Abstract
Rationale & objective
End-stage kidney disease (ESKD) negatively affects patients’ physical, emotional, and social functioning. Furthermore, adjustment to dialysis require substantial lifestyle changes that may further impact on patients physical and emotional well-being. However, the relationship between Health-Related Quality of life impairment with future adverse outcomes in dialysis is not well characterized. Our study aims to investigate the relationship between Health-Related Quality of Life (HRQoL) and patients’ survival and hospitalization rates within a large European dialysis network.
Methods
A historical cohort study was conducted to evaluate association of HRQoL with hospitalization and mortality rates over a 12-month follow-up period. Patients responded to a self-administered survey as part of a Continuous Quality Improvement Program implemented in clinics affiliated with the Spanish FMC-Nephrocare organization. Health-Related Quality of Life (HRQoL) was measured with the KDQOL-36. Potential confounders included socio-demographic characteristics, comorbidities, biochemical parameters, dialysis treatment. We used Cox’s Proportional Hazard regression to assess the hazard of death and Logistic Regression to assess the likelihood of hospital admissions during 12-month follow-up period.
Results
A total of 2280 (51.5%) completed the self-administrated survey, and 1838 patients met the inclusion/exclusion criteria of the study. Higher HRQoL scores were associated with significantly lower mortality and hospitalization risk. Risk estimates were robust to adjustment for potential confounders.
Conclusions
Several dimensions of HRQoL are associated with patient-centered outcomes (i.e., mortality and hospitalizations at 1 year). Patient-Reported Outcomes contribute unique pieces of information characterizing patients’ health. Residual confounding cannot be fully ruled out; moreover, the high attrition rate could result in selection bias, which may limit the generalizability of the findings to a broader population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12882-024-03835-0.
Keywords: Health-Related Quality of Life, Hospitalization, Mortality, Dialysis, End Stage Kidney Disease
Introduction
End-stage kidney disease (ESKD) strongly interferes with patients’ emotional, physical, and social functions due to the coexistence of multiple comorbidities. Additionally, the psycho-social and physical burden of renal replacement therapy, a life-saving treatment, further impacts these aspects of their lives [1].
Recent research on the relationship between Health-Related Quality of Life (HRQoL) and clinical outcomes in dialysis patients has primarily focused on identifying the clinical factors that influence HRQoL in patients with end-stage renal disease (ESRD). Several studies have examined how variables such as comorbidities, treatment modalities, and socio-demographic factors affect patients’ quality of life [2–5]. However, fewer studies have explored the reverse relationship-namely, the impact of HRQoL on clinical outcomes like hospitalizations and mortality [6–8]. This gap in the literature reflects a limited understanding of whether and how improving HRQoL can lead to better clinical outcomes. While previous studies have hinted at this association, much of the research is dated and aligns with the direction of our work. Our study aims to contribute to this field by assessing how improvements in HRQoL can positively influence key clinical outcomes, including reducing hospitalization rates and improving survival among dialysis patients. Therefore, achieving the highest level of functioning and HRQoL is becoming an increasingly important goal of dialysis [9].
To achieve this goal, the KDIGO 2012 Clinical Practice Guidelines [10], recommend that healthcare providers engage in Continuous Quality Improvement Programs (CQIP) focused on medical Key Performance Indicators (KPIs) (e.g. dialysis quality, laboratories parameters or vascular access rates) based on the assumption that such amelioration should translate into improvement in patients’ outcomes. Supporting KDIGO recommendations, we have recently shown that improvement in intermediate clinical endpoints such as, but not exclusively, phosphorus, calcium, PTH, hemoglobin, pre-dialysis blood pressure, fluid status, is associated with longer patients’ survival [11] and may be associated with improved HRQoL [12].
Despite considerable improvements in haemodialysis (HD) treatment over the last decades, the management of HD patients’ comorbidities and risk factors remains challenging, and it is still associated with high mortality rates [13] and HRQoL impairment.
The publication of Patient Reported Outcome Measures (PROM) specific guidelines [14–16] and emerging evidence suggests that patients’ HRQoL may be considered an independent endpoint for continuous quality improvement [17]. Furthermore, the use of PROM in clinical practice may enhance communication between healthcare professionals and patients, enable standardized symptoms monitoring, and inform prevention and treatment strategies improving biological functions and hard outcomes [18–25].
To better characterize the suitability of PROM as independent key performance and outcome indicator in nephrology, we sought to evaluate the relationship between HRQoL with patients’ survival and hospitalization rate controlling for potential confounders and medical key performance indicators adopted in the CQIP of a large European dialysis network.
Materials and methods
Design, population and setting
We conducted an historical cohort study assessing the occurrence of hospitalization and death along a 12-month follow up among patients responding to a self-administered survey as part of a Continuous Quality Improvement Program carried out in clinics belonging to the Spanish FMC-Nephrocare organization. The study adheres to the principles enunciated in the declaration of Helsinki and following modifications. The Ethical Committee of the Hospital Clinic-Barcelona, Spain, approved the study protocol.
All adult patients receiving dialysis treatment in one of the participating centers in September 2019 have been invited to fill in a self-administered questionnaire packet. The attending nurse provided assistance to patients who were unable to complete the questionnaire themselves due to sensory impairments. We excluded from the present analysis all patients who were unable to read and write in Spanish, with severe cognitive impairment preventing collection of survey data, those who did not consent their data be used for secondary research, incident dialysis patients (i.e., started dialysis since less than 90 days), lost-to-follow up patients and those whose clinical record could not be matched to survey data.
Endpoint definition
The primary endpoint was all cause mortality in the 12-month follow up period after survey completion date. Patients were censored at the end of follow up, kidney transplantation, definitive transfer outside the provider’s network. Secondary endpoints were all cause hospitalization and overall hospitalization length over the same follow-up period.
Quality of life measures
Health-Related Quality of life (HRQoL) assessment was performed using KDQoL™-36 (Kidney Disease Quality of Life-36) survey, a kidney disease-specific HRQoL measure consisting of 36 items. The questionnaire allows the computation of the Physical and Mental Composite Scores (PCS and MCS respectively) of the RAND Short Form 12 (SF-12) as a generic core plus three additional specific subscales to assess the level of burden of kidney disease (BKD), symptoms/problems of kidney disease (SKD), and effects of kidney disease (EKD). All measures were scored on a scale of 0–100 points, with higher values representing better HRQoL. Consistent with Piepert et al. [8] we also computed the Kidney Summary Score (KSS) using a scoring equation derived from principal component analysis and transformed it to a 0-100 T-score with mean fixed at the value of 50 and standard deviation (SD) at 10. The five scores of KDQoL™-36 described above have been computed using SAS® code provided by KDQoL Working Group (KDQoL™-36 Scoring Program version 2.0).
Covariates
We abstracted all clinical information from EuCliD (European Clinical Database), the clinical information system of the Fresenius Medical Care clinic network. Demographic and life-style variables were assessed at survey completion date; we also obtained the last biochemical biomarker essay results recorded up to 6 months prior to survey data collection. Vascular access and dialysis modality were defined as the most frequent class over 6 months before survey completion date. Etiology of kidney disease was categorized in four classes: metabolic syndrome if renal impairment was due to diabetes and/or hypertension; glomerulonephritis if renal impairment was due to inflammatory diseases; polycystic syndrome; all other causes. Finally, comorbidities were evaluated by the occurrence of ICD10 codes before survey date. The full list of all variables included in the analyses is provided in Table 1.
Table 1.
Baseline characteristics of patients included in the study sample. Baseline values of all variables included in the statistical analyses
| Variable | Cohort description (N = 1838) |
|---|---|
| Mean ± std or N (%) | |
| Age (year) | 68.8 ± 14.4 |
| Vintage (months) | 62.6 ± 73.0 |
| Gender (M) | 1116 (60.7%) |
| Smoking Status: no smoker | 465 (25.3%) |
| Smoking Status: smoker or ex-former | 454 (24.7%) |
| Smoking Status: unknown | 919 (50%) |
| Vascular Access: Fistula or Graft | 1218 (66.3%) |
| Vascular Access: Catheter | 620 (33.7%) |
| Dialysis Modality: HD | 812 (44.2%) |
| Dialysis Modality: HDF | 1026 (55.8%) |
| Etiology: Metabolic Syndrome | 537 (29.2%) |
| Etiology: Glomerulonephritis | 378 (20.6%) |
| Etiology: Polycystic | 102 (5.6%) |
| Etiology: Other | 821 (44.7%) |
| Atherosclerotic heart disease | 9% (166) |
| Congestive heart failure | 30% (559) |
| Cerebrovascular Disease | 14% (256) |
| Peripheral Vascular Disease | 24% (434) |
| Dysrhythmia | 25% (463) |
| Cardiovascular Diseases (others) | 32% (595) |
| COPD | 13% (238) |
| Gastrointestinal bleeding | 11% (196) |
| Peripheral vascular disease | 8% (149) |
| Hypertension | 63% (1149) |
| Cancer | 5% (95) |
| Diabetes | 40% (742) |
| Albumin (g/dl) | 3.9 ± 0.3 |
| Phosphate (mg/dl) | 4.4 ± 1.3 |
| Calcium (mg/dl) | 9.0 ± 0.7 |
| Haemoglobin (g/dl) | 11.5 ± 1.2 |
| Kt/V | 1.9 ± 0.4 |
| PTH (ng/l) | 337 ± 300 |
| Albumin: < 3.5 g/dl | 173 (9.4%) |
| Albumin: >=3.5 g/dl | 1665 (90.6%) |
| Phosphate: <2.5 mg/dl | 71 (3.9%) |
| Phosphate: >=2.5 mg/dl and < 5.5 mg/dl | 1492 (81.2%) |
| Phosphate: >=5.5 mg/dl | 275 (15%) |
| Calcium: <8.4 mg/dl | 376 (20.5%) |
| Calcium: >=8.4 mg/dl and < 10 mg/dl | 1376 (74.9%) |
| Calcium: >=10 mg/dl | 86 (15%) |
| Haemoglobin: <10 g/dl | 141 (7.7%) |
| Haemoglobin: >=10 g/dl and < 12 g/dl | 1230 (66.9%) |
| Haemoglobin: >=12 g/dl | 467 (25.4%) |
| Kt/V: <1.2 | 47 (2.6%) |
| Kt/V: >=1.2 and < 1.4 | 108 (5.9%) |
| Kt/V: >=1.4 | 1683 (91.6%) |
| PTH: <130 ng/l | 365 (19.9%) |
| PTH: >=130 ng/l and < 575 ng/l | 1233 (67.1%) |
| PTH: >=575 ng/l | 240 (13.1%) |
COPD: Chronic Obstructive Pulmonary Disease; HD: Hemodialysis; HDF Hemodiafiltration
Statistical analysis
Survival analysis was conducted using Cox’s proportional hazard model, incorporating all potential confounders and the HRQoL score, each included one at a time. The proportional hazards assumption for each Cox regression model has been tested and verified. From each Cox regression model Harrell’s concordance index was obtained as an indicator of the goodness of fit of the built model. We further assessed the association between hospitalization risk and length of stay with generalized linear models. We used logistic regression to evaluate the likelihood of at least one hospitalization during the 12-month follow up. From the logistic regression model, we computed the area under the ROC curve (ROC-AUC) as an indicator of the goodness of fit of the model. We modeled correlates of length of stay with a negative binomial distribution function and log link function. Linearity assumptions for continuous independent variables, such as age and HRQoL scores, has been assessed for each implemented general linear model.
For each outcome, we separately constructed one model for each KDQoL-36 score. We used hazard ratio (HR) for the Cox regression models (death), odds ratio (OR) for the logistic models (hospitalization), and incidence rate ratio (IRR) for the negative binomial models (hospitalization days). The computed HR, OR, and IRR represent the change in the hazard of death or hospitalization for each 0.5 standard deviation (SD) change in HRQoL score.
All models were adjusted for an outcome risk score (ORS) constructed as the likelihood of endpoint occurrence given the full set of potential confounders selected among non-modifiable patients’ characteristics and the medical key performance indicators described in Appendix (supplementary material). All analyses have been conducted with SAS 9.4®.
Results
Sample characteristics
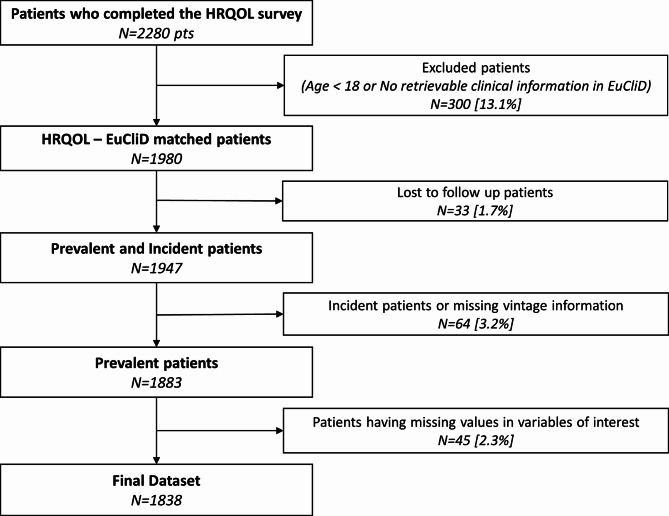
Among 4431 patients undergoing dialysis in Spanish FMC-Nephrocare clinics in September 2019, a total of 2280 (51.5%) completed the self-administrated HRQoL questionnaire, and 1838 patients met the inclusion/exclusion criteria of the study (Fig. 1).
Fig. 1.
Flow chart of the study sample. Flow chart of the study cohort of patients depicting all the exclusion criteria applied to the collected sample
Sample characteristics are reported in Table 1. The mean age was 68.8 ± 14.4 years and the 39.3% of patients were female, 66.3% used an arteriovenous fistula as a vascular access and the 55.8% received hemodiafiltration as the primary dialysis technique (Table 1).
Health-related quality of life
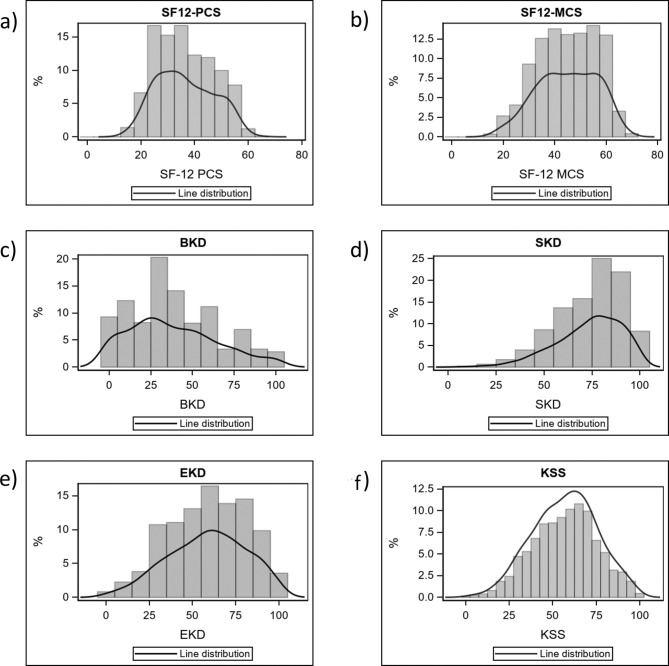
The average scores for the five KDQoL-36 measures were 36.4 ± 10.7 (PCS), 44.9 ± 11.6 (MCS), 38.2 ± 26.3 (BKD), 58.5 ± 22.6 (EKD) and 73.5 ± 17.2 (SKD). The mean KSS was 57.0 ± 18.5. Figure 2 shows the distribution of scores in the study sample.
Fig. 2.
Distribution of KDQoL-36 scores in the study sample. (a) Histogram of SF-12 physical composite score (PCS) values; (b) histogram of SF-12 mental composite score (MCS) values; (c) histogram of burden of kidney disease (BKD) values; (d) histogram of symptoms of kidney disease (SKD) values; (e) histogram of effects of kidney disease (EKD) values; (f) histogram of Kidney Disease Scale (KSS) values, a summary score derived from the 24 disease specific items of the KDQoL-36
Health‑related quality of life and survival
We observed 176 (9.6%) deaths during the 12-month study period. The incidence rate (occurrence of new cases of a disease or condition in a specific population over a defined period of time) of death was 10.6 events per 100 patient-years [95% CI 9.1–12.3]. The ORS representing the probability of death given baseline patients’ characteristics accurately predicted the occurrence of the primary endpoint as demonstrated by the high concordance index estimate (C-statistics, 95% confidence interval: 74.9%, 71.5-78.3%). Age, diabetic nephropathy, catheter use as vascular access, HD as a dialysis modality, active smoking, low serum calcium, low serum albumin, history of congestive heart failure, and chronic obstructive pulmonary disease were associated with increased risk of death. The HR representing the association between baseline clinical parameters and mortality risk are reported in Supplementary Table 1 (supplementary material).
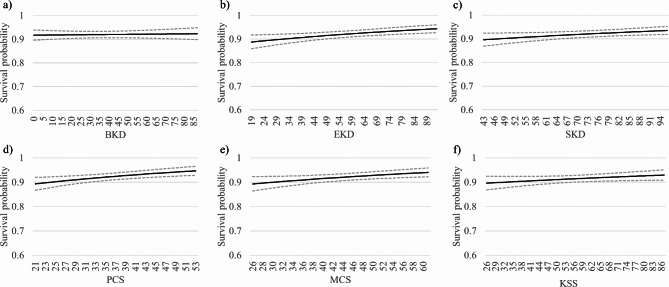
In a second analytic step, we added KDQoL-36™ scores in one model specification for each scale. In all models, higher HRQoL scores were associated with longer patients’ survival beyond adjustment for potential clinical confounders. The association was statistically significant for all scales but the BKD. Hazard ratio estimates indicated that each half standard deviation increase in HRQoL score was associated with 7.4–11.4% decrease in mortality risk (Table 2; Fig. 3).
Table 2.
Association between Health-Related Quality of Life and Mortality. Estimates based on Cox Proportional Hazard Regression. The outcome risk score (ORS) model includes socio-demographic variables, labs, medical prescriptions, dialysis treatment parameters and comorbidities. We added KDQoL-36 scales to the initial ORS model individually. BKD, burden of kidney disease; SKD, symptoms of kidney disease; EKD, effects of kidney disease; PCS, physical composite score; MCS, mental composite score; KSS, Kidney Disease Scale, a summary score derived from the 24 disease specific items of the KDQoL-36
| Model | Concordance Index | Coefficient Estimate | Mean QoL Score | SD QoL Score | Hazard Ratio (HR)* | p-value Coefficient |
|---|---|---|---|---|---|---|
| ORS | 0.7489 | - | - | - | - | - |
| ORS + BKD | 0.7495 | -0.0009 | 38.2 | 26.3 | 0.9885 | n.s. |
| ORS + SKD | 0.7530 | -0.0093 | 73.5 | 17.2 | 0.9232 | < 0.05 |
| ORS + EKD | 0.7574 | -0.0098 | 58.5 | 22.6 | 0.8952 | < 0.05 |
| ORS + PCS | 0.7540 | -0.0227 | 36.4 | 10.7 | 0.8857 | < 0.05 |
| ORS + MCS | 0.7453 | -0.0174 | 44.9 | 11.6 | 0.9038 | < 0.05 |
| ORS + KSS | 0.7542 | -0.0083 | 57.0 | 18.5 | 0.9258 | < 0.05 |
*HR = exp(SD/2*Coefficient Estimate). SD: standard deviation; ORS: Outcome Risk Score; BKD, burden of kidney disease; SKD, symptoms of kidney disease; EKD, effects of kidney disease; PCS, physical composite score; MCS, mental composite score; KSS, Kidney Disease Scale, summary score derived from the 24 disease specific items of the KDQoL-36
Fig. 3.
Plots of the relationship between survival and KDQoL-36 scores in the study sample. The relationship between survival and KDQoL-36 scores was estimated through Cox regression analysis. Dashed lines outline 95% confidence interval of the estimated values. (a) BKD, burden of kidney disease; (b) EKD, effects of kidney disease; (c) SKD, symptoms of kidney disease; (d) PCS, physical composite score; (e) MCS, mental composite score; (f) KSS, Kidney Disease Scale, a summary score derived from the 24 disease specific items of the KDQoL-36
Health related quality of life and hospitalizations
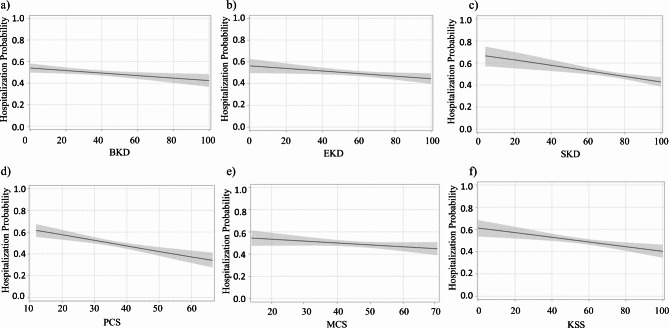
A total of 909 hospitalizations were registered within the follow-up period with 49% of patients having at least one hospitalization corresponding to an incidence rate of 54.8 (95% CI 51.3–58.4) per 100 patient-years. Hospitalization days per patients were 2.57 (95% CI 0.44–8.12). Median length of stay in the hospital was 10 days with interquartile range equal to 4–21 days for patients having at least one hospitalization. Patients using a catheter as the vascular access for dialysis, as well as those with a history of chronic obstructive pulmonary disease and diabetes had higher hospitalization risk according to our ORS model. The concordance index estimates for the hospitalization model indicated modest accuracy of endpoint prediction (C-statistics, 95% confidence interval: 62.7%, 60.1-65.3%). For all scales but the SF-12 Mental composite Score, we observed a statistically significant association between higher HRQoL scores and reduced hospitalization risk. The reduction in risk associated with half standard deviation increase in HRQoL score ranged from 5.2 to 10.5%, depending on the scale under consideration. Odds Ratio (OR) estimates and plots showing the relationship between HRQoL score and hospitalization risk are reported in Table 3; Fig. 4. The odds ratio values representing the association between baseline clinical parameters and hospitalization risk are reported in Supplementary Table 2 (supplementary material).
Table 3.
Association between Health-Related Quality of Life and Hospital admissions. Estimates based on generalized linear models. The outcome risk score (ORS) model includes socio-demographic variables, labs, medical prescriptions, dialysis treatment parameters and comorbidities. We added KDQoL-36 scales to the initial ORS model individually. BKD, burden of kidney disease; SKD, symptoms of kidney disease; EKD, effects of kidney disease; PCS, physical composite score; MCS, mental composite score; KSS, Kidney Disease Scale, a summary score derived from the 24 disease specific items of the KDQoL-36
| Model | ROC-AUC | Coefficient Estimate | Mean QoL Score | SD QoL Score | Odds Ratio (OR)* | p-value Coefficient |
|---|---|---|---|---|---|---|
| ORS | 0.6272 | - | - | - | - | - |
| ORS + BKD | 0.6321 | -0.0047 | 38.2 | 26.3 | 0.9403 | < 0.05 |
| ORS + SKD | 0.6354 | -0.0102 | 73.5 | 17.2 | 0.9162 | < 0.05 |
| ORS + EKD | 0.6298 | -0.0047 | 58.5 | 22.6 | 0.9480 | < 0.05 |
| ORS + PCS | 0.6344 | -0.0207 | 36.4 | 10.7 | 0.8951 | < 0.05 |
| ORS + MCS | 0.6195 | -0.0068 | 44.9 | 11.6 | 0.9611 | n.s. |
| ORS + KSS | 0.6338 | -0.0085 | 57.0 | 18.5 | 0.9243 | < 0.05 |
*OR = exp(SD/2*Coefficient Estimate). ROC-AUC: Area under the ROC curve; SD: standard deviation; ORS: Outcome Risk Score; BKD, burden of kidney disease; SKD, symptoms of kidney disease; EKD, effects of kidney disease; PCS, physical composite score; MCS, mental composite score; KSS, Kidney Disease Scale, summary score derived from the 24 disease specific items of the KDQoL-36
Fig. 4.
Plots of the relationship between the number of hospitalizations and KDQoL-36 scores in the study sample. The relationship between hospitalization incidence and KDQoL-36 scores was estimated through logistic regression analysis. Dashed lines outline 95% confidence interval of the estimated values. (a) BKD, burden of kidney disease; (b) EKD, effects of kidney disease; (c) SKD, symptoms of kidney disease; (d) PCS, physical composite score; (e) MCS, mental composite score; (f) KSS, Kidney Disease Scale, a summary score derived from the 24 disease specific items of the KDQoL-36
We observed a similar pattern of association when considering the relationship between KDQoL-36 scales and hospitalization overall length of stay. The IRR representing the association between baseline clinical parameters and hospitalization days risk are reported in Supplementary Table 3 (supplementary material).
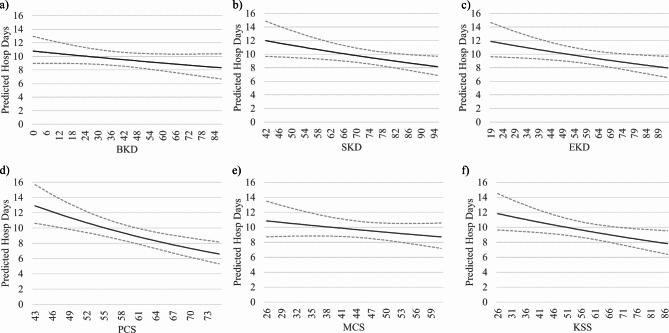
For all scales but the SF-12 Mental composite Score and the Burden of Kidney Disease scale, we observed a statistically significant association between higher HRQoL scores and reduced hospitalization length of stay. The reduction in risk rates associated with half standard deviation increase in HRQoL scores ranged from 5.4 to 11%, depending on the scale under consideration. IRR estimates and plots showing the relationship between HRQoL score and hospitalization risk are reported in Table 4; Fig. 5.
Table 4.
Association between Health-Related Quality of Life and Hospital overall length of stay. Estimates based on generalized linear models. The outcome risk score (ORS) model includes socio-demographic variables, labs, medical prescriptions, dialysis treatment parameters and comorbidities. We added KDQoL-36 scales to the initial ORS model individually. BKD, burden of kidney disease; SKD, symptoms of kidney disease; EKD, effects of kidney disease; PCS, physical composite score; MCS, mental composite score; KSS, Kidney Disease Scale, a summary score derived from the 24 disease specific items of the KDQoL-36
| Model | AIC | Coefficient Estimate | Mean QoL Score | SD QoL Score | Risk Ratio (RR)* | p-value Coefficient |
|---|---|---|---|---|---|---|
| ORS | 9614 | - | - | - | - | - |
| ORS + BKD | 9406 | -0.0032 | 38.2 | 26.3 | 0.9588 | n.s. |
| ORS + SKD | 9412 | -0.0065 | 73.5 | 17.2 | 0.9456 | < 0.05 |
| ORS + EKD | 9488 | -0.0057 | 58.4 | 22.6 | 0.9376 | < 0.05 |
| ORS + PCS | 8553 | -0.0217 | 36.4 | 10.7 | 0.8904 | < 0.05 |
| ORS + MCS | 8566 | -0.0058 | 44.9 | 11.6 | 0.9669 | n.s. |
| ORS + SKK | 9279 | -0.0068 | 57.0 | 18.5 | 0.9390 | < 0.05 |
*RR = exp(SD/2*Coefficient Estimate). AIC: Akaike Information Criterion; SD: standard deviation; ORS: Outcome Risk Score; BKD, burden of kidney disease; SKD, symptoms of kidney disease; EKD, effects of kidney disease; PCS, physical composite score; MCS, mental composite score; KSS, Kidney Disease Scale, summary score derived from the 24 disease specific items of the KDQoL-36
Fig. 5.
Plots of the relationship between the number of hospitalization days and KDQoL-36 scores in the study sample. The relationship between hospitalization days and KDQoL-36 scores was estimated through negative binomial regression analysis. Dashed lines outline 95% confidence interval of the estimated values. (a) BKD, burden of kidney disease; (b) EKD, effects of kidney disease; (c) SKD, symptoms of kidney disease; (d) PCS, physical composite score; (e) MCS, mental composite score; (f) KSS, Kidney Disease Scale, a summary score derived from the 24 disease specific items of the KDQoL-36
Discussion
In this study, we show that higher HRQoL scores were associated with longer patient survival over a period of 12 months of follow up. In addition, a significantly lower risk of hospitalization and length of stay was observed in patients with higher perception of their quality of life. Among the HRQOL metrics adopted for the study, the PCS was the most strongly associated with the study endpoints, in line with previous studies and with comparable scores in the different quality of life scales [7, 26, 27].
We observed as much as 11.4% reduction in mortality risk and 10.5% reduction in hospitalization risk for 5-point increase in PCS, corresponding to the minimal clinical important difference for the scale. The physical composite score taps dimensions related to physical impairment which have been shown to correlate with patients’ frailty, a strong antecedent of mortality in elderly population [28, 29]. Our risk estimates are remarkably overlapping with those reported in earlier DOPPS analysis where a 25% reduction in mortality was noted for each 10-point increase in PCS scores. The association observed was as strong as that observed for well-established risk factors for mortality among haemodialysis patients such as the decrease of 1 mg/dl of albumin [27], or inadequate dialysis dose as measured by Kt/V [30] and larger than that observed by chronic kidney disease (CKD) related mineral bone disorders [31].
Consistent with previous findings, we observed a strong association between worse mental health scores and poor survival [32]. Low MCS scores have been shown to strongly correlate with clinical depression in the general population and among patients with chronic diseases [33]. Mood disorders are a known yet overlooked risk factor for reduced survival in haemodialysis patients, partially due to impaired self-care, adherence to nutritional or therapeutic adherence. Depression and mood disorders have a complex etiology among HD patients including both biochemical and functional alterations dependent on organ failure and psychosocial interference of the disease on social and affective life [34–37]. Managing mood disorders in HD patients is difficult because of the limitations on antidepressant use. However, a recent meta-analysis of randomized controlled trials has demonstrated that cognitive-behavioral therapy can significantly improve depressive symptoms in ESKD patients. Whether such treatments would also translate into prolonged patients’ survival is yet to be demonstrated [38].
We observed a similar, yet less pronounced pattern of association between HRQoL scores and hospitalizations, both in terms of admission rate and overall length of stay. The magnitude of association observed for all scales replicated the results obtained in earlier studies [7, 26, 27]. However, the association between hospitalization risk and the mental component score, did not reach statistical significance in our study.
For years nephrologists have focused on extending patients life and reducing the burden of morbidity among dialysis patients. Dialysis is a life-saving treatment and its introduction in the late sixties dramatically changed the life expectancy and well-being of patients with end stage kidney disease. However, in the latest years technological advances has translated into small, if any, improvements in hard outcomes [38–40]. In this context, a greater interest into healthcare service optimization and HRQoL improvement has grown.
We previously demonstrated that a continuous quality improvement program based on optimization of intermediate endpoints may significantly prolong patients survival [11] and is also associated with improved Health-Related Quality of Life [12]. Evidence suggested that this association may be mediated by variations in symptoms burden and functional capacity rather than by improved satisfaction with care [12]. Given that patients’ health-related quality of life in CKD is a function of glomerular filtration [41–47], it may be argued that the association observed in our study simply reflects CKD-related impairment in bodily functions. The present study expands this body of evidence by showing that the association between health-related quality of life and hard outcomes such as mortality and hospitalization is not explained out by differences in intermediate medical outcomes, biomarkers of CKD-related derangement and case-mix. These findings suggest that, beyond PROMs potential usefulness as outcomes measures for continuous quality improvement programs [23], routine assessment of PROMs in clinical practice can uncover important health trajectories otherwise overlooked by current standard of care.
To increase our knowledge on this topic, nowadays an important effort is being made. Ongoing clinical trials progress are evaluating the technical and clinical viability of PROMs, their adequate feedback, and their impact on improving patient care [9, 48, 49].
An extensive use of PROMs will allow the nephrology community to have an overall view of HD patients, identifying new patient-centered targets that could be validated as quality indicators [24].
Strengths and limitations
Our analysis has several strengths. First, it is a large, real-world evidence study enabling extensive adjustment for potential confounders. Second, we were able to characterize patients’ health by exploiting a wide array of medical information collected during standard clinical practice in a managed healthcare organization. This circumstance enables high quality of data collected through standardized clinical procedures for key intermediate medical endpoints. Supporting KDIGO recommendations, we have recently shown that improvement in intermediate clinical endpoints such as, but not exclusively, phosphorus, calcium, PTH, hemoglobin, pre-dialysis blood pressure, fluid status, is associated with longer patients’ survival and may be associated with improved HRQoL [5].
Our large sample enabled extensive statistical adjustment which lend credibility to our association estimates. This study, however, is subject to some limitations. Observational studies cannot provide definitive evidence of causality. The mechanistic pathways underlying the relationship between health-related quality of life, survival, and hospitalization risk, are not well characterized. Our data cannot shed light on whether poor HRQoL is a marker of unmeasured pathology, or it affects medical endpoints through behavioral or bio-psycho-social mechanisms potentially amenable of intervention. It should be borne in mind that a sizeable share of our patients was unable or unwilling to participate. In fact, patients with cognitive impairment, who were frail with high comorbidity burden have been excluded from the survey, a circumstance that may introduce selection bias and limit the generatability of the results to the Spanish population on hemodialysis.
Conclusions
Several dimensions of HRQOL are associated with patient-centered outcomes (i.e. mortality and hospitalizations at 1 year). Such associations are robust when adjusting to a variety of traditional risk factors. Inclusion of HRQoL in prediction models for mortality and hospitalization slightly increase accuracy. Whether changes in HRQoL directly influence survival and hospitalization rates (for example, through behavioral changes) or merely indicate underlying physiological issues that are not addressed by clinical, anthropometric, and socio-demographic factors remains an area for further research.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Author contributions
IBG wrote the first draft of the paper, contributed to interpretation of the results and to manuscript preparation, review and editing as well as to protocol and study design. JIT performed the statistical analyses, contributed to results interpretation, manuscript preparation, review and editing. ARB, DJCS, SOP, ASP, KS and MEBS designed the study and contributed to conceptualization and interpretation of data from a clinical point of view. LN and SS designed the study and supervised the research. All authors discussed the results, revised, and approved the submitted version of the article.
Funding
There was no external funding for the work presented in this manuscript. Fresenius Medical Care internally supported the conduct of the analysis and manuscript composition, which included employee salaries and company infrastructure.
Data availability
The dataset used in the presented analyses is not publicly available. The dataset was captured from a private electronic medical record system that is restricted to use by only authorized employees of Fresenius Medical Care. The dataset can be made available upon reasonable request to access the dataset, which would require an agreement to be established between Fresenius Medical Care Clinical Board and an external institution of any applicable requestor. Requests to access the datasets should be directed to Luca Neri, luca.neri@freseniusmedicalcare.com.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Ethical approval was obtained from the Ethical Committee of the Hospital Clinic-Barcelona, Spain with approval number HCB/2024/0442. All subjects consented in writing that their data could be used for secondary research and analysis. Each patient was informed of their right to withdraw their consent at any time. Given the retrospective, registry-based nature of the research, a study-specific informed consent was waived by the Ethical Committee.
Consent for publication
Not applicable.
Clinical trial number
Not applicable.
Competing interests
All authors are employees of Fresenius Medical Care. All the authors declare no commercial or financial conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Stefano Stuart and Luca Neri contributed equally to this work.
References
- 1.Kittiskulnam P, Sheshadri A, Johansen KL. <ArticleTitle Language=“En”>Consequences of CKD on Functioning. Semin Nephrol. 2016;36(4):305–18. 10.1016/j.semnephrol.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma S, Kalra D, Rashid I, et al. Assessment of Health-Related Quality of Life in Chronic Kidney Disease Patients: A Hospital-Based Cross-Sectional Study. Med. 2023;59(10). 10.3390/medicina59101788. [DOI] [PMC free article] [PubMed]
- 3.Chuasuwan A, Chuasuwan A, Pooripussarakul S, et al. Comparisons of quality of life between patients underwent peritoneal dialysis and hemodialysis: A systematic review and meta-analysis. Health Qual Life Outcomes. 2020;18(1):1–11. 10.1186/s12955-020-01449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown EA, Zhao J, McCullough K, et al. Burden of Kidney Disease, Health-Related Quality of Life, and Employment Among Patients Receiving Peritoneal Dialysis and In-Center Hemodialysis: Findings From the DOPPS Program. Am J Kidney Dis. 2021;78(4):489–e5001. 10.1053/j.ajkd.2021.02.327. [DOI] [PubMed] [Google Scholar]
- 5.Raoofi S, Pashazadeh Kan F, Rafiei S, et al. Hemodialysis and peritoneal dialysis-health-related quality of life: systematic review plus meta-analysis. BMJ Support Palliat Care. 2023;13:365–73. 10.1136/bmjspcare-2021-003182. [DOI] [PubMed] [Google Scholar]
- 6.Mapes DL, Lopes AA, Satayathum S et al. Health-Related Quality of Life as a Predictor of Mortality and Hospitalization: The Dialysis Outcomes and Practice Patterns Study (DOPPS). Vol 64.; 2003. 10.1046/j.1523-1755.2003.00072.x [DOI] [PubMed]
- 7.Pei M, Aguiar R, Pagels AA et al. Health-related quality of life as predictor of mortality in end-stage renal disease patients: an observational study. 10.1186/s12882-019-1318-x [DOI] [PMC free article] [PubMed]
- 8.Peipert JD, Nair D, Klicko K, Schatell DR, Hays RD. Kidney disease quality of life 36-item short form survey (KDQOL-36) normative values for the United States dialysis population and new single summary score. J Am Soc Nephrol. 2019;30(4):654–63. 10.1681/ASN.2018100994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong A, Manns B, Hemmelgarn B, et al. Standardised outcomes in nephrology - Haemodialysis (SONG-HD): Study protocol for establishing a core outcome set in haemodialysis. Trials. 2015;16(1). 10.1186/s13063-015-0895-7. [DOI] [PMC free article] [PubMed]
- 10.Official JOurnal Of the InternatiOnal SOciety Of NephrOlOgy KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. [DOI] [PubMed]
- 11.Garbelli M, Ion Titapiccolo J, Bellocchio F, Stuard S, Brancaccio D, Neri L. Prolonged patient survival after implementation of a continuous quality improvement programme empowered by digital transformation in a large dialysis network. Nephrol Dial Transpl. 2022;37(3):469–76. 10.1093/ndt/gfab160. [DOI] [PubMed] [Google Scholar]
- 12.Neri L, Ponce P, Matias N, Stuard S, Cromm K. Clinical target achievement is associated with better quality of life among dialysis patients: results from a continuous quality improvement program in a Portuguese healthcare network. Qual Life Res. 2020;29(10):2705–14. 10.1007/s11136-020-02543-0. [DOI] [PubMed] [Google Scholar]
- 13.Himmelfarb J, Vanholder R, Mehrotra R, Tonelli M. The current and future landscape of dialysis. Nat Rev Nephrol. 2020;16(10). 10.1038/s41581-020-0315-4. [DOI] [PMC free article] [PubMed]
- 14.Calvert M, Kyte D, Mercieca-Bebber R, et al. Guidelines for Inclusion of Patient-Reported Outcomes in Clinical Trial Protocols. JAMA. 2018;319(5):483. 10.1001/jama.2017.21903. [DOI] [PubMed] [Google Scholar]
- 15.Nair D, Wilson FP. Patient-Reported Outcome Measures for Adults With Kidney Disease: Current Measures, Ongoing Initiatives, and Future Opportunities for Incorporation Into Patient-Centered Kidney Care. Am J Kidney Dis. 2019;74(6):791–802. 10.1053/j.ajkd.2019.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns DJP, Arora J, Okunade O, et al. International Consortium for Health Outcomes Measurement (ICHOM): Standardized Patient-Centered Outcomes Measurement Set for Heart Failure Patients. JACC Hear Fail. 2020;8(3):212–22. 10.1016/j.jchf.2019.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perl J, Karaboyas A, Morgenstern H, et al. Association between changes in quality of life and mortality in hemodialysis patients: Results from the DOPPS. Nephrol Dial Transpl. 2017;32(3):521–7. 10.1093/ndt/gfw233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyce MB, Browne JP. Does providing feedback on patient-reported outcomes to healthcare professionals result in better outcomes for patients? A systematic review. Qual Life Res. 2013;22(9):2265–78. 10.1007/s11136-013-0390-0. [DOI] [PubMed] [Google Scholar]
- 19.van der Willik EM, Hemmelder MH, Bart HAJ, et al. Routinely measuring symptom burden and health-related quality of life in dialysis patients: first results from the Dutch registry of patient-reported outcome measures. Clin Kidney J. 2021;14(6):1535–44. 10.1093/ckj/sfz192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318(2):197. 10.1001/jama.2017.7156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenhalgh J, Gooding K, Gibbons E, et al. How do patient reported outcome measures (PROMs) support clinician-patient communication and patient care? a realist synthesis. J Patient-Reported Outcomes. 2018;2. 10.1186/s41687-018-0061-6. [DOI] [PMC free article] [PubMed]
- 22.Evans JM, Glazer A, Lum R, et al. Implementing a patient-reported outcome measure for hemodialysis patients in routine clinical care perspectives of patients and providers on esas-r:Renal. Clin J Am Soc Nephrol. 2020;15(9):1299–309. 10.2215/CJN.01840220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valderas JM, Kotzeva A, Espallargues M, et al. The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Qual Life Res. 2008;17(2). 10.1007/s11136-007-9295-0. [DOI] [PubMed]
- 24.Aiyegbusi OL, Nair D, Peipert JD, Schick-Makaroff K, Mucsi I. A narrative review of current evidence supporting the implementation of electronic patient-reported outcome measures in the management of chronic diseases. Ther Adv Chronic Dis. 2021;12. 10.1177/20406223211015958. [DOI] [PMC free article] [PubMed]
- 25.Anderson NE, McMullan C, Calvert M, et al. Using patient-reported outcome measures during the management of patients with end-stage kidney disease requiring treatment with haemodialysis (PROM-HD): A qualitative study. BMJ Open. 2021;11(8). 10.1136/bmjopen-2021-052629. [DOI] [PMC free article] [PubMed]
- 26.Lopes AA, Bragg-Gresham JL, Satayathum S, et al. Health-related quality of life and associated outcomes among hemodialysis patients of different ethnicities in the United States: The dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis. 2003;41(3):605–15. 10.1053/ajkd.2003.50122. [DOI] [PubMed] [Google Scholar]
- 27.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: The dialysis outcomes and practice patterns study (DOPPS). Kidney Int. 2003;64(1):339–49. 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 28.Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912–21. 10.1053/j.ajkd.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chowdhury R, Peel NM, Krosch M, Hubbard RE. Frailty and chronic kidney disease: A systematic review. Arch Gerontol Geriatr. 2017;68:135–42. 10.1016/j.archger.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 30.DeOreo PB. Hemodialysis patient-assessed functional health status predicts continued survival, hospitalization, and dialysis-attendance compliance. Am J Kidney Dis. 1997;30(2):204–12. 10.1016/S0272-6386(97)90053-6. [DOI] [PubMed] [Google Scholar]
- 31.Neri L, Kreuzberg U, Bellocchio F, et al. Detecting high-risk chronic kidney disease-mineral bone disorder phenotypes among patients on dialysis: A historical cohort study. Nephrol Dial Transpl. 2019;34(4):682–91. 10.1093/ndt/gfy273. [DOI] [PubMed] [Google Scholar]
- 32.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. Association among SF36 quality of life measures and nutrition, hospitalization, and mortality in hemodialysis. J Am Soc Nephrol. 2001;12(12). 10.1681/asn.v12122797. [DOI] [PubMed]
- 33.Vilagut G, Forero CG, Pinto-Meza A, et al. The mental component of the short-form 12 health survey (SF-12) as a measure of depressive disorders in the general population: Results with three alternative scoring methods. Value Heal. 2013;16(4):564–73. 10.1016/j.jval.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Drayer RA, Piraino B, Reynolds CF, et al. Characteristics of depression in hemodialysis patients: symptoms, quality of life and mortality risk. Gen Hosp Psychiatry. 2006;28(4):306–12. 10.1016/J.GENHOSPPSYCH.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Muthukumaran A, Natarajan G, Thanigachalam D, Sultan SA, Jeyachandran D, Ramanathan S. The Role of Psychosocial Factors in Depression and Mortality Among Urban Hemodialysis Patients. Kidney Int Rep. 2021;6(5):1437. 10.1016/J.EKIR.2021.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farrokhi F, Abedi N, Beyene J, Kurdyak P, Jassal SV. Association Between Depression and Mortality in Patients Receiving Long-term Dialysis: A Systematic Review and Meta-analysis. Am J Kidney Dis. 2014;63(4):623–35. 10.1053/J.AJKD.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 37.Neri L, Brancaccio D, Rocca Rey LA, Rossa F, Martini A, Andreucci VE. Social support from health care providers is associated with reduced illness intrusiveness in hemodialysis patients. Clin Nephrol. 2011;75(2):125–34. 10.5414/CNP75125. [DOI] [PubMed] [Google Scholar]
- 38.Ling C, Evans D, Zhang Y, et al. The effects of cognitive behavioural therapy on depression and quality of life in patients with maintenance haemodialysis: A systematic review. BMC Psychiatry. 2020;20(1). 10.1186/s12888-020-02754-2. [DOI] [PMC free article] [PubMed]
- 39.Locatelli F, Martin-Malo A, Hannedouche T, et al. Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol. 2009;20(3):645–54. 10.1681/ASN.2008060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rocco MV, Lockridge RS, Beck GJ, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80(10):1080–91. 10.1038/KI.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ryom Oh T, Sang Choi H, Kim S et al. Association between health related quality of life and progression of chronic kidney disease. 10.1038/s41598-019-56102-w [DOI] [PMC free article] [PubMed]
- 42.Mujais SK, Story K, Brouillette J, et al. Health-related quality of Life in CKD patients: Correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4(8):1293–301. 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kefale B, Alebachew M, Tadesse Y, Engidawork E. Quality of life and its predictors among patients with chronic kidney disease: A hospital-based cross sectional study. PLoS ONE. 2019;14(2):e0212184. 10.1371/JOURNAL.PONE.0212184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Porter AC, Lash JP, Xie D, et al. Predictors and outcomes of health-related quality of life in adults with CKD. Clin J Am Soc Nephrol. 2016;11(7):1154–62. 10.2215/CJN.09990915/-/DCSUPPLEMENTAL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorodetskaya I, Zenios S, McCulloch CE, et al. Health-related quality of life and estimates of utility in chronic kidney disease. Kidney Int. 2005;68(6):2801–8. 10.1111/J.1523-1755.2005.00752.X. [DOI] [PubMed] [Google Scholar]
- 46.Neri L, Dukes J, Brennan DC, et al. Impaired renal function is associated with worse self-reported outcomes after kidney transplantation. Qual Life Res. 2011;20(10):1689–98. 10.1007/S11136-011-9905-8/METRICS. [DOI] [PubMed] [Google Scholar]
- 47.Neri L, McEwan P, Sennfält K, Baboolal K. Characterizing the relationship between health utility and renal function after kidney transplantation in UK and US: A cross-sectional study. Health Qual Life Outcomes. 2012;10(1):1–8. 10.1186/1477-7525-10-139/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pagels AA, Stendahl M, Evans M. Patient-reported outcome measures as a new application in the Swedish Renal Registry: health-related quality of life through RAND-36. Clin Kidney J Published online. 2019. 10.1093/ckj/sfz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duncanson E, Bennett PN, Viecelli A, et al. Feasibility and acceptability of e-PROMs data capture and feedback among patients receiving haemodialysis in the Symptom monitoring with Feedback Trial (SWIFT) pilot: Protocol for a qualitative study in Australia. BMJ Open. 2020;10(11). 10.1136/bmjopen-2020-039014. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset used in the presented analyses is not publicly available. The dataset was captured from a private electronic medical record system that is restricted to use by only authorized employees of Fresenius Medical Care. The dataset can be made available upon reasonable request to access the dataset, which would require an agreement to be established between Fresenius Medical Care Clinical Board and an external institution of any applicable requestor. Requests to access the datasets should be directed to Luca Neri, luca.neri@freseniusmedicalcare.com.