Abstract
Early brain development research is important to public health professionals for understanding early development and strengthening systemic supports for young children's healthy brain growth. This overview describes basic processes of early brain development, including prenatal development and the "fetal programming" of brain and behavior, neural proliferation and the essential influence of experience in the creation of neural networks, the maturational timing of different brain systems and their behavioral consequences, the myelination of neural pathways and its influence on children's action and thinking, and the capacity of the brain to create new neural connections throughout life, which contributes to its continuing adaptability to new experiences. The implications of this research for public health and for strengthening support for early brain development are considered throughout this discussion.
Introduction
The brain is the most complex biological system in the human body, by far. It consists of billions of nerve cells of different cell types that are interlinked by trillions of connections, organized into distinct neural circuits and located in regions that differ structurally and functionally. Consequently, brain development is a prolonged process, beginning in the first two to three weeks after conception and lasting through early adulthood. This extended maturation derives from the complexity of neurobiological growth, of course, but it is also due to the brain's continuous incorporation of experience. In other words, the brain's development is shaped throughout life both by genetic guidance and by experience -- the classic nature-nurture dynamic.
Understanding the early development of the brain is important for public health professionals. It is important not only to inform understanding of early childhood development, but also because the work of public health is crucial to supporting healthy brain development in the early years and preventing and remediating many of the threats that exist. In this article, my goal is to highlight the central processes of early brain development and their relevance to public health.
Brain Development Begins Early. Very Early.
Because its development is so foundational to the rest of human growth, the brain develops rapidly early in life. Whereas a newborn's brain is already 26% of its adult weight, by age five, the brain has reached 88% of its adult weight.1 The latter figure helps to account for the well-known claim that the brain is 90% developed by the age of five. That claim is mistaken because brain weight is not a very informative index of its maturation (after all, men's brains are 10% heavier than women's). More important than estimating how heavy the brain has become by various ages is understanding the foundations for life-long functioning that are established by its early growth.
Some of the most important foundations are established prenatally. The nine months of prenatal development witnesses the most rapid growth of the brain. Beginning early in prenatal growth, during the third gestational week, new neurons (nerve cells) and other supportive cells are generated at an astonishing rate -- by some estimates, several hundred thousand each minute -- and then migrate to their eventual destinations in the fetal brain.2 Throughout this process of neural proliferation and migration, neurons continue to develop through gene expression within the cell and by the influence of adjacent neurons. By the 23rd week of gestation, synapses (contacts between neurons permitting the transmission of activation, a.k.a. neural communication) begin to proliferate as the structural organization of the brain begins to form. Before birth, the vast majority of neurons that populate the brain have been created.
It is a truism that the more rapid pace of development, the greater the vulnerability of the organism. Consistent with this truism is the vulnerability of prenatal brain development to a variety of hazards, including environmental toxins (such as pesticide and lead exposure), drugs (e.g., alcohol, cannabis), and maternal viral infection (such as influenza and herpes simplex) that can potentially have long-lasting developmental consequences, sometimes because of the epigenetic processes (i.e., changes in gene expression) they trigger.3 Some prenatal influences are hazardous because they provoke developmental adaptations in the fetus that may have negative long-term consequences for health and morbidity. According to the developmental origins of health and disease (DOHaD) theory, certain early influences, such as prenatal undernutrition, can program changes in fetal development in anticipation of the requirements of the postnatal environment.4 In this view, "fetal programming" of physical and neurobiological systems derives from fetal sensitivity to aspects of the intrauterine environment that signal conditions of life outside the womb, and fetal development adapts to enhance the chance of survival after birth.
This was evocatively seen in the Dutch Hunger Winter of 1945, when official rations for citizens in the occupied Netherlands were dramatically cut by Nazi authorities in reprisal for a strike on the Dutch railways. The children born to women who were pregnant during this period have been studied into adulthood to determine the long-term effects of this time-limited period of profound nutritional insufficiency that was followed by the immediate restoration of an adequate diet (when the Allies liberated the Netherlands). Researchers discovered that the children born to mothers who were malnourished early in their pregnancy were at significantly greater risk as adults for a variety of chronic health and mental health problems compared with children of different gestational age or same-sex siblings.5 One explanation is that fetal metabolism, growth rate, and organ system development early and quickly adapted to conditions of limited food intake in preparation for encountering comparably deprived circumstances after birth. Children were biologically unprepared for the conditions of nutritional adequacy into which they were born, however, and this led to a range of physical and mental health problems that endured throughout life.6
Guided by this view, researchers have been concerned not only with nutritional adequacy but also with other prenatal influences, such as the effects of maternal stress on the developing fetus. This concern is warranted given that 15% to 20% of pregnant women experience depression or anxiety symptoms, and these rates increase in the context of other stresses of pregnancy, such as difficult employment conditions, strain in close relationships, and low income.7 Chronic maternal stress has direct effects on the fetal brain: it increases cortisol levels in the brain and contributes to the development of heightened stress reactivity in newborns.8 These consequences may derive from fetal programming, since prenatal exposure to elevated stress hormones is a powerful signal of environmental adversity for which developing biological systems adapt in preparation for birth into a challenging world. One study showed that by six months after birth, the infants of mothers who were prenatally depressed showed weaker connections between the amygdala and the prefrontal cortex, which suggests compromised emotion regulation beginning early in infancy and heightened sensitivity to cues of threat and danger.9 The behavioral effects of fetal exposure to maternal stress can be enduring, and include heightened emotional reactivity and enhanced risk for depression or anxiety as late as adolescence.10
Developing an Adaptive Brain
Fetal programming shows that from the beginning, the brain incorporates experience into its architecture. This is even more evident in another feature of early brain development: the overproduction and refinement of neural connections.
As earlier noted, synapses begin to form prenatally, connecting neurons in communicative networks. Because synapses are a basic means by which the brain develops, they are formed continuously as the brain matures (and, indeed, throughout life). But early in life, the production of synapses is especially rapid, and can be considered (in the technical language of developmental neuroscientists) "exuberant." By one estimate, more than one million synapses are formed every second in the early years.11 If this seems excessive, it is true: up to 40% more synapses are overproduced early in life than will be retained in the mature brain.12 Consequently, the young brain is a more active and densely packed organ than it will be at maturity.
Overproduction of synapses, which derives from the genetic guidance of brain development, is a good thing. It affords considerable capacity for early growth because synapses can become enlisted into developing and consolidating a potentially expansive range of skills and knowledge. But overproduction has liabilities, including poorer signal-to-noise in neural communication (i.e., lots of incoming transmissions reaching neurons when only a subset is important), and some forms of mental disability are associated with atypical rates of proliferation of synaptic connections.13 Therefore, an important subsequent step in brain development is the progressive reduction and elimination of synapses to increase the efficiency of neural processing.
Experience guides the process of synaptic pruning by strengthening neural connections when they are activated. When the brain responds to stimulation, it activates specific synaptic networks, and these networks become strengthened in the process. By contrast, synapses that are rarely activated eventually wither and disappear. The brain of a Parisian baby overhearing French spoken at home develops consolidated synaptic networks related to the perception of French speech sounds, for example, but synapses for Russian or Chinese speech perception, rarely activated, wither. In this manner, early experiences help to determine the retention of connections in the developing brain (this is the basis for the well-known maxim, "use it or lose it") and the result is a brain that consolidates skills that are relevant to the world in which the child is living.
In fact, we can observe this process both behaviorally and biologically. Studies have shown that during their first six months, infants can discriminate speech sounds found in languages all over the world, including sounds that their parents cannot differentiate (a young Japanese infant can discriminate, for example, between the sounds /l/ and /r/, even though her parents cannot readily do so). But by the end of the first year, the baby's speech perception has become more narrowly channeled to the sounds of the home language, and infants can no longer discriminate foreign speech sounds.14 In other words, infants become perceptually fine-tuned to the language system that they are overhearing and preparing to learn. (The same occurs with the child's own sounds: by 10 months, infants have incorporated the sounds of the home language into their babbling.15) This developmental change can also be observed by neuroimaging brain regions involved in speech perception. One research group found that at seven months, the brain became active in response to speech sounds from different languages. Between seven and 11 months, however, there was an increase in activation to speech in the home language, and by the end of the first year, infants showed neural activation only to sounds of the home language.16
This illustrates the profound adaptability, or plasticity, of the developing brain. Since the fetal brain cannot predict whether it will be born in Paris, Kiev, Tokyo, Los Angeles, or elsewhere, the brain at birth is prepared through the overproduction of synapses to discriminate the sounds of any human language. But months of experience overhearing the language -- or languages -- spoken at home guide the pruning of synapses and reorganization of brain areas governing speech perception to focus on the home language, and the capacity for universal speech discrimination is lost. The benefits of this can be observed a few months later, in the "vocabulary explosion" beginning at 18 months, when the rewired brain is now attuned to this language and new words are acquired at a very rapid rate.
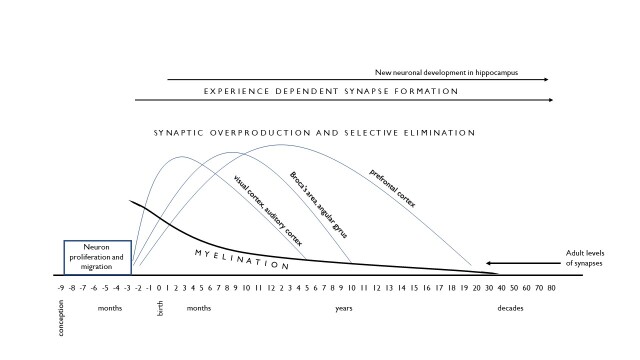
Retaining lots of unneeded synapses is unhelpful, therefore, since the desired developmental outcome is efficient learning. Consequently, the dual processes of exuberant overproduction followed by the experience-guided elimination of synapses occurs throughout the brain to increase the efficiency of neural processing and adapt the brain's capacities to the world in which the child lives. Importantly, the timing and pace of this process varies for different brain regions, as shown in Figure 1 using three brain regions for illustration. The figure shows that synaptic overproduction begins prenatally in areas relevant to basic sensory skills -- seeing and hearing -- with postnatal experience contributing to the refinement of these connections, completed by early childhood. Language areas of the brain (such as Broca's area and the angular gyrus) have a more extended period of synaptic pruning as language skills continue to be refined until age 11 or 12. Even longer is the developmental timetable for the prefrontal cortex, an area important to self-regulation, which does not reach maturity until early adulthood (despite the fervent hopes of parents).
Figure 1.
Development of the Human Brain
This figure illustrates, therefore, that the timing of these dual developmental processes varies for different brain regions and in a manner that accords with what we see behaviorally: young children achieve sensory acuity by kindergarten (a necessary skill for the acquisition of other abilities, such as reading), show a longer timetable for first (and second) language acquisition, and by adolescence are still mastering capacities for self-control in thinking and behavior. Many developmental changes in children's behavior -- growing motor coordination and fine-motor control, advances in thinking and reasoning, skills in social and emotional understanding -- likewise follow advances in synaptic pruning in relevant brain areas. Note, however, that the figure also illustrates how the first five years witnesses major advances in virtually all of these developmental domains. In this respect, the importance of early childhood is underscored by the neurobiological advances that are establishing a foundation for many developmental achievements that are occurring in childhood and at later ages.
The figure also illustrates another important aspect of early brain development: the myelination of neural connections. Myelin is a fatty protein sheath covering neurons that provides insulation and speeds the conduction of neural activation (in some areas of the brain, over 100-fold).17 Whereas synaptic overproduction and pruning refine the brain's neural networks, myelination contributes speed and efficiency to them. The timetable for myelination is similar to the one described for synapse development, with earlier advances in sensory and motor regions and much later development in brain regions governing complex information processing and self-regulation. One can observe the effects of myelination in children's behavior, from the growing speed of coordinated movement to the increased quickness and dexterity of thinking. Although neural transmission can occur in unmyelinated neurons, the efficiency of myelinated neural networks is significantly greater, and it is myelination that significantly advances the efficiency of neural transmission.
Neural Networks
Before proceeding further, a clarification. The discussion thus far has referred to neural "networks" because that is how neuroscientists think about brain functioning. Specifically, the brain processes governing most behavior involve connections between neurons that are often widely distributed throughout the brain. This is especially true of the complex behaviors that are most interesting, such as those associated with learning or emotion. The view that the brain functions in neural networks contrasts with the more common locationist view of the brain that attributes specific behaviors to particular brain structures (e.g., the amygdala is where fear responding occurs; the hippocampus is where memory occurs). By contrast with the locationist view, the brain processes involved with emotions like fear network systems associated with sensory evaluation, cognitive appraisal, short- and long-term memory, and other processes that are widely distributed throughout the brain.
This is important because a view of brain functioning in neural networks not only underscores the amazing complexity of how the brain functions, but also provides greater insight into the development and remediation of behavioral problems. The development of emotion regulation is not, for example, primarily due to maturation of the amygdala, but due to a range of other brain systems we don't usually see as relevant to emotion, such as areas of the prefrontal cortex that support self-regulation and which take much longer to mature. Understanding the network of brain systems involved in emotion regulation might give parents and practitioners greater patience for understanding the challenges of young children in emotion management, and also provide insights that can help in remedial and therapeutic interventions when problems of emotion management are more severe and enduring.
Two Kinds of Experience
At this point it is possible to answer a question that is often posed about brain development: why isn't it all in the genes? In other words, given its importance, why isn't the entire development of the brain encoded in the human genome?
One reason is that the entire genome is simply inadequate for mapping the trillions of connections in a typical adult brain. Another reason, however, is that a "genetic blueprint" would make brain development inflexible, and render children poorly suited to any of the incredibly diverse environments in which people live. As research has shown, by incorporating experience into the architecture of brain development, individual brains become tailored to the young child's lived experience in their individual environments.
There are, however, two kinds of "experience" relevant to early brain development.
The first kind of experience contributes to experience-expectant development. These are experiences that are pervasive in life and, as a consequence, the developing brain "expects" to encounter these experiences as part of its development. Examples include exposure to patterned light, gravity, the sound of human voices and language, touch on skin surfaces, social contact, and others. Although these are common, universal experiences, brain development is built on these exposures occurring when relevant neurobiological systems are maturing. Their failure to occur, or to occur at the right time, can result in the loss of relevant brain capacities. Consequently, experience-expectant development is often viewed in terms of sensitive periods during which certain environmental exposures must occur if brain development is to proceed normally.
Ordinarily there is no reason for concern about this in normal circumstances. But when young children live in profound deprivation early in life, essential experience-expectant exposures may be lacking. When a 13-year-old girl with the pseudonym Genie was rescued by Los Angeles County child welfare authorities in 1970, the authorities were astonished at the grossly neglectful conditions in which she had lived her entire life, lacking normal human contact, language exposure, and even opportunities for normal motor development. Many of her developmental deficits proved to be remediable with therapeutic assistance, but the psychologists assisting in her care concluded differently with respect to language. Although Genie's vocabulary development surpassed researchers' expectations, she never acquired mastery of basic grammar and language pragmatics. Since her rescue occurred when she was at the close of the period of synaptic pruning in regions of the brain relevant to language development (see Figure 1), her irreversible deficits in certain domains of language use were not unexpected.18
The second kind of experience contributes to experience-dependent development. These are experiences affecting the brain that can vary across individuals. Their influence can occur at any age (not just during an early sensitive period) and they can be virtually any kind of experience that leads to the formation of new synapses or modification of existing synaptic connections in the brain. Needless to say, the range of potential experiences contributing to this kind of development is extremely broad: learning how to play the piano, indulging an interest in poetry, joining a support group, or developing deeper understanding of American history are examples. Experience-expectant development constitutes life-long brain plasticity, and helps to account for the increasingly individualized character of brain growth as the years proceed. Although the brain completes its maturation by early adulthood (see Figure 1), evidence that the brain continues to be capable of changing throughout life attests to the life-long importance of experience-dependent development. Neurobiological studies with adults suggest, for example, that new neurons and synaptic connections develop in the hippocampus, a brain structure important for learning and memory, in response to experiences promoting new learning.19
The profound developmental potential afforded by experience-expectant development is one reason the human brain is such a powerful learning organ, and it helps to account for the astonishingly early and rapid mental advances of early childhood. This developmental potential is one reason that parents who can afford them enlist enrichment experiences for their young children, such as art, music, or other kinds of classes or tutoring, even though there is little or no evidence that such experiences can accelerate early brain development beyond normal limits.20 There is, however, abundant evidence that deprivation and adversity can undermine healthy brain development. Unfortunately, experience-dependent development is undiscriminating with respect to the kinds of experiences affecting the brain's growth: it incorporates harm as well as enrichment into its developing architecture.21 Deprivation of important supports (such as nurturant care) can also significantly undermine the developing brain through the loss of opportunities that would otherwise strengthen important neural networks.
Implications for Public Health Practitioners
A quarter-century ago, the American public was rocked by a revolution in their understanding of early childhood development that focused on early brain development and its implications.22 In the years since, developmental science has modified but not fundamentally changed the account that parents and others learned then. Early brain development is foundational to the growth of life-long capacities, and many of these foundations are established prenatally. Early experiences are important to the development and refinement of neural connections in the early years, although the hazards of adverse experiences are much clearer than are the benefits of enrichment. The brain's adaptability, or plasticity, is one of its superpowers for tailoring its development to the child's experience in a specific environment, and this continues to be a strength beyond early childhood as the brain continues to develop in later life. The brain's adaptability can be a liability, however, when it incorporates into its architecture the effects of abuse, deprivation, and trauma. In light of the brain's rapid growth, identifying and remediating developmental problems early in life is important before these problems become consolidated, and doing so can be far more cost-effective than trying to remediate them later.
Understanding the implications of developmental neuroscience for parents, practitioners, and policymakers has been as important as understanding the science, and the past 25 years have witnessed slow progress on important science-based initiatives for children, as well as the excesses of overpromising and the liabilities of ignoring important needs.22 For public health practitioners, however, the implications of the science may be self-evident. Improving the prenatal care of expectant mothers, with particular attention to the hazards to fetal brain development of nutritional inadequacy, maternal stress, and toxic exposures. Strengthening the supports for young children within their families, especially in the availability of nurturant relationships, adequate nutrition, safe environments, and learning opportunities. Early intervention when young children's environments are unsafe or neglectful. Expanding programs to address the multiple threats deriving from childhood poverty, a multisystemic stressor with documented effects on developing children's brains and behavior.23 Early detection of developmental problems, coupled with state-of-the-science interventions to remediate them. Enlarging the availability of early learning opportunities, but with an important proviso. Early childhood programs to support young children's learning need to be buttressed by strong primary education opportunities to build on that foundation. The research is clear that early education cannot alone lead to high school graduation and other later achievements.
There are other implications of the research on early brain development. The insights on the developing brain that have been emerging during the past quarter century provide continuing catalysts for the responsibilities of those of us committed to young children's healthy development.
Acknowledgement
This article is an adapted version of chapter 3, "Dispatches from the Laboratory," from The Brain Development Revolution: Science, the Media, and Public Policy. Figure 1 is also taken from the chapter.
References
- 1.Dekaban, A. S., & Sadowsky, D. (1978, October). Changes in brain weights during the span of human life: Relation of brain weights to body heights and body weights. Annals of Neurology, 4(4), 345–356. 10.1002/ana.410040410 [DOI] [PubMed] [Google Scholar]
- 2.Cowan, W. M. (1979, September). The development of the brain. Scientific American, 241(3), 112–133. 10.1038/scientificamerican0979-112 [DOI] [PubMed] [Google Scholar]
- 3.Kundakovic, M., & Jaric, I. (2017, March 18). The epigenetic link between prenatal adverse environments and neurodevelopmental disorders. Genes, 8(3), 104. 10.3390/genes8030104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doi, M., Usui, N., & Shimada, S. (2022, March 15). Prenatal environment and neurodevelopmental disorders. Frontiers in Endocrinology (Lausanne), 13, 860110. 10.3389/fendo.2022.860110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lumey, L. H., Stein, A. D., Kahn, H. S., van der Pal-de Bruin, K. M., Blauw, G. J., Zybert, P. A., & Susser, E. S. (2007, December). Cohort profile: The Dutch Hunger Winter families study. International Journal of Epidemiology, 36(6), 1196–1204. 10.1093/ije/dym126 [DOI] [PubMed] [Google Scholar]
- 6.Schulz, L. C. (2010, September 28). The Dutch Hunger Winter and the developmental origins of health and disease. Proceedings of the National Academy of Sciences of the United States of America, 107(39), 16757–16758. 10.1073/pnas.1012911107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunkel Schetter, C., & Tanner, L. (2012, March). Anxiety, depression and stress in pregnancy: Implications for mothers, children, research, and practice. Current Opinion in Psychiatry, 25(2), 141–148. 10.1097/YCO.0b013e3283503680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monk, C., Lugo-Candelas, C., & Trumpff, C. (2019). Prenatal developmental origins of future psychopathology: Mechanisms and pathways. Annual Review of Clinical Psychology, 15, 16.1-16.28. [DOI] [PMC free article] [PubMed]
- 9.Posner, J., Cha, J., Roy, A. K., Peterson, B. S., Bansal, R., Gustafsson, H. C., et al. Monk, C. (2016, November 1). Alterations in amygdala-prefrontal circuits in infants exposed to prenatal maternal depression. Translational Psychiatry, 6(11), e935. 10.1038/tp.2016.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis, E. P., & Thompson, R. A. (2014). Prenatal foundations: Fetal programming of health and development. Zero to Three, 34, 6–11. [Google Scholar]
- 11.Center for the Developing Child. (2024). Brain architecture. Retrieved July 8, 2024 from https://developingchild.harvard.edu/science/key-concepts/brain-architecture/
- 12.Huttenlocher, P. R., & Dabholkar, A. S. (1997, October 20). Regional differences in synaptogenesis in human cerebral cortex. The Journal of Comparative Neurology, 387(2), 167–178. [DOI] [PubMed] [Google Scholar]
- 13.Lepeta, K., Lourenco, M. V., Schweitzer, B. C., Martino Adami, P. V., Banerjee, P., Catuara-Solarz, S., et al. Seidenbecher, C. (2016, September). Synaptopathies: Synaptic dysfunction in neurological disorders - A review from students to students. Journal of Neurochemistry, 138(6), 785–805. 10.1111/jnc.13713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuhl, P. K. (2004, November). Early language acquisition: Cracking the speech code. Nature Reviews. Neuroscience, 5(11), 831–843. 10.1038/nrn1533 [DOI] [PubMed] [Google Scholar]
- 15.de Boysson-Bardies, B., Halle, P., Sagart, L., & Durand, C. (1989, February). A crosslinguistic investigation of vowel formants in babbling. Journal of Child Language, 16(1), 1–17. 10.1017/S0305000900013404 [DOI] [PubMed] [Google Scholar]
- 16.Rivera-Gaxiola, M., Silva-Pereyra, J., & Kuhl, P. K. (2005, March). Brain potentials to native and non-native speech contrasts in 7- and 11-month-old American infants. Developmental Science, 8(2), 162–172. 10.1111/j.1467-7687.2005.00403.x [DOI] [PubMed] [Google Scholar]
- 17.Johnson, M. H., & de Haan, M. (2015). Developmental cognitive neuroscience: An introduction (4th Ed.). New York: Wiley-Blackwell. [Google Scholar]
- 18.Rymer, R. (1994). Genie: A scientific tragedy (2nd Ed.). New York: Harper Perennial. [Google Scholar]
- 19.Erickson, K. I., Voss, M. W., Prakash, R. S., Basak, C., Szabo, A., Chaddock, L., . . . Kramer, A. F. (2011). Exercise training increases size of hippocampus and improves memory. PNAS Proceedings of the National Academy of Sciences, 108, 3017-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tierney, A. L., & Nelson, C. A., III. (2009, November 1). Brain development and the role of experience in the early years. Zero to Three, 30(2), 9–13. [PMC free article] [PubMed] [Google Scholar]
- 21.Kim-Spoon, J., Herd, T., Brieant, A., Peviani, K., Deater-Deckard, K., Lauharatanahirun, N., et al. King-Casas, B. (2021, April). Maltreatment and brain development: The effects of abuse and neglect on longitudinal trajectories of neural activation during risk processing and cognitive control. Developmental Cognitive Neuroscience, 48, 100939. 10.1016/j.dcn.2021.100939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson, R. A. (2023). The brain development revolution: Science, the media, and public policy. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 23.Evans, G. W., Chen, E., Miller, G. E., & Seeman, T. (2012). How poverty gets under the skin: A life course perspective. In R. King & V. Mahalmes (Eds.), The Oxford Handbook of Poverty and Child Development (pp. 13-36). New York: Oxford University Press. [Google Scholar]