Abstract
Background
Type 2 diabetes has traditionally been a risk factor for worse prognosis after myocardial infarction (MI), but major advances have been made in its treatment, and the use of secondary preventive measures has intensified. We evaluated the short- and long-term mortality rates of patients with type 2 diabetes after MI and explored the associations between the characteristics of patients with type 2 diabetes and MI mortality.
Methods
Mortality rates among consecutive MI patients with type 2 diabetes using oral antidiabetic medication (n = 13,152; 40% female; mean age 73.6 years) and MI patients without diabetes (n = 77,669) treated in Finland from 2004 to 2018 were retrospectively studied using a combination of national registries (median follow-up 5.7 years). Differences between groups were balanced with multivariable adjustments and propensity score matching.
Results
Mortality was higher in patients with type 2 diabetes than in the propensity score-matched controls without diabetes at 30 days (12.6% versus 12.0%: p = 0.013), at 1 year (22.4% versus 21.4%; p = 0.001), and at 15 years (83.2% vs. 73.4%; HR 1.20; 95% CI 1.17–1.24; p < 0.0001) after MI. In subgroup analyses, type 2 diabetes was associated with a poorer prognosis across the spectrum of MI patients. The excess mortality risk was attenuated by increasing age but was similar in both sexes. Male sex, age, cardiovascular and noncardiovascular co-morbidities, lack of revascularization, a longer duration of diabetes, and baseline insulin therapy were associated with increased mortality in patients with type 2 diabetes. The one-year prognosis of patients with type 2 diabetes improved during the study period, but the mortality gap compared to patients without diabetes was not altered.
Conclusions
Type 2 diabetes had a negative impact on both short- and long-term outcome after MI, but effect sizes were relatively small. Patients with longer duration of diabetes or need for insulin therapy are still at particular risk.
Graphic abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-024-02479-6.
Keywords: Type 2 diabetes, Myocardial infarction, Mortality
Background
Patients with type 2 diabetes are at increased risk of cardiovascular disease (CVD) and myocardial infarction (MI) [1]. In addition, evidence suggests that the risk of death following an MI is considerably higher in patients with diabetes than in individuals without diabetes [2–6]. However, this evidence, originates mainly from before the reperfusion era and from cohorts of patients in which no distinctions were made between types of diabetes or different treatments, and timely data on long-term prognosis is scarce. Earlier cohorts have found poorer in-hospital [7] and one-year outcomes [8] in patients with type 2 diabetes after MI. Advances in treatment, including timely reperfusion, along with more effective secondary prevention therapies, have since led to improvements in the prognosis of MI [9]. Diabetes care has also advanced, for example, with new pharmacotherapies (such as sodium-glucose co-transporter 2 [SGLT2] inhibitors and glucagon-like peptide-1 [GLP-1] analogues, which improve cardiovascular outcomes) [10], enhanced glucose monitoring [11], and more aggressive CVD risk management [12], improving the outlook for many patients with type 2 diabetes.
Data are scarce on the impact of type 2 diabetes on the prognosis of MI in the current era, with improved treatments for both MI and diabetes. Therefore, the aim of this study was to investigate the impact of type 2 diabetes on short- and long-term mortality after MI. In addition, we explored the association between the characteristics of type 2 diabetes patients and MI mortality.
Methods
Data sources
The data were collected and combined from the multiple nationwide registries mandated by law. Diagnostic codes (International Classification of Diagnosis / ICD-10) for hospital admissions, outpatient visits in specialist medical care, and emergency room admissions as well as operational codes (Nordic Classification of Surgical Procedures) were collected from the Care Register for Health Care in Finland (CRHC) [13]. Cancer diagnosis data was obtained from the Finnish Cancer Registry. The CRHC and Finnish Cancer Registry data spanned from Jan 1st 2004 to index MI.
Antidiabetic medication purchases (Anatomical Therapeutic Chemical/ATC-codes) and purchase dates were obtained from a national drug purchase database. In Finland, antidiabetic drugs are available only with a prescription from a pharmacy, and hence their purchases are recorded in this database [14].
From a registry maintained by the Social Insurance Institution of Finland, we obtained data on special reimbursements for prescription medications, including entitlement codes and underlying ICD-10 diagnoses. This registry spans from Jan 1st 1964 to index event. All patients in Finland with appropriately diagnosed diabetes are entitled to special reimbursement by the state for their antidiabetic medications [15]. Special reimbursement needs to be applied for and is granted by the Social Insurance Institution of Finland after a review of the medical certificate submitted by the treating physician, which describes the rationale for the diagnosis of diabetes.
Data on mortality date was obtained from the Statistics Finland (until Dec 31st 2021).
Study patients
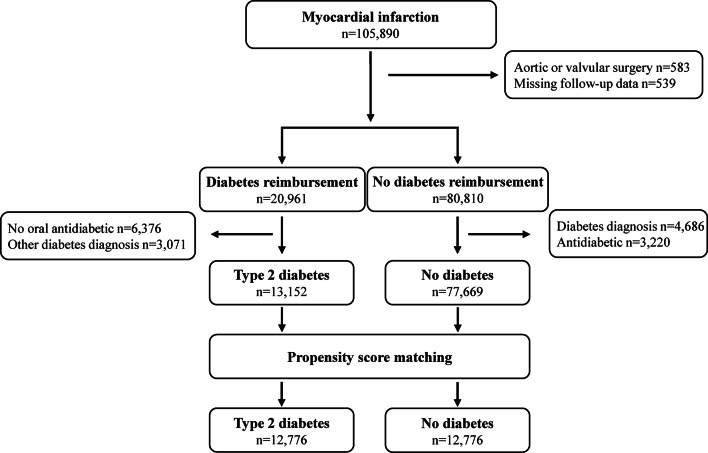
We studied patients with an incident MI admitted to hospitals in Finland between July 1, 2004, and December 31, 2018. Patient data were retrospectively collected from the CRHC. The index MI was identified with ICD-10 code I21 as the primary discharge diagnosis. The sensitivity and positive predictive value of MI diagnoses in this registry from 1998 to 2002 has been shown to be 76–81% and 86–90%, respectively [16]. Data obtained from all 20 Finnish hospitals treating MI patients (including five university hospitals with emergency cardiac surgery available) were included. Patients treated with aortic or valvular surgery (0.6%) and patients lost to follow-up (0.5%) were excluded (Fig. 1).
Fig. 1.
Study flow-chart
Definitions
Type 2 diabetes was identified when all of the following conditions were met: (1) entitlement for special reimbursement for antidiabetic medications (code 103 or 215), (2) diagnosis of type 2 diabetes (ICD-10 code E11), (3) use of an oral antidiabetic medication within six months prior to index MI, and (4) no diagnosis of other types of diabetes (ICD-10 codes E10, E12, E13, E14) (Fig. 1). Patients without entitlement to special reimbursement for antidiabetic medications, no diabetes diagnosis, and no purchases of antidiabetic medications within six months to one year prior to MI were selected as controls without diabetes.
Baseline comorbidities, revascularizations during index MI admission, and type of index MI were identified, as previously described [13, 17]. Baseline usage of antidiabetic medications was defined as medication purchase within six months prior to the index MI. Medications were detected using the ATC codes (Supplement Table 1). Duration of diabetes was calculated from the date of the entitlement for special reimbursement for antidiabetics and categorized as < 5 years, 5–10 years, or ≥ 10 years.
The outcome of interest was death at 30 days, one year, and 15 years after the index MI. Outcome data were available up to December 31, 2021. The median duration of follow-up was 5.7 years (interquartile range [IQR]: 2.7–9.7).
Statistical analysis
The outcome of patients with type 2 diabetes were compared to the outcome of patients without diabetes (non-adjusted, multivariable-adjusted, and propensity score-matched analyses separately). In addition, we explored the associations between mortality and the baseline characteristics of patients with type 2 diabetes.
Differences between the study groups were analyzed using t-, chi-squared, and McNemar’s tests. The effect sizes were evaluated using standardized mean differences (SMD). The outcomes were studied using the Kaplan–Meier method and Cox regression. Schoenfeld residuals were used for visual confirmation of proportional hazard assumptions.
For matching, logistic regression was used to create propensity scores based on baseline age, sex, comorbidities (hypertension, heart failure, atrial fibrillation, previous MI, cerebrovascular disease, chronic pulmonary disease, malignancy, peripheral vascular disease, dementia, valvular disease, rheumatic disease, previous coronary artery bypass grafting (CABG), psychotic disorder, renal failure, alcohol abuse, liver disease, paralysis, and coagulopathy), revascularization by percutaneous coronary intervention (PCI) or CABG, the presence of ST-elevation, treatment in a university hospital, and year of index MI (categorized as 2004–2009, 2010–2014, or 2015−2018). The variables were selected based on previous knowledge [15, 18] and clinical experience. Patients with type 2 diabetes were 1:1 matched to patients without any type of diabetes by using the optimal matching method with a caliper set at 0.1 times the standard deviation of the propensity score [19]. No replacement was used in matching. The E-value for estimating the potential impact of unmeasured confounding was calculated as previously described [20].
Multivariable regression models included the same adjusting variables that were used for propensity score (year of index MI used when modeling 30-day and one-year outcome). Multivariable-adjusted subgroup analyses were performed for men and women, patients aged < 60 years, 60–79 years and ≥ 80 years, and patients with and without heart failure, atrial fibrillation, cerebrovascular disease, chronic pulmonary disease, malignancy, peripheral vascular disease, presence of ST-elevation, revascularization (none, PCI, or CABG), and year of MI (for studying 30-day and one-year outcome; classified as 2004–2009, 2010–2014, or 2015–2018). The results were given as the median, mean, percentage, hazard ratio (HR) with a 95% confidence interval (CI), interquartile range (IQR), or ± SD. Statistical significance was inferred as a p value < 0.05. SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA) was used for analyses.
Results
A total of 13,152 patients with type 2 diabetes and 77,669 patients without diabetes were included.
The median duration of diabetes before the index MI was 7.5 years (range 0–45.1 years), with a duration of < 5 years in 34.5%, 5–10 years in 28.2%, ≥ 10 years in 37.3% of the patients. A single class of oral antidiabetic medication was used by 57.9%, a combination of two medications by 35.8%, and a combination of ≥ 3 by 6.3% of the patients with type 2 diabetes before the index MI. Metformin was the most commonly used oral antidiabetic (79.4%), followed by dipeptidyl peptidase 4 inhibitors and sulfonylureas (Supplemental Table 2). Insulin was used by 34.9% of the patients. The use of SGLT2 inhibitors and GLP-1 analogues was rather uncommon in the cohort (2.9% and 2.4%, respectively).
The patients with type 2 diabetes were older, more often women, and had a higher frequency of baseline comorbidities than the MI patients without diabetes in the nonmatched cohort (Table 1). ST-elevation was less frequent in MI in the patients with type 2 diabetes. The patients with type 2 diabetes were less frequently revascularized by PCI but had CABG more often than the patients without diabetes (Table 1). Propensity score matching resulted in 12,776 pairs of patients with type 2 diabetes and control patients with comparable baseline features (Table 1).
Table 1.
Baseline features of myocardial infarction (MI) patients with type 2 diabetes and and without diabetes. Cohorts of all patients and propensity score–matched patients. CABG = coronary artery bypass surgery. PCI = percuteneus coronary intervention. SMD = standardized mean difference
| Variable | All patients | Matched patients | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Type 2 diabetes |
No diabetes | |SMD| | P-value | Type 2 diabetes | No diabetes | |SMD| | P-value | ||
| n = 13,152 | n = 77,669 | n = 12,776 | n = 12,776 | ||||||
| Age, years (SD) | 73.6 (10.2) | 70.3 (13.1) | 0.251 | < 0.0001 | 73.5 (10.2) | 73.6 (11.1) | 0.014 | 0.345 | |
| Women | 39.6% | 36.4% | 0.066 | < 0.0001 | 39.8% | 40.0% | 0.010 | 0.291 | |
| Comorbidities | |||||||||
| Hypertension | 75.6% | 45.1% | 0.658 | < 0.0001 | 74.9% | 75.2% | 0.012 | 0.154 | |
| Heart failure | 34.4% | 19.8% | 0.335 | < 0.0001 | 33.2% | 33.4% | 0.003 | 0.503 | |
| Atrial fibrillation | 22.0% | 14.9% | 0.183 | < 0.0001 | 21.4% | 21.8% | 0.010 | 0.271 | |
| Previous MI | 19.3% | 13.7% | 0.153 | < 0.0001 | 18.6% | 18.4% | 0.006 | 0.314 | |
| Cerebrovascular disease | 17.5% | 11.0% | 0.185 | < 0.0001 | 17.0% | 17.1% | 0.003 | 0.598 | |
| Chronic pulmonary disease | 16.0% | 13.1% | 0.083 | < 0.0001 | 15.6% | 15.5% | 0.003 | 0.664 | |
| Malignancy | 15.5% | 12.8% | 0.079 | < 0.0001 | 15.3% | 15.1% | 0.006 | 0.597 | |
| Peripheral vascular disease | 13.3% | 6.3% | 0.239 | < 0.0001 | 11.9% | 11.6% | 0.011 | 0.198 | |
| Dementia | 7.5% | 5.9% | 0.063 | < 0.0001 | 7.4% | 7.3% | 0.003 | 0.642 | |
| Valvular disease | 7.3% | 5.7% | 0.067 | < 0.0001 | 7.1% | 6.9% | 0.006 | 0.462 | |
| Rheumatic disease | 6.3% | 6.5% | 0.009 | 0.345 | 6.2% | 6.1% | 0.003 | 0.678 | |
| Previous CABG | 6.2% | 2.6% | 0.176 | < 0.0001 | 5.2% | 5.0% | 0.006 | 0.321 | |
| Psychotic disorder | 4.7% | 3.1% | 0.081 | < 0.0001 | 4.3% | 4.0% | 0.019 | 0.164 | |
| Renal failure | 4.5% | 2.6% | 0.103 | < 0.0001 | 4.2% | 3.9% | 0.020 | 0.107 | |
| Alcohol abuse | 2.4% | 3.1% | 0.044 | < 0.0001 | 2.4% | 2.3% | 0.004 | 0.625 | |
| Liver disease | 1.4% | 0.9% | 0.048 | < 0.0001 | 1.3% | 1.1% | 0.013 | 0.199 | |
| Paralysis | 0.5% | 0.4% | 0.015 | 0.102 | 0.5% | 0.5% | 0.005 | 0.719 | |
| Coagulopathy | 0.5% | 0.4% | 0.015 | 0.091 | 0.5% | 0.5% | 0.005 | 0.721 | |
| Revascularization | 50.8% | 56.4% | 0.113 | < 0.0001 | 51.0% | 50.9% | 0.008 | 0.452 | |
| PCI | 43.0% | 50.6% | 0.152 | < 0.0001 | 43.3% | 43.4% | 0.004 | 0.533 | |
| CABG | 8.3% | 6.5% | 0.070 | < 0.0001 | 8.2% | 8.1% | 0.003 | 0.784 | |
| ST-elevation MI | 27.1% | 39.2% | 0.258 | < 0.0001 | 27.6% | 27.6% | 0.002 | 0.889 | |
| Treatment in University hospital | 47.4% | 49.6% | 0.043 | < 0.0001 | 47.3% | 47.4% | 0.003 | 0.837 | |
| Year of MI | 0.161 | < 0.0001 | 0.013 | 0.616 | |||||
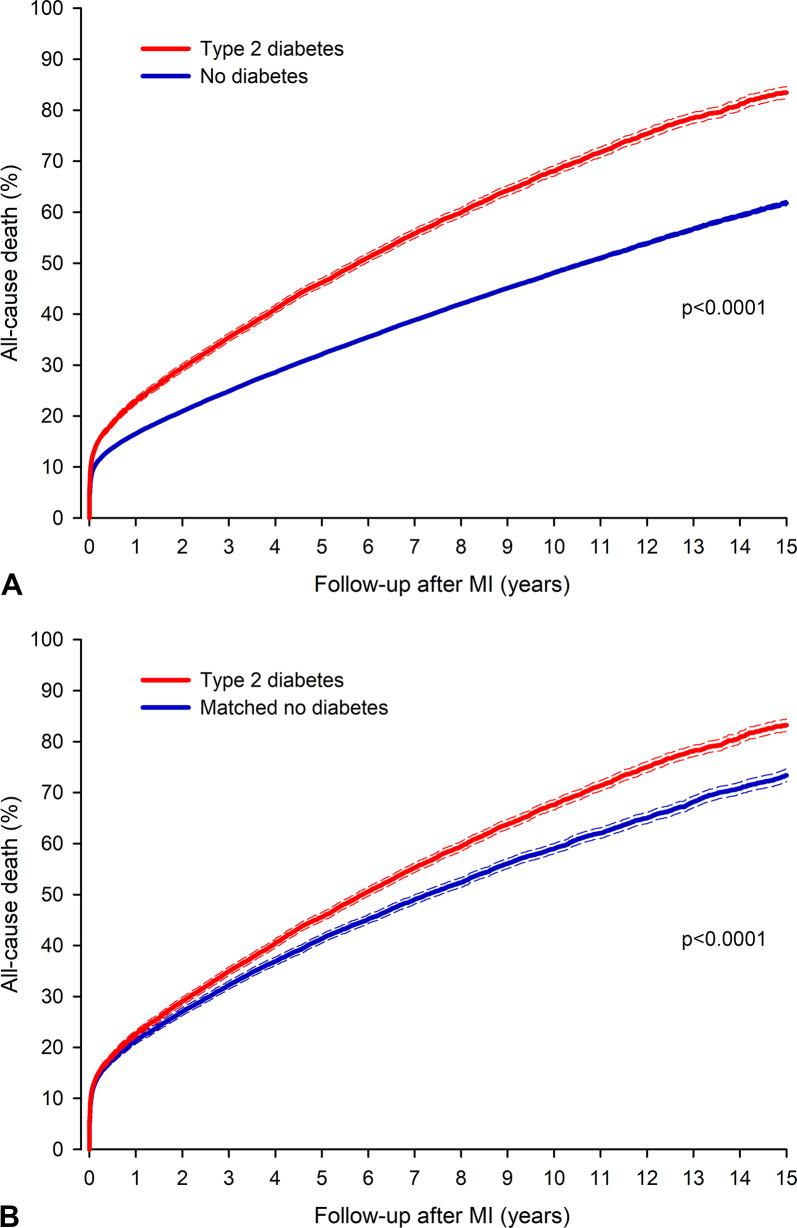
A total of 44,452 patients in the nonmatched cohort died (8,150 in the type 2 diabetes group) during the follow-up of 569,701 patient years. Nonadjusted all-cause mortality was 12.7% in the patients with type 2 diabetes vs. 9.6% in the patients without diabetes at 30 days (p < 0.0001), 22.7% vs. 16.6%, respectively, at one year (p < 0.0001), and 83.5% vs. 61.8%, respectively, at 15 years following MI (HR 1.67; 95% CI 1.63–1.70; p < 0.0001) (Fig. 2). The multivariable-adjusted hazard ratio (HR) for death after MI in patients with type 2 diabetes compared to control patients was 1.16 (95% CI 1.09–1.22; p < 0.0001) at 30 days, 1.16 (95% CI 1.11–1.21; p < 0.0001) at one year, and 1.25 (95% CI 1.22–1.29; p < 0.0001) at 15 years following MI.
Fig. 2.
All-cause mortality after myocardial infarction (MI) in patients with type 2 diabetes mellitus and patients without diabetes in non-adjusted cohort (A) and propensity matched cohort (B). Dashed lines represent 95% confidence interval
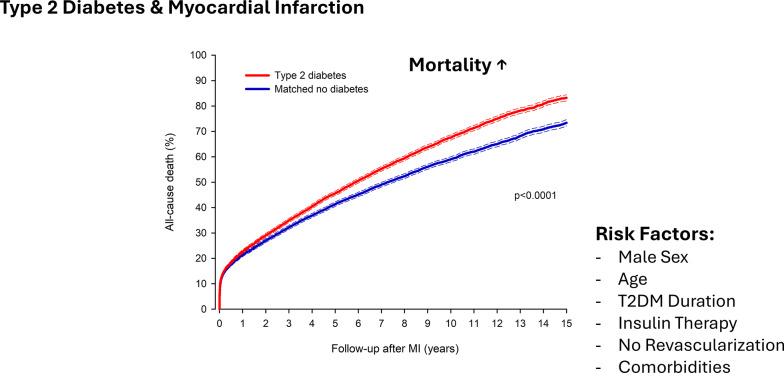
The all-cause mortality in the matched cohort was 12.6% in the type 2 diabetes group versus 12.0% in the matched control group at 30 days (HR 1.07; 95% CI 1.01–1.12; p = 0.013) and 22.4% versus 21.4% at one year (HR 1.07; 95% CI 1.03–1.11; p = 0.001). The difference in mortality between the type 2 diabetes group and the matched control group after MI increased after the first post-MI year (Fig. 2). At 15 years, the corresponding mortalities were 83.2% vs. 73.4% (HR 1.20; 95% CI 1.17–1.24; p < 0.0001). The E-value for death at 15 years was 1.69 (95% CI 1.62–1.79).
Type 2 diabetes was associated with a poorer adjusted prognosis compared to the prognosis for patients without diabetes in patient subgroups by sex, age, heart failure, atrial fibrillation, cerebrovascular disease, chronic pulmonary disease, malignancy, peripheral vascular disease, presence of ST-elevation, and revascularization (Table 2). The excess hazard of death in patients with type 2 diabetes was attenuated by increasing age (Table 2)
Table 2.
Multivariable adjusted hazard ratios for all-cause death at 30 days, 1 year, and 15 years after myocardial in patients with type 2 diabetes versus patients without diabetes in the subgroups
| Type 2 diabetes vs. no diabetes | |||||||
|---|---|---|---|---|---|---|---|
| 30-day mortality | 1-year mortality | 15-year mortality | |||||
| adj.HR (95%CI) | P-value | adj.HR (95%CI) | P-value | adj.HR (95%CI) | P-value | ||
| All patients | 1.16 (1.09–1.22) | < 0.0001 | 1.16 (1.11–1.21) | < 0.0001 | 1.25 (1.22–1.29) | < 0.0001 | |
| Sex | 0.787* | 0.881* | 0.051* | ||||
| Women | 1.16 (1.08–1.26) | 0.0002 | 1.16 (1.09–1.23) | < 0.0001 | 1.21 (1.16–1.27) | < 0.0001 | |
| Men | 1.15 (1.07–1.24) | 0.0003 | 1.16 (1.10–1.23) | < 0.0001 | 1.29 (1.24–1.33) | < 0.0001 | |
| Age (years) | < 0.0001* | < 0.0001* | < 0.0001* | ||||
| < 60 | 2.77 (2.10–3.67) | < 0.0001 | 2.50 (1.99–3.14) | < 0.0001 | 2.32 (2.06–2.62) | < 0.0001 | |
| 60–79 | 1.36 (1.25–1.48) | < 0.0001 | 1.40 (1.32–1.49) | < 0.0001 | 1.50 (1.45–1.55) | < 0.0001 | |
| ≥ 80 years | 1.00 (0.93–1.07) | 0.942 | 0.99 (0.94–1.05) | 0.716 | 1.01 (0.97–1.05) | 0.648 | |
| Heart failure | 0.0002* | 0.0002* | < 0.0001* | ||||
| No | 1.29 (1.19–1.39) | < 0.0001 | 1.26 (1.19–1.34) | < 0.0001 | 1.36 (1.31–1.40) | < 0.0001 | |
| Yes | 1.05 (0.97–1.13) | 0.217 | 1.08 (1.03–1.15) | 0.005 | 1.14 (1.09–1.18) | < 0.0001 | |
| Atrial fibrillation | 0.534* | 0.179* | < 0.0001* | ||||
| No | 1.17 (1.10–1.25) | < 0.0001 | 1.18 (1.13–1.24) | < 0.0001 | 1.31 (1.27–1.35) | < 0.0001 | |
| Yes | 1.13 (1.02–1.24) | 0.019 | 1.11 (1.04–1.20) | 0.004 | 1.11 (1.05–1.17) | 0.0002 | |
| Cerebrovascular disease | < 0.0001* | 0.0004* | < 0.0001* | ||||
| No | 1.22 (1.16–1.30) | < 0.0001 | 1.21 (1.15–1.26) | < 0.0001 | 1.30 (1.26–1.33) | < 0.0001 | |
| Yes | 0.94 (0.83–1.06) | 0.290 | 1.01 (0.93–1.11) | 0.756 | 1.09 (1.03–1.16) | 0.004 | |
| Chronic pulmonary disease | 0.575* | 0.842* | 0.0001* | ||||
| No | 1.15 (1.08–1.22) | < 0.0001 | 1.16 (1.11–1.22) | < 0.0001 | 1.28 (1.25–1.32) | < 0.0001 | |
| Yes | 1.19 (1.05–1.35) | 0.006 | 1.15 (1.05–1.26) | 0.003 | 1.11 (1.04–1.19) | 0.001 | |
| Malignancy | 0.502* | 0.092* | < 0.0001* | ||||
| No | 1.15 (1.08–1.22) | < 0.0001 | 1.18 (1.13–1.24) | < 0.0001 | 1.30 (1.26–1.34) | < 0.0001 | |
| Yes | 1.20 (1.07–1.34) | 0.002 | 1.09 (1.00-1.19) | 0.059 | 1.07 (1.01–1.13) | 0.041 | |
| Peripheral vascular disease | 0.338* | 0.830* | 0.550* | ||||
| No | 1.17 (1.10–1.24) | < 0.0001 | 1.16 (1.11–1.21) | < 0.0001 | 1.26 (1.22–1.29) | < 0.0001 | |
| Yes | 1.09 (0.96–1.24) | 0.188 | 1.17 (1.07–1.29) | 0.001 | 1.23 (1.15–1.31) | < 0.0001 | |
| ST-elevation MI | 0.001* | 0.001* | 0.0001* | ||||
| No | 1.08 (1.01–1.16) | 0.018 | 1.11 (1.06–1.17) | < 0.0001 | 1.21 (1.17–1.24) | < 0.0001 | |
| Yes | 1.31 (1.19–1.44) | < 0.0001 | 1.30 (1.20–1.41) | < 0.0001 | 1.40 (1.33–1.47) | < 0.0001 | |
| Revascularization | < 0.0001* | < 0.0001* | < 0.0001* | ||||
| None | 1.08 (1.02–1.15) | 0.012 | 1.10 (1.05–1.15) | < 0.0001 | 1.17 (1.13–1.21) | < 0.0001 | |
| PCI | 1.57 (1.39–1.78) | < 0.0001 | 1.43 (1.31–1.57) | < 0.0001 | 1.44 (1.38–1.50) | < 0.0001 | |
| CABG | 1.15 (0.84–1.57) | 0.386 | 1.31 (1.05–1.64) | 0.016 | 1.39 (1.27–1.53) | < 0.0001 | |
| Year of MI | 0.891* | 0.208* | - | ||||
| 2004–2009 | 1.14 (1.05–1.24) | 0.002 | 1.15 (1.08–1.23) | < 0.0001 | |||
| 2010–2014 | 1.15 (1.05–1.27) | 0.004 | 1.12 (1.04–1.20) | 0.003 | |||
| 2015–2018 | 1.18 (1.06–1.31) | 0.002 | 1.23 (1.14–1.32) | < 0.0001 | |||
*Interaction p-value. Variables adjusted for included baseline age, sex, comorbidities (hypertension, heart failure, atrial fibrillation, previous MI, cerebrovascular disease, chronic pulmonary disease, malignancy, peripheral vascular disease, dementia, valvular disease, rheumatic disease, previous CABG, psychotic disorder, renal failure, alcohol abuse, liver disease, paralysis, and coagulopathy), revascularization by PCI or CABG, the presence of ST-elevation, and treatment in a university hospital. For 30-day and 1-year mortality rates, additional adjustment was made for the year of index MI (categorized as 2004–2009, 2010–2014, or 2015−2018). The variable used to create a specific subgroup was not adjusted for in that subgroup analysis
. The one-year outcome of patients with type 2 diabetes improved over the study period (mortality was 28.4% in 2004–2009, 21.1% in 2010–2014, and 19.6% in 2015–2018) (Table 3). However, the HR for one-year mortality in patients with type 2 diabetes versus controls did not change during the study period (Table 2).
Table 3.
Association of baseline features in patients with type 2 diabetes with all-cause death at 30 days, 1 year, and 15 years after myocardial infarction (MI)
| Variable | Association with mortality | |||||||
|---|---|---|---|---|---|---|---|---|
| 30-days | 1-year | 15-years | ||||||
| adj.HR (95%CI) | P-value | adj.HR (95%CI) | P-value | adj.HR (95%CI) | P-value | |||
| Age, years | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| < 60 | Reference | Reference | Reference | |||||
| 60–79 | 1.55 (1.18–2.03) | 0.002 | 1.86 (1.49–2.31) | < 0.0001 | 2.26 (2.01–2.54) | < 0.0001 | ||
| ≥ 80 | 2.65 (2.00-3.50) | < 0.0001 | 3.10 (2.47–3.89) | < 0.0001 | 4.60 (4.07–5.20) | < 0.0001 | ||
| Women | 0.95 (0.86–1.05) | 0.344 | 0.92 (0.85–0.99) | 0.025 | 0.91 (0.86–0.95) | < 0.0001 | ||
| Co-morbidities | ||||||||
| Hypertension | 0.95 (0.85–1.06) | 0.344 | 1.00 (0.92–1.09) | 0.953 | 0.99 (0.94–1.04) | 0.654 | ||
| Heart failure | 1.44 (1.29–1.60) | < 0.0001 | 1.66 (1.54–1.80) | < 0.0001 | 1.70 (1.62–1.78) | < 0.0001 | ||
| Atrial fibrillation | 1.12 (1.01–1.25) | 0.040 | 1.15 (1.06–1.25) | 0.001 | 1.17 (1.11–1.24) | < 0.0001 | ||
| Previous MI | 1.05 (0.94–1.18) | 0.392 | 1.11 (1.02–1.21) | 0.012 | 1.14 (1.08–1.20) | < 0.0001 | ||
| Cerebrovascular disease | 0.95 (0.84–1.08) | 0.422 | 1.04 (0.96–1.14) | 0.356 | 1.12 (1.06–1.19) | < 0.0001 | ||
| Chronic pulmonary disease | 1.13 (1.00-1.28) | 0.045 | 1.13 (1.03–1.24) | 0.008 | 1.10 (1.04–1.17) | 0.002 | ||
| Malignancy | 1.29 (1.14–1.47) | < 0.0001 | 1.33 (1.22–1.46) | < 0.0001 | 1.21 (1.13–1.28) | < 0.0001 | ||
| Peripheral vascular disease | 1.30 (1.14–1.47) | < 0.0001 | 1.42 (1.30–1.56) | < 0.0001 | 1.50 (1.41–1.60) | < 0.0001 | ||
| Dementia | 1.56 (1.36–1.79) | < 0.0001 | 1.51 (1.36–1.68) | < 0.0001 | 1.59 (1.47–1.72) | < 0.0001 | ||
| Valvular disease | 1.24 (1.06–1.45) | 0.007 | 1.33 (1.18–1.48) | < 0.0001 | 1.26 (1.16–1.37) | < 0.0001 | ||
| Rheumatic disease | 1.05 (0.87–1.28) | 0.600 | 1.11 (0.96–1.27) | 0.150 | 1.12 (1.03–1.23) | 0.011 | ||
| Prior CABG | 0.69 (0.55–0.86) | 0.001 | 0.73 (0.63–0.85) | < 0.0001 | 0.89 (0.81–0.97) | 0.011 | ||
| Psychotic disorder | 1.38 (1.14–1.69) | 0.001 | 1.25 (1.06–1.48) | 0.007 | 1.22 (1.10–1.36) | 0.0002 | ||
| Renal failure | 1.35 (1.13–1.62) | 0.001 | 1.36 (1.19–1.56) | < 0.0001 | 1.41 (1.27–1.55) | < 0.0001 | ||
| Alcohol abuse | 1.42 (1.05–1.93) | 0.023 | 1.27 (0.99–1.62) | 0.065 | 1.28 (1.09–1.50) | 0.002 | ||
| Liver disease | 0.95 (0.62–1.45) | 0.802 | 1.21 (0.92–1.60) | 0.176 | 1.08 (0.87–1.35) | 0.487 | ||
| Paralysis | 1.39 (0.82–2.36) | 0.221 | 1.33 (0.89–1.99) | 0.164 | 1.16 (0.70–1.90) | 0.564 | ||
| Coagulopathy | 2.27 (1.48–3.48) | 0.0002 | 2.20 (1.57–3.08) | < 0.0001 | 1.79 (1.33–2.40) | 0.0001 | ||
| Duration of diabetes | 0.069 | 0.0004 | < 0.0001 | |||||
| < 5 years | Reference | Reference | Reference | |||||
| 5–10 years | 1.02 (0.89–1.16) | 0.792 | 0.99 (0.90–1.09) | 0.801 | 1.09 (1.03–1.15) | 0.005 | ||
| > 10 years | 1.15 (1.01–1.31) | 0.037 | 1.17 (1.06–1.29) | 0.001 | 1.26 (1.19–1.34) | < 0.0001 | ||
| Insulin therapy | 1.09 (0.97–1.22) | 0.146 | 1.06 (0.98–1.16) | 0.144 | 1.07 (1.02–1.13) | 0.007 | ||
| Revascularization | < 0.0001 | < 0.0001 | < 0.0001 | |||||
| None | Reference | Reference | Reference | |||||
| PCI | 0.36 (0.31–0.41) | < 0.0001 | 0.35 (0.32–0.39) | < 0.0001 | 0.54 (0.51–0.57) | < 0.0001 | ||
| CABG | 0.33 (0.25–0.46) | < 0.0001 | 0.37 (0.30–0.45) | < 0.0001 | 0.56 (0.51–0.61) | < 0.0001 | ||
| ST-elevation MI | 2.11 (1.90–2.34) | < 0.0001 | 1.62 (1.49–1.76) | < 0.0001 | 1.17 (1.11–1.24) | < 0.0001 | ||
| University Hospital | 0.91 (0.82-1.00) | 0.051 | 0.94 (0.87–1.01) | 0.100 | 0.93 (0.89–0.97) | 0.001 | ||
| Year of MI | < 0.0001 | < 0.0001 | - | - | ||||
| 2004–2009 | Reference | Reference | ||||||
| 2010–2014 | 0.85 (0.76–0.95) | 0.006 | 0.81 (0.74–0.89) | < 0.0001 | ||||
| 2015–2018 | 0.75 (0.66–0.85) | < 0.0001 | 0.78 (0.71–0.85) | < 0.0001 | ||||
Results of multivariable adjusted analyses. CABG = coronary artery bypass grafting. PCI = percutaneus coronary intervention
In patients with type 2 diabetes, higher age, male sex, baseline cardiovascular comorbidities, malignancy, renal failure, dementia, chronic pulmonary disease, dementia, coagulopathy, and the presence of ST-elevation were associated with one-year death (Table 3). Alcohol abuse, cerebrovascular disease, and rheumatic disease were additional risk factors for long-term mortality (Table 3). Previous MI was associated with higher mortality and previous CABG with lower mortality after MI. A longer duration of diabetes prior to the index event was associated with a higher risk of death at both one year and 15 years (Table 3). Patients with baseline insulin therapy in addition to oral antidiabetic medication had poorer long-term outcomes, irrespective of the duration of diabetes. Revascularization with either PCI or CABG was associated with significantly better short- and long-term prognoses.
Discussion
In this nationwide registry study of Finnish MI patients who were treated between 2004 and 2018, the risk of death was higher among patients with type 2 diabetes compared to patients without diabetes. In contrast to many previous studies, after accounting for differences in baseline characteristics with propensity score matching, the effect size of the impact of type 2 diabetes on mortality after MI, and in particular, short-term and intermediate-term mortality, was small (a 7% increase in 30-day and one-year mortality and a 20% increase in 15-year mortality). Within the MI cohort with type 2 diabetes, insulin treatment and a longer duration of diabetes stood out as factors associated with poorer prognoses following MI.
In previous studies conducted before or during the early 2000s, in-hospital or 30-day post-MI mortality rates were not shown to unequivocally increase among patients with diabetes, but longer-term mortality rates increased by 1.3–1.7-fold [6, 21–28]. Compared to these results, our one-year and 15-year HRs for mortality in the propensity score-matched cohort seem low (1.07 and 1.20, respectively), and may imply a slowly narrowing mortality gap between MI patients with and without type 2 diabetes. In more recent European studies from time periods overlapping with our study period, the results have varied; among Polish acute coronary syndrome (ACS) patients with type 2 diabetes, the HR for three-year mortality was more similar to our estimate —1.20 (95% CI 1.14, 1.27) but among UK and Italian MI patients with diabetes, the long-term mortality risk increased by 1.5–1.6-fold after adjustments [5, 29, 30].
We evaluated temporal trends in mortality rates in the time periods 2004–2009, 2010–2014, and 2015–2018 and, while absolute mortality rates improved, HRs remained similar, suggesting that prognosis following MI in patients with diabetes may have already improved earlier than 2004–2018. This hypothesis is reinforced by a Finnish nationwide registry study among ACS patients conducted between 1988 and 2002, in which the HRs for one-year mortality after MI were substantially higher than our results (point estimates varied between 1.56 and 2.87 in men and between 1.45 and 5.84 among women across age groups) [7]. Previous evaluations of temporal trends in MI prognosis among patients with diabetes have suggested either no improvement or a small improvement in the excess mortality rates over time [6, 23, 25, 31].
The reasons for the relatively small difference in post-MI survival between patients with and without diabetes—which contrasts with previous studies—may be speculated upon. Differences in study designs and the extent of adjustments may play a role. Previously, the worse post-MI survival among patients with diabetes has been explained by several factors, including delays in diagnosis due to more prevalent nonspecific presenting symptoms, poorer treatment in the acute phase of MI and in post-MI risk factor management, and more advanced coronary artery disease at diagnosis [32]. We adjusted for differences in rates and mode of revascularization and in the proportion of patients with NSTEMI and STEMI, but not directly for differences in the extent of coronary artery atherosclerosis. Survival disadvantage related to type 2 diabetes was more pronounced among patients with STEMI compared to those with NSTEMI, as well as among revascularized compared to non-revascularized patients. This may imply that diabetes is associated with more severe coronary disease in the setting of STEMI, but not so much in NSTEMI, which has become more common during the past few decades [33]. Patients with STEMI and diabetes also have higher rates of reduced left ventricular ejection fraction and lower estimated glomerular filtration rates than STEMI patients without diabetes, and these prognostic factors may drive the increased in-hospital mortality rates [34]. In patients with STEMI who underwent PCI, the diabetes-related increased mortality risk seems to be mediated by mechanisms other than impaired epicardial or myocardial reperfusion [35].
Evidence also suggests that the gaps in post-MI care among patients with type 2 diabetes may be closing; for example, patients with diabetes seem to be titrated to high-intensity statins following MI at a similar rate as the non-diabetic controls [36]. The outlook for many patients with type 2 diabetes has improved with the advent of new pharmacotherapies with favorable cardiovascular effects, such as SGLT2 inhibitors and GLP-1 analogues [10], enhanced glucose monitoring [11], and intensified CVD risk management [12]. Although the use of SGLT2 inhibitors and GLP-1 analogues was quite rare before the index MI in our cohort, the use of SGLT2 inhibitors, in particular, increased during the study period in Finland [37].
Similar to previous studies [13, 38], we found that female sex was associated with better outcomes after MI in patients with type 2 diabetes. However, in some previous studies, the deleterious effect of diabetes on in-hospital and long-term mortality after MI compared to patients without diabetes was confined to women or more pronounced in women [6, 24, 31, 39]. In contrast, we found no interaction between type 2 diabetes and sex in terms of short- or long-term mortality after adjustments. Type 2 diabetes had a comparably negative impact on one-year and 15-year survival regardless of sex when accounting for age, comorbidities, revascularization, diabetes duration, and insulin use. The reasons for this discrepancy with previous studies remain speculative but may be related to differences in study design, covariable adjustment, or healthcare systems.
Although increasing age was associated with an increased mortality risk among the patients with type 2 diabetes, the excess long-term mortality risk compared to patients without diabetes was more pronounced (more than two-fold) among individuals aged < 60 years, and not apparent among individuals aged ≥ 80 years. This finding is consistent with the results of previous studies [7] and suggests that type 2 diabetes negatively impacts prognosis, especially in younger MI patients; therefore, special attention should be paid to glycemic control and secondary preventive measures in these patients.
Longer disease duration and poor glycemic control are risk factors for developing CVD in type 2 diabetes [1, 40]. We found both longer duration of diabetes and the need for insulin therapy to be risk factors for death after MI. Our results corroborate previous findings on the negative impact of diabetes duration [30, 41] and insulin therapy [42–44] on MI prognosis. The need for insulin treatment may be a sign of poor glycemic control, which is associated with an increased risk of mortality and adverse cardiovascular events after STEMI and after PCI [45, 46]. The current and previous findings underline the importance of measures to postpone the development of occult type 2 diabetes in populations at risk and good glycemic control in patients with type 2 diabetes.
Our study has some notable strengths, including the inclusion of a nationwide MI cohort and the use of propensity score matching based on a plethora of important covariables to account for confounding factors. Nevertheless, residual confounding is possible. We lacked data on some possible confounders, such as the extent of coronary artery disease, body mass index, and smoking status. Based on the analysis of E-value, unmeasured confounders would have to have had a minimum strength of an association of 1.6 on the risk ratio scale with both the exposure and the outcome to explain away our main finding [20]. To maximize the specificity in detecting patients with type 2 diabetes, we included only patients who used oral antidiabetics before the index MI. Thus, our cohort includes patients with established type 2 diabetes who are taking peroral antidiabetic medication but excludes patients who were newly diagnosed with diabetes during MI admission, those not taking antidiabetic medication, and those treated solely with insulin. Previous data indicate better outcomes after MI among patients with type 2 diabetes who did not need antidiabetic medication and received diet treatment alone [3]. Our results are not generalizable to prediabetic patients or to patients with substantially improved glycemic control and no further need for antidiabetic medication.
Conclusions
Short- and long-term mortality after MI remains higher in type 2 diabetes patients compared with individuals without diabetes, although the effect sizes were small in this contemporary MI cohort. This may indicate that advances in treatment and CVD prevention measures have resulted in a narrowing mortality gap for patients with type 2 diabetes after MI, but there is still a need to optimize secondary prevention among this vulnerable patient population. The excess mortality risk in type 2 diabetes was more pronounced among younger age groups, but the excess risk was similar in men and women. A longer duration of diabetes and the need for insulin therapy were found to be risk factors for a poor prognosis after MI.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- ACS
Acute coronary syndrome
- CABG
Coronary artery bypass grafting
- CI
Confidence interval
- CRHC
Care Register for Health Care in Finland
- CVD
Cardiovascular disease
- GLP-1
Glucagon-like peptide-1
- HR
Hazard ratio
- IQR
Interquartile range
- MI
Myocardial infarction
- NSTEMI
Non-ST-elevation myocardial infarction
- PCI
Percutaneous coronary intervention
- SGLT2
Sodium-glucose co-transporter 2
- STEMI
ST-elevation myocardial infarction
Author contributions
AMK contributed to the design and interpretation of the study, and together with VK, drafted the first version of the manuscript. MJ and PR contributed to the design and interpretation of the study and critically revised the manuscript to include important intellectual content. VK contributed to design, data acquisition, analysis, and interpretation, and critically revised the manuscript. All authors approved the final version of the manuscript. VK is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding
This study was supported by grant funding from the Finnish Foundation for Cardiovascular Research and the Finnish Governmental VTR funding.
Data availability
Requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Findata at http://findata.fi/en.
Declarations
Ethics approval and consent to participate
The data were obtained from Findata, including the CRHC data, antidiabetic medication purchase data prior to MI, special reimbursement entitlement data, and cancer data in the Finnish Cancer Registry (permission THL/164/14.02.00/2021); mortality data were obtained from Statistics Finland (permission TK-53-484-20). The included registries are mandatory by law and cover the entire Finnish population. Ethical board review and informed consent were waived by law due to the study design. The participants were not contacted. The legal basis for the processing of personal data is of public interest and scientific research (EU General Data Protection Regulation [GDPR] 2016/679, Article 6(1)I and Article 9(2)(j); Data Protection Act, Sects. 4 and 6).
Consent for publication
Not applicable.
Competing interests
AMK has received speaker fees from Boehringer-Ingelheim, Abbvie, and Sanofi; has participated in the advisory boards of Pfizer and Boehringer-Ingelheim; and has received congress sponsorship from Abbvie and Johnson & Johnson, which are all unrelated to this work. MJ has received speaker fees from Amgen, AstraZeneca, Boehringer Ingelheim, Novartis, and NovoNordisk. VK: None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shah AD, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3(2):105–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chun BY, Dobson AJ, Heller RF. The impact of diabetes on survival among patients with first myocardial infarction. Diabetes Care. 1997;20(5):704–8. [DOI] [PubMed] [Google Scholar]
- 3.Gustafsson I, Hildebrandt P, Seibaek M, et al. Long-term prognosis of diabetic patients with myocardial infarction: relation to antidiabetic treatment regimen. The TRACE Study Group. Eur Heart J. 2000;21(23):1937–43. [DOI] [PubMed] [Google Scholar]
- 4.Miettinen H, Lehto S, Salomaa V, et al. Impact of diabetes on mortality after the first myocardial infarction. The FINMONICA myocardial infarction Register Study Group. Diabetes Care. 1998;21(1):69–75. [DOI] [PubMed] [Google Scholar]
- 5.Alabas OA, Hall M, Dondo TB, et al. Long-term excess mortality associated with diabetes following acute myocardial infarction: a population-based cohort study. J Epidemiol Community Health. 2017;71(1):25–32. [DOI] [PubMed] [Google Scholar]
- 6.Eliasson M, Jansson JH, Lundblad D, Näslund U. The disparity between long-term survival in patients with and without diabetes following a first myocardial infarction did not change between 1989 and 2006: an analysis of 6,776 patients in the Northern Sweden MONICA Study. Diabetologia. 2011;54(10):2538–43. [DOI] [PubMed] [Google Scholar]
- 7.Winell KM, Pääkkönen R, Pietilä A, Niemi MK, Reunanen AR, Salomaa VV. Case fatality rates after first acute coronary syndrome in persons treated for type 2 diabetes show an improving trend. Diabetologia. 2010;53(3):472–80. [DOI] [PubMed] [Google Scholar]
- 8.Ahmed B, Davis HT, Laskey WK. In-hospital mortality among patients with type 2 diabetes mellitus and acute myocardial infarction: results from the national inpatient sample, 2000–2010. J Am Heart Assoc 2014;3(4). [DOI] [PMC free article] [PubMed]
- 9.Martin SS, Aday AW, Almarzooq ZI, et al. 2024 Heart Disease and Stroke statistics: a report of US and Global Data from the American Heart Association. Circulation. 2024;149(8):e347–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Diabetes Association Professional Practice C. 9. Pharmacologic approaches to Glycemic Treatment: standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S158–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Diabetes Association Professional Practice C. 7. Diabetes Technology: Standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S126–S144. [DOI] [PMC free article] [PubMed]
- 12.American Diabetes Association Professional Practice C. 10. Cardiovascular Disease and Risk Management: standards of Care in Diabetes-2024. Diabetes Care. 2024;47(Suppl 1):S179–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerola AM, Palomäki A, Rautava P, Kytö V. Less revascularization in young women but impaired long-term outcomes in young men after myocardial infarction. Eur J Prev Cardiol. 2022;29(10):1437–45. [DOI] [PubMed] [Google Scholar]
- 14.Prami T, Khanfir H, Deleskog A, et al. Clinical factors associated with initiation of and persistence with ADP receptor-inhibiting oral antiplatelet treatment after acute coronary syndrome: a nationwide cohort study from Finland. BMJ Open. 2016;6(11):e012604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kerola AM, Juonala M, Palomäki A, Semb AG, Rautava P, Kytö V. Case fatality of patients with type 1 diabetes after myocardial infarction. Diabetes Care. 2022;45(7):1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pajunen P, Koukkunen H, Ketonen M, et al. The validity of the Finnish Hospital Discharge Register and causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2005;12(2):132–7. [DOI] [PubMed] [Google Scholar]
- 17.Palomäki A, Kerola AM, Malmberg M, Rautava P, Kytö V. Patients with rheumatoid arthritis have impaired long-term outcomes after myocardial infarction: a nationwide case-control registry study. Rheumatology (Oxford). 2021;60(11):5205–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerola AM, Semb AG, Juonala M, Palomäki A, Rautava P, Kytö V. Long-term cardiovascular prognosis of patients with type 1 diabetes after myocardial infarction. Cardiovasc Diabetol. 2022;21(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kytö V, Sipilä J, Ahtela E, Rautava P, Gunn J. Mechanical Versus Biologic prostheses for Surgical aortic valve replacement in patients aged 50 to 70. Ann Thorac Surg. 2020;110(1):102–10. [DOI] [PubMed] [Google Scholar]
- 20.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the e-value. Ann Intern Med. 2017;167(4):268–74. [DOI] [PubMed] [Google Scholar]
- 21.Koek HL, Soedamah-Muthu SS, Kardaun JW, et al. Short- and long-term mortality after acute myocardial infarction: comparison of patients with and without diabetes mellitus. Eur J Epidemiol. 2007;22(12):883–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Löwel H, Köenig W, Engel S, Hormann A, Keil U. The impact of diabetes mellitus on survival after myocardial infarction: can it be modified by drug treatment? Results of a population-based myocardial infarction register follow-up study. Diabetologia. 2000;43(2):218–26. [DOI] [PubMed] [Google Scholar]
- 23.Norhammar A, Lindbäck J, Ryden L, et al. Improved but still high short- and long-term mortality rates after myocardial infarction in patients with diabetes mellitus: a time-trend report from the Swedish Register of Information and Knowledge about Swedish Heart Intensive Care Admission. Heart. 2007;93(12):1577–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nedkoff L, Knuiman M, Hung J, Briffa TG. Improving 30-day case fatality after incident myocardial infarction in people with diabetes between 1998 and 2010. Heart. 2015;101(16):1318–24. [DOI] [PubMed] [Google Scholar]
- 25.Nauta ST, Deckers JW, Akkerhuis KM, van Domburg RT. Short- and long-term mortality after myocardial infarction in patients with and without diabetes: changes from 1985 to 2008. Diabetes Care. 2012;35(10):2043–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patel PA, Cubbon RM, Sapsford RJ, et al. An evaluation of 20 year survival in patients with diabetes mellitus and acute myocardial infarction. Int J Cardiol. 2016;203:141–4. [DOI] [PubMed] [Google Scholar]
- 27.Gruppetta M, Calleja N, Fava S. Long-term survival after acute myocardial infarction and relation to type 2 diabetes and other risk factors. Clin Cardiol. 2010;33(7):424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matetic A, Doolub G, Bharadwaj A, et al. Differential Impact of Type 1 and type 2 diabetes Mellitus on outcomes among 1.4 million US patients undergoing percutaneous coronary intervention. Cardiovasc Revasc Med. 2022;38:83–8. [DOI] [PubMed] [Google Scholar]
- 29.Fojt A, Kowalik R, Gierlotka M, Gasior M, Smeding C, Opolski G. Three-year mortality after acute myocardial infarction in patients with different diabetic status. Pol Arch Intern Med 2021;131(11). [DOI] [PubMed]
- 30.Baviera M, Genovese S, Colacioppo P, et al. Diabetes mellitus duration and mortality in patients hospitalized with acute myocardial infarction. Cardiovasc Diabetol. 2022;21(1):223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedkoff L, Knuiman M, Hung J, Briffa TG. Long-term all-cause and cardiovascular mortality following incident myocardial infarction in men and women with and without diabetes: temporal trends from 1998 to 2009. Eur J Prev Cardiol. 2016;23(12):1273–81. [DOI] [PubMed] [Google Scholar]
- 32.Byrne RA, Rossello X, Coughlan JJ, et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38):3720–826. [DOI] [PubMed] [Google Scholar]
- 33.Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121(7):863–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marenzi G, Cosentino N, Genovese S, et al. Reduced cardio-renal function accounts for most of the In-Hospital morbidity and mortality risk among patients with type 2 diabetes undergoing primary percutaneous coronary intervention for ST-Segment Elevation myocardial infarction. Diabetes Care. 2019;42(7):1305–11. [DOI] [PubMed] [Google Scholar]
- 35.Brener SJ, Mehran R, Dressler O, Cristea E, Stone GW. Diabetes mellitus, myocardial reperfusion, and outcome in patients with acute ST-elevation myocardial infarction treated with primary angioplasty (from HORIZONS AMI). Am J Cardiol. 2012;109(8):1111–6. [DOI] [PubMed] [Google Scholar]
- 36.Giustino G, Colantonio LD, Brown TM, et al. Titration to high-intensity statin therapy following Acute myocardial infarction in patients with and without diabetes Mellitus. Cardiovasc Drugs Ther. 2018;32(5):453–61. [DOI] [PubMed] [Google Scholar]
- 37.Wikström K, Lamidi ML, Rautiainen P, Tirkkonen H, Laatikainen T. Type 2 diabetes medication and HbA1c levels in North Karelia Finland, 2013–2019. Diabet Med. 2022;39(9):e14866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kytö V, Nuotio M, Rautava P. Sex difference in the Case Fatality of older myocardial infarction patients. J Gerontol Biol Sci Med Sci. 2022;77(3):614–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-de-Andres A, Jimenez-Garcia R, Hernandez-Barrera V, et al. Are there sex differences in the effect of type 2 diabetes in the incidence and outcomes of myocardial infarction? A matched-pair analysis using hospital discharge data. Cardiovasc Diabetol. 2021;20(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huo X, Gao L, Guo L, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4(2):115–24. [DOI] [PubMed] [Google Scholar]
- 41.Yaliqin N, Yu ZX, Aimaier S, Adi D, Ma YT. Impact of Duration of Diabetes Mellitus on Long-term outcome in type 2 Diabetic patients with primary percutaneous coronary intervention after the first myocardial infarction. Cardiology 2024. [DOI] [PubMed]
- 42.Hoebers LP, Claessen BE, Woudstra P, et al. Long-term mortality after primary percutaneous coronary intervention for ST-segment elevation myocardial infarction in patients with insulin-treated versus non-insulin-treated diabetes mellitus. EuroIntervention. 2014;10(1):90–6. [DOI] [PubMed] [Google Scholar]
- 43.Zuanetti G, Latini R, Maggioni AP, Santoro L, Franzosi MG. Influence of diabetes on mortality in acute myocardial infarction: data from the GISSI-2 study. J Am Coll Cardiol. 1993;22(7):1788–94. [DOI] [PubMed] [Google Scholar]
- 44.Bundhun PK, Li N, Chen MH. Adverse cardiovascular outcomes between insulin-treated and non-insulin treated diabetic patients after percutaneous coronary intervention: a systematic review and meta-analysis. Cardiovasc Diabetol. 2015;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Li X, Zhang Y, et al. Impact of glycemic control status on patients with ST-segment elevation myocardial infarction undergoing percutaneous coronary intervention. BMC Cardiovasc Disord. 2020;20(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma PK, Agarwal S, Ellis SG, et al. Association of glycemic control with mortality in patients with diabetes mellitus undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7(4):503–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests to access the dataset from qualified researchers trained in human subject confidentiality protocols may be sent to Findata at http://findata.fi/en.