Abstract
Background
Cerebral palsy (CP) is one of the most common motor-postural disorders in childhood. It occurs due to impairment in the developing brain—before, during, or after birth—and has a significant burden on the public health system. This study aimed to investigate the potential risk factors and detect the associated CP-related disorders.
Methods
This case-control study was conducted on 46 children with CP and 175 matched healthy children less than three years old who referred to the Children’s Hospital, Tabriz, Iran in 2022. Then, a checklist related to the mother’s medical history during current and previous pregnancies, a questionnaire related to perinatal factors of the newborn, types of CP, concurrent disorders, the Gross Motor Function Classification System (GMFCS), and Age and Stage Questionaire (ASQ) were completed. Data was analyzed using Statistical Package for the Social Sciences) SPSS(-21 software by descriptive and analytical statistics consisted of Chi-square, Independent t-test, and Binary logistic regression.
Results
Finally, 35 children with CP and 122 healthy children completed the study and were analyzed. The mean (standard deviation: SD) age of children in the CP group was 15.3 (6.2) and in the healthy group was 14.4 (6.6) months (p = 0.635). Spastic CP (82.9%) was the common type, and the most common prevailing form of the involved limb was quadriplegia (54.3%). The severity of the functional disorder in 39.3% of CP cases was at levels 4 and 5 (severe form). The most prevalent comorbidities were inability to walk (31.4%), speech delay (22.9%), epilepsy (11.4), and strabismus (8.6%). Children with CP had abnormal development in gross motor (82.9%), problem-solving (68.6%), personal-social (65.7%), fine motor (60%), and communication (54.3%). Moreover, duration of pregnancy (p = 0.023), birth weight lower than 2500 g (p = 0.002), problems in the current pregnancy [adjusted odds ratio (aOR) [95% CI]: 3.06 (1.87 to 8.54); p = 0.013] and problems in previous pregnancy ([aOR (95% CI): 4.8 (1.6 to 14.2); p = 0.005) were potential risk factors.
Conclusion
Due to accompanying movement, vision, and speech problems, especially high developmental disorders in children with CP, necessary measures to prevent the identified risk factors are very important.
Keywords: Cerebral palsy, Risk factors, Functional status, Neurodevelopment, Young children, Iran
Background
Cerebral Palsy (CP) is one of the most common causes of physical disability in children, which causes motor disability and postural control [1]. The prevalence of this disorder has been reported to be about 2 to 4 cases per 1,000 live births worldwide [2] and approximately 2 cases per 1000 live births in Iran [1]. Despite numerous advances in fields of treatment and intervention in perinatology, the incidence rate of CP in full-term infants has remained constant in recent decades. The number of premature infants is still increasing, which resulted from the improvement in the survival of them due to technical and interventional advances [3]. Brain damage leading to CP can occur before birth, at birth, one month after birth, or in the first years of a child’s life during brain development [4].
The types of CP are: spastic quadriplegia, spastic diplegia, spastic monoplegia or hemiplegia, dyskinetic, and ataxia. The types of CP are classified into spastic and non-spastic categories based on physiological indicators (including hypotonic, atonic, athetoid or dyskinetic, and compound) or spatial indicators (hemiplegia, diplegia, quadriplegia, and double hemiplegia) [5]. About 85% of children suffering from CP are spastic, the most common of which is diplegia, which includes 34% of all children with CP. Impairment e in communication and social interaction has been observed in 58% of children with CP [6].
CP is often associated with sensory, cognitive, communication, behavioral, epileptic, and skeletal problems. CP originates from the central nervous system, and this disorder causes disturbance in the movement and condition of body organs that are in a static state [7–9]. Disorders observed in children with CP during infancy include seizures, hypotonia, fontanel enlargement, and less common disorders such as restlessness, feeding problems, and excessive tremors or abnormal crying [7]. Primary and secondary disorders in the visual cortex of the brain can lead to blindness, amblyopia, visual field disorders, lack of visual recognition, and lack of visual tracking. The rate of visual impairment has been reported in 42% of CP patients [6]. The prevalence of hearing deficiencies in these children is lower than that of vision because auditory signals are mainly processed in the brain stem and are less affected by the damage caused by lack of oxygen to the brain. It has been reported as 7 to 37% which includes sensorineural, conductive, mixed, and unspecified types [10]. Autistic disorders are seen in 6.9% of children with non-spastic CP, and also 41% of epilepsy cases are associated with CP. In several studies, seizures are present in 15 to 60% of children with CP [11–13]. Speech disorders are also seen in more than 80% of children with CP [14]. Of course, the prevalence of these disorders has a significant relationship with the type and intensity of motor involvement. Delayed cognitive development and lower-than-average IQ are seen in 50 to 75% of these children. About half of children with CP have digestive and nutritional problems [11].
Studies conducted on CP show that CP cannot be cured, but some of its symptoms and complications can be treated [15]. Conventionally, most treatments and interventions in CP are based on a medical approach that focuses on reducing spasticity and preventing shortness and deformities. This approach emphasizes conservative interventions (splints and cast), drug treatment (baclofen and botulism toxin), and surgical treatment [16].
Low birth weight (LBW) and preterm birth have been known as the most common causes of CP in developed countries [4]. Multiple pregnancies [17], assisted reproductive technology (ART) [18], inflammation and infection during pregnancy, hyperbilirubinemia and kernicterus, the presence of medical problems and diseases in the mother, and childbirth complications are among the risk factors of CP [19]. In most cases, CP is caused by congenital abnormalities of the brain, and less than 10% are caused by factors such as intrauterine asphyxia, uterine and placental infections, amniotic fluid problems, maternal infections, and maternal fever during childbirth. Urinary infections increase the risk of CP in children with normal birth.Furthermore, numerous evidence indicate that genetic factors also play a key role [14]. A small percentage of CP is caused by brain damage that occurs more than 28 days after birth, and this condition is called acquired CP and is usually associated with meningitis or head injury [20].
In a case-control study conducted by Inaloo et al., the type of delivery was mentioned as the risk factors for CP. Cesarean section (C/S) was twice in the CP group and has been mentioned as a risk of CP [21]. In a cross-sectional study conducted in Turkey, no significant difference was observed in C/S, breech delivery, and normal delivery between the studied groups [22]. In a meta-analysis study by Callaghan et al., which examined 9 case studies and 4 cohort studies, no significant relationship was observed between CP and C/S [23]. According to the studies, the risk factors of CP can be different somewhat based on geographical and socio-cultural differences. Identifying preventable risk factors in this area seems necessary to reduce the aforementioned problems for newborns, families, and society. Therefore, the aim of this study was to investigate potential risk factors, neurodevelopment, and motor function in CP children under 3 years of age in the northwestern region of Iran.
Methods
This study was conducted in Mardani Azar Children’s Hospital and Tabriz Comprehensive Growth and Development Center in 2021–2022 after obtaining ethical permission to conduct the research from the Regional Research Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1400.897) in two phases. The study was conducted in accordance with the Declaration of Helsinki [24]. The first phase of this project was a cross-sectional study aimed to investigate the frequency of CP types in children under three (CP classification based on muscle tone and the involved limb). Based on this, CP types include: spastic, dyskinetic (athetoid, ataxic, and chorea), hypotonic, or mixed, and were divided into diplegic, quadriplegic, hemiplegic, triplegic, and monoplegic typesaccording to the involved limb. The inclusion criteria were a diagnosis of CP under three years. Exclusion criteria were myopathy, spine defects, encephalitis, meningitis, chromosomal abnormalities, genetic syndromes, chronic systemic diseases, or malnutrition. It should be mentioned that CP was diagnosed by a pediatrician who specialized in child development. Data were collected through the medical files available in the children’s hospital and additional information was collected through contacting people. The sample size was based on the total number of available children for this phase. The sampling method was simple and accessible.
Children’s developmental process was evaluated by the Age and Stage Questionnaire (ASQ) and gross motor function score by the Gross Motor Functional Classification System (GMFCS). The ASQ is a valid questionnaire and screening tool for examining the development of children, which examines the ability of children aged 2 to 60 months in important areas such as communication, gross and fine movements, problem-solving, and personal social domains [25]. In the study of Vameghi et al., the reliability and validity of the ASQ questionnaire were investigated and the reliability with Cronbach’s alpha was determined between 0.76 and 0.86, and the inter-observer reliability was 0.93. Validity determined by factor analysis was satisfactory [26]. GMFCS is a standard measure for gross motor classification in children with CP aged 1 to 12 years. GMFCS is a five-level system designed to reflect differences in gross motor performance. Differentiation between levels of motor function is based on functional limitations, the need for assistive technology, including mobility devices (such as walkers, crutches, and canes) and wheeled mobility, and to a much lesser extent, quality of movement. It has been designed for four age groups: less than two years, two to four years, four to six years, and six to twelve years [27]. The stability of GMFCS has been examined by Palisano et al., and the weighted kappa coefficient between the first and last rating was 0.84 for children less than 6 years old and 0.89 for children at least 6 years old [28].
Also, associated disorders (speech and language problems, speech delay, swallowing disorder, drooling from the mouth or damage in controlling the movements of the tongue and lips), visual problems (strabismus, blurred vision, blindness, limitation of eye movement and lack of eye coordination), walking disability, cognitive disorders, epilepsy, hearing problems and mental retardation) were fully investigated. Descriptive statistics including frequency and percentage were used to report the types of disorders associated with CP.
The second phase of the project was an analytical study (case-control) aimed to investigate the risk factors among children with CP. After examining the frequency of types of CP and other variables studied for the study group, five times the number of children under three years old diagnosed with CP were examined as a control group [29]. The control group was selected from children under three years of age who were referred to the specialized children’s clinic for other reasons. Thee exclusion criteria in this group were children diagnosed with CP, chromosomal abnormalities, myopathies, spine defects, encephalitis, meningitis, genetic syndromes, chronic systemic diseases, or malnutrition who were selected after assimilation in terms of age and sex. After obtaining a informed consent from the parents, a questionnaire was completed for each child. It contained medical history before, during, and after birth, which was completed with the help of the mother and the existing medical record. Also, another questionnaire was completed for the mother, which included examining the medical history and history of the mother in the previous and current pregnancy. The mother’s medical history in the current pregnancy included premature rupture of the amniotic sac, history of infertility treatment, multiple births, preterm parturition in the current pregnancy, and cervical insufficiency, multipar and nullipar. The medical history of the previous pregnancy included intrauterine death, previous infant death, preterm delivery, history of congenital malformation, cervical insufficiency, and miscarriage. The mother’s medical history included diabetes, hypertension, chronic disease, epilepsy, infections, hypothyroidism, hyperthyroidism, kidney disease, consanguineous marriage, and disability in the family. To determine the validity of the tools used in this research, content and face validity were used. So that before starting the study the researcher provided the questionnaires to 8 members of the academic staff of the research group and after collecting their opinions, the necessary corrections were made to the tools based on the obtained feedback. These questionnaires were completed for both case and control groups.
Data was analyzed using Statistical Package for the Social Sciences) SPSS(-21 software. To compare the investigated variables in two groups, Chi-square statistical tests as well as Fisher’s exact test and independent t-test were used. Kolmogorov-Smirnov was used to test the normality of the ASQ scores. Problem-solving and gross motor domains had non-normal distribution. To check the relationship between the investigated factors with CP in children, the Odds Ratio using the univariate logistic regression and then the multivariate logistic regression model adjusting the confounding variables was used. The Hosmer-Lemshow test was used to check the fitness of the model. A p-value less than 0.05 was considered as significant.
Results
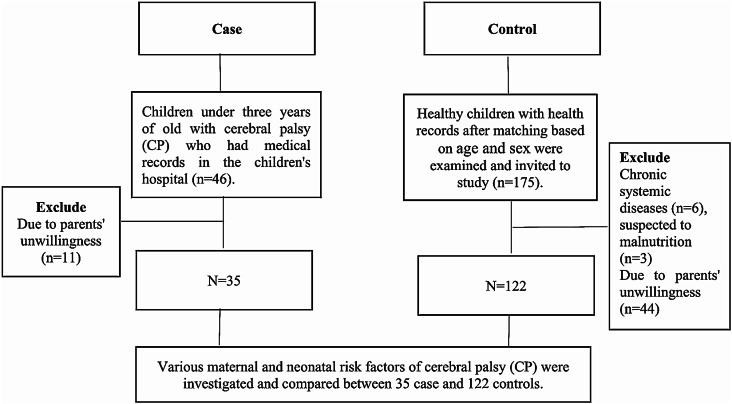
In this study, 46 children under three years old with CP who had medical records in the children’s hospital and were eligible for the study were identified and examined. Due to parents’ unwillingness to participate in the study, 11 children were excluded, and 35 CP children were included in the study. Then 5 times the number of these children, i.e. 175 healthy children with health records were examined and invited to study after matching with the affected group in terms of age and sex. Nine of them were not eligible for the study and 44 of the children did not want to participate in the study. Finally 122 healthy children were included in the study and were examined in terms of study variables (Fig. 1).
Fig. 1.
The flowchart of the study
Based on the results of Table 1, in terms of gender, 51.4% of the children in the CP group and 52.5% in the healthy group belonged to boys (p = 0.530). The mean (standard deviation: SD) and median (min, max) age of children in the CP group was 15.3 (6.2) and 14.0 (8.0, 36.0) and in the healthy group was 14.4 (6.6) months and 12.0 (6.0, 36.0) (p = 0.635). Two-thirds (65.8%) of children in the CP group and 76.2% of children in the healthy group were born by cesarean section (p = 0.448). Maternal parity was multiparous in more than half of the cases in both groups (p = 0.431). There was no significant difference between the two groups in terms of mother’s and father’s education level, father’s and mother’s occupation, history of mother’s problems in previous pregnancy, number of children, mother’s medical history, and maternal problems (p > 0.05). However, there was a significant difference between the two groups in terms of pregnancy duration (p < 0.001), birth weight (p < 0.001), and neonatal problems (p = 0.010). So, the percentage of children with pregnancy duration less than 34 weeks (12 (34.3%) vs. 9 (7.4%)), birth weight less than 1500 g (5 (14.7%) vs. 2 (1.7%)), and a history of neonatal problems (12 (34.3%) vs. 16 (13.1%)) in CP was significantly more than healthy group.
Table 1.
The demographic characteristics of study subjects (n = 157)
| Variable | Healthy (n = 122) | CP (n = 35) | P-value | ||
|---|---|---|---|---|---|
| n | Percent | N | Percent | ||
| Gender | 0.530 | ||||
| Female | 58 | 47.5 | 17 | 48.6 | |
| Male | 64 | 52.5 | 18 | 51.4 | |
| Age (month)* Mean ± SD | 14.4 | 6.6 | 15.3 | 6.2 | 0.635 |
| Education of mother | 0.647 | ||||
| College or above | 15 | 12.4 | 5 | 14.3 | |
| High school | 65 | 53.7 | 20 | 57.1 | |
| primary - middle school | 39 | 32.2 | 9 | 25.7 | |
| illiterate | 2 | 1.7 | 1 | 2.9 | |
| Education of father | 0.917 | ||||
| College or above | 13 | 10.8 | 6 | 17.1 | |
| High school | 60 | 50.0 | 15 | 42.9 | |
| primary - middle school | 45 | 37.5 | 12 | 34.3 | |
| illiterate | 2 | 1.7 | 2 | 5.7 | |
| Occupation of mother | 0.711 | ||||
| Employed | 8 | 6.6 | 3 | 8.6 | |
| Unemployed | 113 | 93.4 | 32 | 91.4 | |
| Occupation of father | 0.623 | ||||
| Employed | 24 | 19.7 | 5 | 14.3 | |
| Self-employed | 98 | 80.3 | 30 | 85.7 | |
| Number of children* Mean ± SD | 1.74 | 0.64 | 1.94 | 0.90 | 0.144 |
| Type of Delivery | 0.448 | ||||
| Vaginal | 28 | 23.7 | 12 | 34.3 | |
| Elective Cesarean | 85 | 72.0 | 22 | 62.9 | |
| Emergency cesarean | 5 | 4.2 | 1 | 2.9 | |
| Maternal parity | 0.431 | ||||
| Primipara | 49 | 40.2 | 11 | 31.4 | |
| Multipara | 73 | 59.8 | 24 | 68.6 | |
| Pregnancy duration | < 0.001 | ||||
| < 34 weeks | 9 | 7.4 | 12 | 34.3 | |
| 34–36 weeks | 4 | 3.3 | 2 | 5.7 | |
| Term (37–42) | 108 | 89.3 | 21.0 | 60.0 | |
| Birth weight | < 0.001 | ||||
| < 1500 g | 2 | 1.7 | 5 | 14.7 | |
| 1500–2500 g | 7 | 5.8 | 9 | 26.5 | |
| 2500–4000 g | 112 | 92.6 | 20 | 58.8 | |
| Previous gestational problem | 13 | 10.7 | 8 | 22.9 | 0.088 |
| Maternal medical history | 19 | 15.6 | 10 | 28.6 | 0.089 |
| Neonatal problem | 16 | 13.1 | 12 | 34.3 | 0.010 |
| Maternal problem | 11 | 9.0 | 6 | 17.1 | 0.146 |
*These two variables were described as mean & standard deviation
The medical history of the mother included diabetes, kidney disease, hypothyroidism, hyperthyroidism, chronic disease, epilepsy, infections, family history of marriage, history of disability in the family, and hypertension. Neonatal factors included neonatal infection, hospitalization in a neonatal intensive care unit for more than three days, neonatal seizures, microcephaly, hospitalization for more than 25 days during infancy, intraventricular hemorrhage, periventricular leukemia, history of neonatal respiratory problems, and heart disease at birth
In children with CP, the most type of involvement (82.9%) was spastic. Regarding limb involvement, most of them (54.3%) belonged to quadriplegia type. Regarding speech-language problems, the most common associated speech-language problem (22.9%) was speech delays (. In terms of vision, 8.6% had strabismus. Also, 31.4% of them had an inability to walk and 11.4% of them had epilepsy. The severity of CP based on the GMFCS scale was level II (39.3%) and then level IV (32.2%). It should be noted that the severity of the functional disorder was in levels 4 and 5 in approximately 40% of CP cases (severe form) (Table 2).
Table 2.
The frequency of different types of CP, severity, and associated disorders
| Variable | Subgroup | N | Percent |
|---|---|---|---|
| Type of CP | Spastic | 29 | 82.9 |
| Ataxic | 2 | 5.7 | |
| Hypotonic | 4 | 11.4 | |
| Involved limb | Diplegic | 10 | 28.6 |
| Quadriplegia | 19 | 54.3 | |
| Hemiplegia | 6 | 17.1 | |
|
Speech-Language problems |
Speech delay | 8 | 22.9 |
| Swallowing disorders | 1 | 2.9 | |
| Vision problems | Strabismus | 3 | 8.6 |
| Other problems | Restriction of Eye Movement | 2 | 5.7 |
| Inability to walk | 11 | 31.4 | |
| Cognitive impairment | 1 | 2.9 | |
| Epilepsy | 4 | 11.4 | |
| Hearing problem | 2 | 5.7 | |
| Mental retardation | 3 | 8.6 | |
| GMFCS | I | 2 | 7.1 |
| II | 11 | 39.3 | |
| III | 4 | 14.3 | |
| IV | 9 | 32.2 | |
| v | 2 | 7.1 |
GMFCS: Gross Motor Functional Classification System (7 childs aed under 12 months and excluded from this test)
ASQ developmental screening questionnaire showed that from the range of 0–50, the mean (SD) developmental score in the domains of communication, gross motor, fine motor, problem-solving, and personal-social domain was 27.2 (21.5), 12.8 (15.9), 27.1 (19.4), 23.8 (22.2), and 26.7 (21.7), respectively. The lowest score was related to e gross motor domain, where 82.9% of them had neurodevelopmental problems based on their age. Two-thirds of CP children had problems in problem-solving and personal-social domains, and more than half of them had problems in fine motor and communication (Table 3).
Table 3.
The score of ASQ in different neurodevelopment domains and frequency of children with neurodevelopmental problems (2 standard deviations below average)
| ASQ Domains | Mean (SD) | Median | P25, P75 |
|---|---|---|---|
| Communication | 27.2 (21.5) | 20.0 | 5.0, 45.0 |
| Having problem | Yes | n = 19 | 54.3% |
| No | n = 16 | 45.7% | |
| Gross Motor | 12.8 (15.9) | 5.0 | 0, 20.0 |
| Having problem | Yes | n = 29 | 82.9% |
| No | n = 6 | 17.1% | |
| Fine Motor | 27.1 (19.4) | 30.0 | 10.0, 45.0 |
| Having problem | Yes | n = 21 | 60% |
| No | n = 14 | 40% | |
| Problem Solving | 23.8 (22.2) | 20.0 | 0, 45.0 |
| Having problem | Yes | n = 24 | 68.6% |
| No | n = 11 | 31.4% | |
| Personal-Social | 26.7 (21.7) | 25.0 | 5.0, 50.0 |
| Having problem | Yes | n = 23 | 65.7% |
| No | n = 12 | 34.3% |
ASQ: Ages and Stages Questionnaire, Second Edition,
Univariate logistic regression showed that among the investigated factors, pregnancy duration, birth weight, and neonatal problems were the predictors of CP. In the multivariate regression model, among the investigated factors, pregnancy duration, birth weight, maternal complications during the current pregnancy and maternal problems during the previous pregnancy remained in the model and were identified as predictors of CP (Table 4).
Table 4.
Results of logistic regression for un-adjusted and adjusted odds ratios (ORs) for participant characteristics
| Variables | Un-Adjusted | Adjusted | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | Lower | Upper | P-value | OR | Lower | Upper | P-value | |
| Children Number | 1.46 | 0.87 | 2.45 | 0.148 | - | - | - | - |
| Pregnancy Duration | ||||||||
| Less than 33 weeks | Ref | |||||||
| 34 to 36 | 0.37 | 0.05 | 2.51 | 0.313 | 0.161 | 0.008 | 1.155 | 0.065 |
| Term (37–42) | 0.14 | 0.05 | 0.39 | < 0.001 | 0.139 | 0.025 | 0.758 | 0.023 |
| Birth Weight | ||||||||
| 2500–4000 g | Ref | |||||||
| 1500–2500 g | 7.20 | 2.40 | 21.55 | < 0.001 | 19.44 | 2.85 | 132.26 | 0.002 |
| < 1500 g | 14.00 | 2.53 | 77.20 | 0.002 | 28.09 | 2.13 | 369.45 | 0.011 |
| Neonatal Problem | ||||||||
| Yes | 3.45 | 1.44 | 8.28 | 0.005 | - | - | - | - |
| No | Ref | |||||||
| Maternal Problem | ||||||||
| Yes | 2.08 | 0.71 | 6.12 | 0.180 | 3.06 | 1.87 | 8.54 | 0.013 |
| No | Ref | |||||||
| Previous Gestational Problem | ||||||||
| Yes | 2.48 | 0.93 | 6.59 | 0.068 | 4.80 | 1.62 | 14.21 | 0.005 |
| No | Ref | |||||||
| Maternal Medical History | ||||||||
| Yes | 2.16 | 0.89 | 5.26 | 0.085 | - | - | - | - |
| No | Ref | |||||||
Mother’s problems in the current pregnancy were: pre-term delivery, post-term delivery, premature rupture of the amniotic membranes, history of infertility treatment, multiple fetuses, cervical insufficiency, and parity of the mother. Mother’s problems in previous pregnancy were: history of intrauterine fetal death, preterm delivery, post-term delivery, history of congenital malformation, cervical insufficiency, and miscarriage. The medical history of the mother included diabetes, kidney disease, hypothyroidism, hyperthyroidism, chronic disease, epilepsy, infections, family history of marriage, history of disability in the family, and hypertension. Neonatal factors included neonatal infection, hospitalization in a neonatal intensive care unit for more than three days, neonatal seizures, microcephaly, hospitalization for more than 25 days during infancy, intraventricular hemorrhage, periventricular leukemia, history of neonatal respiratory problems, and heart disease at birth
Discussion
In this study, we investigated the prevalence of different types of CP, associated disorders, and risk factors in children with CP under three years of age. The findings of our study showed that birth weight, gestational lenght, maternal problems during the current pregnancy, and maternal problems during the previous pregnancy were identified as potential predictors of CP. The results of various studies indicate that one of the most common causes of CP is LBW [30]. So that the percentage of CP increases with a decrease in birth weight [31, 32].
In the current study, a birth weight of 1500–2500 g as well as a weight of less than 1500 g compared to a normal weight of 2500–4000 g increased the risk of CP several times, which is consistent with other findings [33, 34]. The prevalence of LBW in the case group (41.2%) was significantly higher than the control group (7.5%). In addition to the birth weight, preterm birth is one of the most common risk factors for CP. The frequency and severity of neurodevelopmental disorders are related to gestational age, and the reports of several studies show that there is a significant relationship between the prevalence of preterm birth and CP [35, 36]. In our study, the prevalence of preterm delivery in the case group (40%) was significantly higher than the control group (10.7%). Moreover, 34.3% of the children in the CP group had a pregnancy duration of ≤ 34 weeks. Compared with gestational age ≤ 34 full weeks, gestational age of 34–37 weeks reduced the risk of CP by 84% and term pregnancy by 86%. Therefore, the risk of CP increased with preterm birth and in proportionally with decreasing fetal age at birth. In the study conducted by Soleimani et al. in Iran, it was shown that the risk of CP increased 17 times with a birth weight less than 1500 g and 5.7 times with a birth weight less than 2500 g.the Pregnancy less than 37 weeks has increased the risk of CP by 22 times and pregnancy less than 30 weeks by 18 times [7]. In another study conducted by Topchizadeh et al. in Tabriz-Iran, children with CP had risk factors of birth weight less than 1500 g in 30%, weight 1500–2500 g in 33.3%, and gestational age less than 33 weeks in 51.3% [37]. In the study by Başaran et al., 73% of children with CP had a gestational age less than 37 weeks, and half of the children weighed less than 2500 g [32]. The results of our study are consistent with the results of other studies [38, 39].
In our study, as previously mentioned, maternal problems during the current pregnancy and previous pregnancy were identified as potential predictors of CP so that the risk of getting CP in the presence of maternal problems (such as pre-term birth, post-term birth, rupture of the amniotic sac, history of infertility treatment, multiple births, cervical insufficiency, multiparity, and nulliparity) in recent pregnancy was more than three times. And if there were problems in the previous pregnancy (such as pre-term delivery, post-term delivery, history of intrauterine death, cervical insufficiency, history of congenital abnormalities, miscarriage) it increased nearly 5 times. Consistent with the findings of our study, in a population-based follow-up study on 1.6 million singletons with a gestational age of 37–44 weeks, a significant increase in the prevalence of CP was observed in children born at or after 42 weeks of gestation compared to the gestational age of 40 weeks and 37–38 [40]. Also, reports of a cohort study conducted by Trønnes et al. on 1.7 million children born between 23 and 43 weeks of gestation showed that deliveries at 42–43 weeks, 34–36 weeks, 31–33 weeks, multiparity, rupture of membranes, cervical conization, congenital anomalies, and IUGR(increased the probability of CP in infants [36]. Reports of a cross-sectional study conducted by Sellier et al. showed that the probability of CP is higher in triplets and quadruplets compared to twins. The possibility of this increase is due to the high probability of preterm delivery [41]. In another study, twins and triplets increased the risk of CP by 12-fold and 14-fold, respectively, compared to singleton pregnancies [42]. Furthermore, in one study, mothers of infants with CP had a higher rate of spontaneous abortion, stillbirths, and LBW [43].
Our investigation regarding the types of CP in these children showed that spastic type was present in 82.9%, hypotonic type in 11.4%, and ataxic type in 5.7% of patients. In this study, spastic CP was recognized as the most common CP, and based on the classification of the involved limb (topographic), quadriplegic type (54.3%) and then diplegia (28.6%) were the most common types.
In a study conducted at Tabriz rehabilitation center in 2009, the most common type of CP was related to spastic (70.3%), and in terms of topography, quadriplegic (46.5%) and diplegic (38.7%) was the most common [37]. The reports of several studies also showed that the most common spastic CP in terms of topography is quadriplegia [44–47], and these studies are consistent with the current study. In the study conducted in Tehran, -Iran, spastic CP was also the most common (80.3), and based on the affected limb hemiplegia (36%) followed by diplegia 31% [7], were the most common ones. In the study by Sajedi et al. in Iran, the highest frequency of CP was reported as diplegia, quadriplegia, and hemiplegia, respectively [48]. In Najjar et al.‘s study, the most prevalent type of CP was spastic diplegia, quadriplegia, and hemiplegia respectively [49].
The symptoms and severity of CP vary among patients and range from independent to completely dependent on daily activities. For this purpose, in this study, we used the GMFCS, a scale that reflects the level of independence and performance of children based on gross movements [50]. In the present study, the severity of gross motor involvement based on the GMFCS scale was at level two (37.1%) and then at level four (31.4%) in more than one-third of the cases. Also, nearly 40% of children with CP had a GMFCS level ≥ 4, indicating significant limitations in two out of five patients. Also, based on the results, 53.6% of children with CP had a GMFCS level of ≥ 3, which indicates significant limitations in half of the children. Children at GMFCS level 4 or 5 are also at greater risk of developing hip and spine problems compared to children at lower GMFCS levels [51]. Also, patients at GMFCS level 4 or 5 are more likely to have simultaneous disorders [52]. In a study conducted by Himmelman et al., 89% of children who were at GMFCS level 5 had two or more comorbid disorders [53].
Since CP is the result of damage to the developing brain, it can affect or disrupt various other brain functions and thus be associated with several diseases [44, 54]. CP has been reported to be associated with several disorders such as vision, hearing, speech, behavior, epilepsy, etc [50].
Walking disability was the most common comorbidity (31.4) in the present study. Various studies on children with CP indicate a clear relationship between the severity of problems and the degree of o walking disability, and these children are different in terms of walking ability [55]. In a study conducted in Iran, 52.7% of patients were unable to walk without assistance [48]. There are several clinical factors in predicting the gait of children with CP, including the type of CP, primary reflexes, gross motor skills, the degree of cognitive impairment, and the underlying cause of CP [56, 57]. Spastic quadriplegia was the dominant form of CP in patients who were not able to walk independently [58, 59]. Hemiplegia and then diplegia had a better prognosis [59, 60]. In the current study, the quadriplegic type was the most common one, and 40% of the children had gross movement limitations. Speech disorders are also common in children with CP, and according to reports, 33–63% of children with CP have experienced some form of speech disorder [61]. In two studies conducted in Iran, the prevalence of this disorder was reported as 47% [62] and 33% [7]. About 70% in Mei et al.‘s study [61] and 35% in Anderson et al.‘s study [63], children with CP were suffering from speech disorders. In our study, the most common associated speech-language problems were speech delays (22.9%) as the second most common comorbidity. The association of epilepsy with CP has been reported between 15 and 60% all over the world [65, 66], and in this study, the prevalence of epilepsy is 11.4%. Based on the evidence, 16–70% of children with CP had some type of vision problems [67]. In our study, the most frequent visual disorder was related to strabismus (8.6%).
In our study, among children with CP, the lowest neurodevelopmental score was related to the domain of gross motor, so that 82.9% of children had disorders in this area. Two-thirds of the children had developmental disorders in problem-solving and personal-social domains, and more than half of the children had developmental disorders in the areas of communication and fine motor. Children with one or more risk factors before, during, or after birth were at risk of developmental delay [68].
LBW and low gestational age are among these risk factors and are associated with neuromotor complications that extend to childhood [69]. In the study conducted by Baskabadi et al. in Mashhad-Iran, premature infants with lower birth weight and lower gestational age were more likely to have developmental delays [70]. Also, in a case-control study on 5-year-old children, Karimi et al. showed that children with a birth weight of 1500–2499 g had developmental delays in the area of problem-solving, gross, and fine motor compared to children with a normal weight [71]. In the study of Ahishakiye et al. [72], the detrimental effect of low gestational age and LBW on the developmental process of children aged 24–36 months was shown in Rwanda. Developmental disorders have a great impact on the child’s personal-social functions. Early diagnosis of developmental delay in high-risk children is an important issue, and screening for developmental status is very important in terms of early detection of these disorders for timely intervention and treatment [73]. In our study, LBW and very LBW (VLBW) (41.2%) and preterm delivery (40%) were the most common risk factors.
The strengths of this research were the use of reliable and reliable tools to collect information on children in the first ages after birth (under 3 years old). One of the limitations of this study is the use of the GMFCS, which is used to assess gross movements such as walking, so it is suggested that another scale such as the Mini-MACS (Mini-MACS) be used in future studies. For children with CP from 1 to 4 years old, it explores how they use their hands to control objects in everyday life. Moreover, due to not recording the umbilical cord artery pH of children with and without CP in their medical files, we could not report the mentioned variable in this study. Therefore, it is recommended to consider this variable in future studies. Finally, the results of this study cannot be generalized to children over three years old.
Conclusions
CP is a multifactorial disease and has a wide range of causes, risk factors, and associated disorders. Based on the results, one of the important predictors of CP that was found in our study was the history of maternal problems in the previous pregnancy. Also, in the current study, significant movement limitations were observed in 40% of children with CP. Since this disease reduces the quality of life of patients, it is hoped that by recognizing these preventable risk factors and taking preventive measures during pre-pregnancy and pregnancy period, and in the case of CP, by quick identifying this disease and associated disorders and taking appropriate rehabilitation measures, we can have significant effects on the health of these children and their quality of life in the future.
Acknowledgements
We sincerely appreciate all the participants in the study and the staff of study settings for their assistance.
Author contributions
A.F.Kh, Sh.O, N.N, and V.T contributed to the design of the study. A.F.Kh, Sh.O, M.H, T.SH, and S.P contributed to the implementation and analysis plan. A.F.Kh, Sh.O, M.H, S.P has written the first draft of this manuscript and V.T, N.N, and T.SH have critically read the text and contributed with inputs and revisions, and all authors read and approved the final manuscript.
Funding
This research was funded by the Vice-Chancellor for Research of Tabriz University of Medical Sciences, Iran (Grant no: 68507). The funding center had no role in the study design, data analysis, or writing this paper.
Data availability
Data will be available from corresponding author upon reasonable request.
Declarations
Ethical approval
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences (IR.TBZMED.REC.1400.897). Written informed consent was obtained from the a parent for study participation before starting data collection.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Vahideh Toopchizadeh Co-first author.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Azizeh Farshbaf-Khalili, Email: farshbafa@tbzmed.ac.ir.
Shirin Osouli-Tabrizi, Email: shirin.osouli@yahoo.com.
References
- 1.Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil. 2006;28(4):183–91. [DOI] [PubMed]
- 2.Sellier E, Platt MJ, Andersen GL, Krägeloh-Mann I, De La Cruz J, Cans C, et al. Decreasing prevalence in cerebral palsy: a multi-site European population-based study, 1980 to 2003. Dev Med Child Neurol. 2016;58(1):85–92. [DOI] [PubMed]
- 3.Oskoui M, Coutinho F, Dykeman J, Jette N, Pringsheim TA. An update on the prevalence of cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol. 2013:55. [DOI] [PubMed]
- 4.Subramaniam ND, Jenifer A, Devi LU, Suresh P. Etiology, outcomes and co-morbidities among cerebral palsy children attending tertiary care hospital, India: a prospective study. Int J Contemp Pediatr. 2019;6(3):1–5.
- 5.Schiariti V, Selb M, Cieza A, O’Donnell M. International classification of functioning, disability and health core sets for children and youth with cerebral palsy: a consensus meeting. Dev Med Child Neurol. 2015;57(2):149–58. [DOI] [PubMed]
- 6.Sajedi F, Soleiman F, Ahmadi M. Cerebral palsy in children. JHC. 2013;15:88–97.
- 7.Soleimani F, Sourtiji H. Evaluation of perinatal and neonatal risk factors of children with cerebral palsy referred from health-care centers in north and east of Tehran. Tehran Univ Med J. 2009;67(6):435–41.
- 8.Das N, Bezboruah G, Das I. Study on the clinical profile of patients with cerebral palsy. IOSR J Dent Med Sci. 2016;15:54–8. [Google Scholar]
- 9.Lestari AF, Sitaresmi MN, Sutomo R, Ridhayani F. Factors affecting the health-related quality of life of children with cerebral palsy in Indonesia: a cross-sectional study. Child Health Nurs Res. 2024;30(1):7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra MR, Adhikari KM, Panigrahi NK. A prospective cohort study of auditory and visual comorbidities in children with cerebral palsy. J Mar Med Soc. 2023;25(1):43–7. [Google Scholar]
- 11.Mesraoua B, Ali M, Deleu D, Al hail H, Melikyan G, Haddad N et al. Epilepsy and cerebral palsy. Neurodevelopment and neurodevelopmental disease. IntechOpen. 2019.
- 12.Hirschberger RG, Kuban KCK, O’Shea TM, Joseph RM, Heeren T, Douglass LM, et al. Co-occurrence and severity of neurodevelopmental burden (cognitive impairment, cerebral Palsy, Autism Spectrum Disorder, and Epilepsy) at age ten years in children born extremely Preterm. Pediatr Neurol. 2018;79:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chiang K-L, Kuo F-C, Cheng C-Y, Chang K-P. Prevalence and demographic characteristics of comorbid epilepsy in children and adolescents with cerebral palsy: a nationwide population-based study. Child’s Nerv Syst. 2019;35(1):149–56. [DOI] [PubMed] [Google Scholar]
- 14.Winter S, Autry A, Boyle C. Trends in the prevalence of cerebral palsy in a Population-based study. Pediatrics. 2003;10:1220–5. [DOI] [PubMed] [Google Scholar]
- 15.Kumari A, Yadav S. Cerebral palsy: a mini review. Int J Ther App. 2012;3(1):15–24. [Google Scholar]
- 16.Amirsalari S, Dalvand H, Dehghan L, Feizy A, Hosseini sa, Shamsoddini A. The efficacy of botulinum toxin type injection in the hamstring and calf muscles with and without serial foot casting in Gait improvement in children with cerebral palsy. Tehran Univ Med J. 2011;69:509–17. [Google Scholar]
- 17.Bonellie S, Currie D, Chalmers J. Comparison of risk factors for cerebral palsy in twins and singletons. Dev Med Child Neurol. 2005;47(9):287–91. [PubMed] [Google Scholar]
- 18.Goldsmith S, Mcintyre S, Badawi N, Hansen M. Cerebral palsy after assisted reproductive technology: a cohort study. Dev Med Child Neurol. 2018;60(1):73–80. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan AH, Thompson SC, Gecz J. Cerebral palsy: causes, pathways, and the role of genetic variants. Am J Obstet Gynecol. 2015;213(6):779–88. [DOI] [PubMed]
- 20.Robertson CMT, Svenson LW, Joffres MR. Prevalence of cerebral palsy in Alberta. Can J Neurol Sci. 2015;25(2):117–22. [DOI] [PubMed]
- 21.Inaloo S, Katibeh P, Ghasemof M. Cerebral palsy in 1–12 year old children in Southern Iran. Iran J Child Neurol. 2016;10(1):35–41. [PMC free article] [PubMed] [Google Scholar]
- 22.Öztürk A, Demirci F, Yavuz T, Yıldız S, Değirmenci Y, Döşoğlu M, et al. Antenatal and delivery risk factors and prevalence of cerebral palsy in Duzce (Turkey). Brain Develop. 2007;29(1):39–4. [DOI] [PubMed] [Google Scholar]
- 23.O’Callaghan M, MacLennan A. Cesarean delivery and cerebral palsy: a systematic review and meta-analysis. Obstet Gynecol. 2013;122(6):1169–75. [DOI] [PubMed] [Google Scholar]
- 24.Cantín M. World medical association declaration of Helsinki: ethical principles for medical research involving human subjects. Reviewing the latest version. Int J Med Surg Sci. 2014;1(4):339–46. [Google Scholar]
- 25.Singh A, Yeh CJ, Boone Blanchard S. Ages and stages Questionnaire: a global screening scale. Bol Med Hosp Infant Mex. 2017;74(1):5–12. [DOI] [PubMed] [Google Scholar]
- 26.Vameghi R, Sajedi F, Kraskian Mojembari A, Habiollahi A, Lornezhad HR, Delavar B. Cross-cultural adaptation, validation and standardization of Ages and Stages Questionnaire (ASQ) in Iranian children. Iran J Public Health. 2013;42(5):522–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Morris C, Bartlett D. Gross motor function classification system: impact and utility. Dev Med Child Neurol. 2004;46(1):60–5. [DOI] [PubMed] [Google Scholar]
- 28.Palisano RJ, Cameron D, Rosenbaum PL, Walter SD, Russell D. Stability of the gross motor function classification system. Dev Med Child Neurol. 2006;48(6):424–8. [DOI] [PubMed] [Google Scholar]
- 29.Tenny S, Kerndt CC, Hoffman MR. Case control studies. 2023 Mar 27. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan–. PMID: 28846237. [PubMed]
- 30.Mohanty T, Joseph SD, Gunasekaran PK, Doreswamy SM, Saini L. Predictors of risk for cerebral palsy: a review. Pediatr Phys Ther. 2023;35(3):347–57. [DOI] [PubMed] [Google Scholar]
- 31.Esih K, Trunk T, Osredkar D, Verdenik I, Neubauer D, Troha Gergeli A, et al. The impact of birth weight on the development of cerebral palsy: a population-based matched case-control study. Early Hum Dev. 2022;165:105533. [DOI] [PubMed] [Google Scholar]
- 32.Başaran A, Kilinç Z, Sari H, Gündüz E. Etiological risk factors in children with cerebral palsy. Med (Baltim). 2023;102(15):e33479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cortese M, Moster D, Wilcox AJ. Term birth weight and neurodevelopmental outcomes. Epidemiology. 2021;32(4):583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soleimani F, Vameghi R, Biglarian A. Antenatal and intrapartum risk factors for cerebral palsy in term and near-term newborns. Arch Iran Med. 2013;16(4):213–6. [PubMed] [Google Scholar]
- 35.Sadowska M, Sarecka-Hujar B, Kopyta I. Cerebral palsy: current opinions on definition, epidemiology, risk factors, classification and treatment options. Neuropsychiatr Dis Treat. 2020;16:1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trønnes H, Wilcox AJ, Lie RT, Markestad T, Moster D, Ad. Risk of cerebral palsy in relation to pregnancy disorders and preterm birth: a national cohort study. Dev Med Child Neurol. 2014;56(8):779–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toopchizadeh V, Barzegar M, Hosseini SM. Cerebral palsy in respect of etiology, type, associated disorders and motor development in Tabriz Children Medical Center. Med J Tabriz Univ Med Sci. 2009;30(4):27–31. [Google Scholar]
- 38.Schieve LA, Tian LH, Rankin K, Kogan MD, Yeargin-Allsopp M, Visser S, et al. Population impact of preterm birth and low birth weight on developmental disabilities in US children. Ann Epidemiol. 2016;26(4):267–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jöud A, Sehlstedt A, Källén K, Westbom L, Rylander L. Associations between antenatal and perinatal risk factors and cerebral palsy: a Swedish cohort study. BMJ Open. 2020;10(8):e038453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moster D, Wilcox AJ, Vollset SE, Markestad T, Lie RT. Cerebral palsy among term and postterm births. JAMA. 2010;304(9):976–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sellier E, Goldsmith S, McIntyre S, Perra O, Rackauskaite G, Badawi N, et al. Cerebral palsy in twins and higher multiple births: a Europe-Australia population-based study. Dev Med Child Neurol. 2021;63(6):712–20. [DOI] [PubMed] [Google Scholar]
- 42.RS. K. Cerebral palsy in twins and multiple births at Sindhudurg Arabian West coast of Maharashtra (large cohort analysis of 206124 births from 1996 to 2014). BVMJ. 2021;1(1):17–21.
- 43.Pharoah PO, Cooke T, Rosenbloom L, Cooke RW. Effects of birth weight, gestational age, and maternal obstetric history on birth prevalence of cerebral palsy. Arch Dis Child. 1987;62(10):1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhati P, Sharma S, Jain R, Rath B, Beri S, Gupta VK, et al. Cerebral palsy in North Indian children: clinico-etiological profile and comorbidities. J Pediatr Neurosci. 2019;14(1):30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.DAR H, Stewart K, Mcintyre S, Paget S. Multiple motor disorders in cerebral palsy. Dev Med Child Neurol. 2024;66:317–25. [DOI] [PubMed]
- 46.Gowda VK, Kumar A, Shivappa SK, Srikanteswara PK, Shivananda, Mahadeviah MS, et al. Clinical profile, predisposing factors, and associated co-morbidities of children with cerebral palsy in South India. J Pediatr Neurosci. 2015;10(2):108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Troon R, laughton, Doets B, Elsinger L. F. Aetiology of cerebral palsy in children presenting at Tygerberg Hospital. SAJCH. 2007;1(2):74 – 7.
- 48.Sajedi F, Togha M, Karimzadeh P. A survey of 200 cases of cerebral palsy in welfare and rehabilitation centers of Tehran. Saudi J Disabil Rehabilitation. 2003;9(1):1–7. [Google Scholar]
- 49.Najar B, Kachroo A, Gattoo I, Hussain S. Cerebral palsy: risk factors, comorbidities and associated MRI findings, a hospital based observational study. Int J Contemp Pediatr. 2015;2(2):90–5. [Google Scholar]
- 50.Abd Elmagid DS, Magdy H. Evaluation of risk factors for cerebral palsy. Egypt J Neurol Psychiat Neurosurg Egypt J Neurol Psychiatry Neurosurg. 2021;57:1–9. [Google Scholar]
- 51.Soo B, Howard JJ, Boyd RN, Reid SM, Lanigan A, Wolfe R, et al. Hip displacement in cerebral palsy. J Bone Joint Surg Am. 2006;88(1):121–9. [DOI] [PubMed] [Google Scholar]
- 52.Jain A, Sponseller PD, Shah SA, Samdani A, Cahill PJ, Yaszay B, et al. Subclassification of GMFCS Level-5 cerebral palsy as a predictor of complications and health-related quality of life after spinal arthrodesis. J Bone Joint Surg Am. 2016;98(21):1821–8. [DOI] [PubMed] [Google Scholar]
- 53.Himmelmann K, Beckung E, Hagberg G, Uvebrant P. Gross and fine motor function and accompanying impairments in cerebral palsy. Dev Med Child Neurol. 2006;48(6):417–23. [DOI] [PubMed] [Google Scholar]
- 54.Allen J, Zareen Z, Doyle S, Whitla L, Afzal Z, Stack M, et al. Multi-organ dysfunction in cerebral palsy. Front Pead. 2021;9:668544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ippersiel P, Dussault-Picard C, Mohammadyari SG, De Carvalho GB, Chandran VD, Pal S, et al. Muscle coactivation during gait in children with and without cerebral palsy. Gait Posture. 2024;108:110–6. [DOI] [PubMed] [Google Scholar]
- 56.Wu YW, Day SM, Strauss DJ, Shavelle RM. Prognosis for ambulation in cerebral palsy: a population-based study. Pediatrics. 2004;114(5):1264–71. [DOI] [PubMed] [Google Scholar]
- 57.Keeratisiroj O, Thawinchai N, Siritaratiwat W, Buntragulpoontawee M, Pratoomsoot C. Prognostic predictors for ambulation in children with cerebral palsy: a systematic review and meta-analysis of observational studies. Disabil Rehabil. 2018;40(2):135–43. [DOI] [PubMed] [Google Scholar]
- 58.Kułak W, Sendrowski K, Okurowska-Zawada B, Sienkiewicz D, Paszko-Patej G. Prognostic factors of the independent walking in children with cerebral palsy. System. 2011;12:29–34. [Google Scholar]
- 59.Shevell MI, Dagenais L, Hall N, Repacq Consortium. The relationship of cerebral palsy subtype and functional motor impairment: a population-based study. Dev Med Child Neurol. 2009;51(11):872–7. [DOI] [PubMed] [Google Scholar]
- 60.Lee JH, Koo JH, Jang DH, Park EH, Sung IY. The functional prognosis of ambulation in each type of cerebral palsy. J Korean Acad Rehabilitation Med. 2006;30(4):315–21. [Google Scholar]
- 61.Mei C, Reilly S, Bickerton M, Mensah F, Turner S, Kumaranayagam D, et al. Speech in children with cerebral palsy. Dev Med Child Neurol. 2020;62(12):1374–82. [DOI] [PubMed] [Google Scholar]
- 62.Soleimani F, Vameghi R, Rassafiani M, AKBAR FN, Nobakht Z. Cerebral palsy: motor types, gross motor function and associated disorders. Iran Rehabilitation J. 2011;11(14):21–31. [Google Scholar]
- 63.Andersen G, Mjøen TR. Prevalence of speech problems and the use of augmentative and alternative communication in children with cerebral palsy: a registry-based study in Norway. Perspect Augmen Alternat Commun. 2010;19(1):12–20.
- 64.Wallace SJ. Epilepsy in cerebral palsy. Dev Med Child Neurol. 2001;43(10):713–7. 10.1017/s0012162201001281. [DOI] [PubMed] [Google Scholar]
- 65.Szpindel A, Myers KA, Ng P, Dorais M, Koclas L, Pigeon N, et al. Epilepsy in children with cerebral palsy: a data linkage study. Dev Med Child Neurol. 2022;64(2):259–65. 10.1111/dmcn.15028. [DOI] [PubMed] [Google Scholar]
- 66.Dos Santos Rufino A, Påhlman M, Olsson I, Himmelmann K. Characteristics and challenges of epilepsy in children with cerebral palsy—A population-based study. J Clin Med. 2023;12(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Galli J, Loi E, Molinaro A, Calza S, Franzoni A, Micheletti S, et al. Age-related effects on the spectrum of cerebral visual impairment in children with cerebral palsy. Front Hum Neurosci. 2022;16:750464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kazeroono S, Keshavarz K, Abasi R, Zoladl M, Asadi H, Sharafieyan S et al. Status of development of premature children from 4 to 12 months in the neonatal intensive care unit (NICU) admission based on the ASQ Questionnaire. Armaghanj. 2014;19(9).
- 69.IG S. Neurodevelopmental outcomes of preterm infants. Clin Exp Pediatr. 2023;66(7):281–7. 10.3345/cep.2022.00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bagheri F, Askari Hosseni Z. Developmental disorders in preterm neonates during the first two years of life using the ages and stages questionnaire. Babol-Jbums. 2016;18(2):7–13. [Google Scholar]
- 71.Karimi M, Fallah R, Dehghanpoor A, Mirzaei M. Developmental status of 5-year-old moderate low birth weight children. Brain Develop. 2011;33(8):651–5. [DOI] [PubMed] [Google Scholar]
- 72.Ahishakiye A, Abimana MC, Beck K, Miller AC, Betancourt TS, Magge H, et al. Developmental outcomes of Preterm and Low Birth Weight toddlers and Term Peers in Rwanda. Ann Glob Health. 2019;85(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.horrami Z, Namdar A. Development Status among One-Year-Old Children Referring to Urban Health Centers of Jahrom: An Assessment based on Ages and Stages Questionnaires., 5(2), 141–150. SALĀMAT-I IJTIMĀĪ (Community Health). 2018;5(2):141 – 50.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available from corresponding author upon reasonable request.