secreting adipokines, and cytokines, including tumor necrosis alpha (TNF-α) factor, leptin, adiponectin, and interleukin-6 (IL-6) [43]. Some new biomarkers were linked with sexual dimorphisms, such as the hepatocyte fetuin-A [44], and high levels of copeptin (the C-terminal portion of the precursor of vasopressin) were associated with a significant risk of future T2DM in women but not in males [45]. Likewise, exosomes (membrane-derived nanovesicles) secreted from both the placenta and adipose tissue may have an effect throughout gestation, mediating a placental response to hyperglycemia and insulin sensitivity [46]. This is supported by the higher levels of exosomes observed in women with gestational DM compared to normal pregnancies across gestation [47].
This study has certain limitations that should be considered. The reproductive history of the subjects was self-reported, which may lead to misclassification of parity, gravidity gestational DM, and use of oral contraceptives, particularly among older women. Further, self-reporting of menopausal status may be associated with some misclassification, and some other risk factors were not assessed, such as salt intake, diet, physical exercise, oral contraceptive use, smoking, alcohol consumption, and lipid profile.
Conclusion
The prevalence of T2DM among Sudanese women was found to be significantly high, with parity and the number of live births being significant predictors of T2DM among adult Sudanese females.
Acknowledgements
The authors would like to thank all the participants who participated in this study.
Author contributions
IRM and IA conceived the study; IRM, OEO, and IA supervised the work, guided the analysis and critically reviewed the manuscript; OEO, and IA prepared the analysis plan, performed the data analysis and wrote the first draft of the manuscript. All authors reviewed and approved the final manuscript.
Funding
None received.
Data availability
The datasets generated and/or analyzed during the current study are not publicly available (because the manuscript is still under the peer review process) but are available from the corresponding author on a reasonable request.
Declarations
Ethical approval and consent to participate
This study complies with the Declaration of Helsinki. The Ethics Committee of the Health Authority in Almatamah, Sudan, provided ethical approval of the study (reference number # 03/2021). A signed written informed consent was collected from each participant.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rahmawati. IDF Diabetes Atlas 2021 _ IDF Diabetes Atlas. IDF Off website. 2021;:1–4.
- 2.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. <ArticleTitle Language=“En”>IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stuebe AM, Mantzoros C, Kleinman K, Gillman MW, Rifas-Shiman S, Seely EW, et al. Gestational glucose tolerance and maternal metabolic profile at 3 years postpartum. Obstet Gynecol. 2011;118:1065–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fowler-Brown AG, De Boer IH, Catov JM, Carnethon MR, Kamineni A, Kuller LH, et al. Parity and the association with diabetes in older women. Diabetes Care. 2010;33:1778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicholson WK, Asao K, Brancati F, Coresh J, Pankow JS, Powe NR. Parity and risk of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2006;29:2349–54. [DOI] [PubMed] [Google Scholar]
- 7.Araneta MRG, Barrett-Connor E. Grand multiparity is associated with type 2 diabetes in Filipino American women, independent of visceral fat and adiponectin. Diabetes Care. 2010;33:385–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo P, Zhou Q, Ren L, Chen Y, Hui Y. Higher parity is associated with increased risk of Type 2 diabetes mellitus in women: A linear dose-response meta-analysis of cohort studies. J Diabetes Complications. 2017;31:58–66. [DOI] [PubMed] [Google Scholar]
- 9.Li P, Shan Z, Zhou L, Xie M, Bao W, Zhang Y, et al. Mechanisms In Endocrinology: Parity and risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Endocrinol. 2016;175:R231–45. [DOI] [PubMed] [Google Scholar]
- 10.Tian Y, Shen L, Wu J, Chen W, Yuan J, Yang H, et al. Parity and the risk of diabetes mellitus among Chinese women: a cross-sectional evidence from the Tongji-Dongfeng cohort study. PLoS ONE. 2014;9:e104810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller NT, Mueller NJ, Odegaard AO, Gross MD, Koh WP, Yuan JM, et al. Higher parity is associated with an increased risk of type-II diabetes in Chinese women: the Singapore Chinese Health Study. BJOG. 2013;120:1483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanley AJG, McKeown-Eyssen G, Harris SB, Hegele RA, Wolever TMS, Kwan J, et al. Association of parity with risk of type 2 diabetes and related metabolic disorders. Diabetes Care. 2002;25:690–5. [DOI] [PubMed] [Google Scholar]
- 13.Manson JE, Rimm EB, Colditz GA, Stampfer MJ, Willett WC, Arky RA, et al. Parity and incidence of non-insulin-dependent diabetes mellitus. Am J Med. 1992;93:13–8. [DOI] [PubMed] [Google Scholar]
- 14.Iversen DS, Støy J, Kampmann U, Voss TS, Madsen LR, Møller N, et al. Parity and type 2 diabetes mellitus: a study of insulin resistance and β-cell function in women with multiple pregnancies. BMJ open diabetes Res care. 2016;4:e000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omar SM, Musa IR, ElSouli A, Adam I. Prevalence, risk factors, and glycaemic control of type 2 diabetes mellitus in eastern Sudan: a community-based study. Ther Adv Endocrinol Metab. 2019;10:2042018819860071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eltom MA, Babiker Mohamed AH, Elrayah-Eliadarous H, Yassin K, Noor SK, Elmadhoun WM, et al. Increasing prevalence of type 2 diabetes mellitus and impact of ethnicity in north Sudan. Diabetes Res Clin Pract. 2018;136:93–9. [DOI] [PubMed] [Google Scholar]
- 17.Alsammani MA, Jafer AM, Khieri SA, Ali AO, Shaaeldin MA. Effect of Grand Multiparity on Pregnancy Outcomes in Women Under 35 Years of Age: a Comparative Study. Med Arch (Sarajevo, Bosnia Herzegovina). 2019;73:92–6. [DOI] [PMC free article] [PubMed]
- 18.Sudan – 5th Sudan Population and Housing Census. 2008 - IPUMS Subset. https://microdata.worldbank.org/index.php/catalog/1014. Accessed 24 Jul 2023.
- 19.Cuschieri S. The STROBE guidelines. Saudi J Anaesth. 2019;13(Suppl 1):S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riley L, Guthold R, Cowan M, Savin S, Bhatti L, Armstrong T, et al. The world health organization STEPwise approach to noncommunicable disease risk-factor surveillance: Methods, challenges, and opportunities. Am J Public Health. 2016;106:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glycemic targets. standards of medical care in Diabetesd2018. Diabetes Care. 2018;41(Suppl 1):S55–64. [DOI] [PubMed] [Google Scholar]
- 22.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16:223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO Consultation on Obesity (1999. Geneva, Switzerland) & World Health Organization. (2000). Obesity: preventing and managing the global epidemic : report of a WHO consultation. World Health Organization. https://iris.who.int/handle/10665/42330 [PubMed]
- 24.OpenEpi Menu. https://www.openepi.com/Menu/OE_Menu.htm. Accessed 24 Mar 2023.
- 25.Cure P, Hoffman HJ, Cure-Cure C. Parity and diabetes risk among hispanic women from Colombia: cross-sectional evidence. Diabetol Metab Syndr. 2015;7. 10.1186/s13098-015-0001-z. [DOI] [PMC free article] [PubMed]
- 26.Naver KV, Lundbye- Christensen S, Gorst- Rasmussen A, Nilas L, Secher NJ, Rasmussen S, et al. Parity and risk of diabetes in a Danish nationwide birth cohort. Diabet Med. 2011;28:43–7. [DOI] [PubMed] [Google Scholar]
- 27.Moazzeni SS, Hizomi Arani R, Asgari S, Azizi F, Hadaegh F. The association of parity/live birth number with incident type 2 diabetes among women: over 15 years of follow-up in The Tehran Lipid and Glucose Study. BMC Womens Health. 2021;21. 10.1186/s12905-021-01519-7. [DOI] [PMC free article] [PubMed]
- 28.Huo Y, Cheng L, Wang C, Deng Y, Hu R, Shi L, et al. Associations between parity, pregnancy loss, and breastfeeding duration and risk of maternal type 2 diabetes: An observational cohort study. J Diabetes. 2021;13:857–67. [DOI] [PubMed] [Google Scholar]
- 29.Abdelsalam KEA, Elamin AAM. Influence of Grand Multiparity on the Levels of Insulin, Glucose and HOMA-IR in Comparison with Nulliparity and Primiparity. Pakistan J Biol Sci PJBS. 2017;20:42–6. [DOI] [PubMed] [Google Scholar]
- 30.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet (London England). 2009;373:1773–9. [DOI] [PubMed] [Google Scholar]
- 31.Kampmann U, Knorr S, Fuglsang J, Ovesen P. Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J Diabetes Res. 2019;2019:5320156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derakhshan A, Tohidi M, Hajebrahimi MA, Saadat N, Azizi F, Hadaegh F. Sex-specific incidence rates and risk factors of insulin resistance and β-cell dysfunction: a decade follow-up in a Middle Eastern population. Diabet Med. 2017;34:245–52. [DOI] [PubMed] [Google Scholar]
- 33.Mosorin ME, Ollila MM, Nordström T, Jokelainen J, Piltonen T, Auvinen J, et al. Former long-term use of combined hormonal contraception and glucose metabolism disorders in perimenopausal women: A prospective, population-based cohort study. Acta Obstet Gynecol Scand. 2023;102:1488–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aguayo-Mazzucato C, Diaque P, Hernandez S, Rosas S, Kostic A, Caballero AE. Understanding the growing epidemic of type 2 diabetes in the Hispanic population living in the United States. Diabetes Metab Res Rev. 2019;35:e3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langenberg C, Lotta LA. Genomic insights into the causes of type 2 diabetes. Lancet. 2018;391:2463–74. [DOI] [PubMed] [Google Scholar]
- 36.Sun MH, Wen ZY, Wang R, Gao C, Yin JL, Chang YJ, et al. Parity and Metabolic Syndrome Risk: A Systematic Review and Meta-Analysis of 15 Observational Studies With 62,095 Women. Front Med. 2022;9:926944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kautzky-Willer A, Harreiter J, Pacini G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr Rev. 2016;37:278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyrovolas S, Koyanagi A, Garin N, Olaya B, Ayuso-Mateos JL, Miret M, et al. Diabetes mellitus and its association with central obesity and disability among older adults: a global perspective. Exp Gerontol. 2015;64:70–7. [DOI] [PubMed] [Google Scholar]
- 39.Mordarska K, Godziejewska-Zawada M. Diabetes in the elderly. Prz menopauzalny = Menopause Rev. 2017;16:38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang AM, Halter JB. Aging and insulin secretion. Am J Physiol Endocrinol Metab. 2003;284:E7–12. [DOI] [PubMed] [Google Scholar]
- 41.Krentz AJ, Viljoen A, Sinclair A. Insulin resistance: a risk marker for disease and disability in the older person. Diabet Med. 2013;30:535–48. [DOI] [PubMed] [Google Scholar]
- 42.Cignarella A, Bolego C, Cignarella A. Mechanisms of estrogen protection in diabetes and metabolic disease. Horm Mol Biol Clin Investig. 2010;4:575–80. [DOI] [PubMed] [Google Scholar]
- 43.Catalano PM. Trying to understand gestational diabetes. Diabet Med. 2014;31:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laughlin GA, Barrett-Connor E, Cummins KM, Daniels LB, Wassel CL, Ix JH. Sex-specific association of fetuin-A with type 2 diabetes in older community-dwelling adults: the Rancho Bernardo study. Diabetes Care. 2013;36:1994–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbasi A, Corpeleijn E, Meijer E, Postmus D, Gansevoort RT, Gans ROB, et al. Sex differences in the association between plasma copeptin and incident type 2 diabetes: the Prevention of Renal and Vascular Endstage Disease (PREVEND) study. Diabetologia. 2012;55:1963–70. [DOI] [PubMed] [Google Scholar]
- 46.Jayabalan N, Nair S, Nuzhat Z, Rice GE, Zuñiga FA, Sobrevia L et al. Cross talk between adipose tissue and placenta in obese and gestational diabetes mellitus pregnancies via exosomes. Front Endocrinol (Lausanne). 2017;8 SEP:239. [DOI] [PMC free article] [PubMed]
- 47.Salomon C, Scholz-Romero K, Sarker S, Sweeney E, Kobayashi M, Correa P, et al. Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes. 2016;65:598–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available (because the manuscript is still under the peer review process) but are available from the corresponding author on a reasonable request.