Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a prevalent chronic disorder in China, impacting a significant proportion of individuals aged > 40 years. In China, the prevalence of and risk factors for COPD among non-smokers remain largely unexplored. In this study, we aimed to determine the prevalence of COPD in non-smokers within the Chinese population and identify potential risk factors associated with COPD in non-smokers.
Methods
Web of Science, PubMed, Embase, Chinese WanFang, Chinese China National Knowledge Infrastructure, and Weipu databases from inception to August 5, 2024, were searched. Studies reporting the percentage of never-smokers among those diagnosed with COPD and investigations exploring the risk factors associated with COPD in never-smokers in China were examined. Summary proportions and odds ratios (OR), along with their corresponding 95% confidence intervals (95% CI), were measured.
Results
In total, 112 investigations with 491,812 participants were included. The percentage of never-smokers in people with COPD was 41.1% (95% CI: 37.5–44.6%). The prevalence of never-smokers among males diagnosed with COPD was 22.3% (95% CI: 18.8–25.7%), which differed from that among women (81.3%, 95% CI: 75.3–87.2%). The results showed an association between the utilization of biomass fuel and the occurrence of COPD in never-smokers (OR: 1.25, 95% CI: 1.06–1.44). Among never-smokers, the data showed a close association between being underweight (OR: 1.89, 95% CI: 1.78–2.00), tuberculosis history (OR: 1.71, 95% CI: 1.53–1.88) and COPD. Never-smokers living in rural areas or those with low educational status were more susceptible to COPD.
Conclusion
This review confirmed the highly different proportions of never-smokers among male and female patients with COPD.
Trial registration
PROSPERO: CRD42023420786.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-20170-z.
Keywords: Chronic obstructive pulmonary disease, Non-smoker, Proportion, Risk factor
Introduction
In recent decades, there has been a worldwide increase in the morbidity and mortality rates associated with chronic obstructive pulmonary disease (COPD). According to the findings of the China Pulmonary Health (CPH) investigation, COPD is a highly prevalent chronic ailment, impacting an estimated 13.7% of people aged > 40 years within the population [1]. Patients with COPD often complain of dyspnea, cough and sputum production, which could be exacerbated in the chronic course of the disease, leading to lung function decline and even death [1]. Notably, COPD presents a significant economic and social challenge in China.
COPD is distinguished by the presence of persistent airflow obstruction, which arises from a multitude of contributing factors, among which cigarette smoking is the most significant [2]. However, COPD can occur in a considerable number of non-smokers, who account for 15–50% of COPD patients globally [3]. Besides the well-documented causative factors (such as cigarette smoking), a variety of other factors are also involved in the susceptibility to or the pathogenesis of COPD [3]. Based on the findings of the international, population-based Burden of Obstructive Lung Disease investigation, 28% of individuals diagnosed with COPD was never-smokers, across 14 countries included in the analysis [4]. However, this proportion rose to 51% in China, as reported in the CPH study [1]. The notably higher prevalence in China, in comparison to that of other countries, warrants further investigation.
To date, the risk factors associated with COPD in non-smokers include genetic susceptibility, exposure to biomass fuel, a history of recurrent lower respiratory tract infections during childhood, intrauterine growth retardation, a history of pulmonary tuberculosis, inadequate nutrition, and low socioeconomic status [3], and these life-course exposures may impact lung function trajectory and COPD development in later life [5]. Recent studies have significantly advanced our understanding of the etiology of COPD and have been instrumental in guiding the screening of high-risk populations. Considering the increasing research in this area, it is imperative to conduct a systematic review and meta-analysis to comprehensively synthesize the existing evidence and delineate the incidence and risk factors of COPD in China.
In this meta-analysis, we systematically reviewed all relevant publications, estimated the overall and sex-specific proportions of non-smokers among patients with COPD, and conducted an assessment of the risk factors associated with COPD within the non-smoking population of China.
Methods
Search strategy
The present investigation employed a systematic review and meta-analysis methodology in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [6]. We searched electronic databases encompassing PubMed, Embase, Chinese China National Knowledge Infrastructure, Web of Science, Chinese WanFang, and Chinese Weipu databases from inception to August 5, 2024, with the following keywords: “Chronic Obstructive Pulmonary Diseases”/“COPD,” “China,” and “never-smokers”/“non-smoking”/“risk factors”/“risk factor.” The selected terms were integrated employing Boolean logical operators (OR, AND, and NOT). Details of the search terms are provided in Additional file 1. In addition, we evaluated review articles and references of the selected articles for additional eligible studies. Neither geographic nor language restrictions were applied in our search. The PROSPERO study registration number is CRD42023420786.
Study selection
The two reviewers, YZ and HLC, identified the studies meeting the specified eligibility criteria: (1) original articles encompassing study designs with no limitations, (2) reporting the prevalence of never-smokers diagnosed with COPD in China, (3) detailing COPD risk factors in never-smokers in China, and (4) unrestricted by language or geographical location. The exclusion criteria for the studies were as follows: (1) investigations with irrelevant topics; (2) studies focusing solely on findings from animal models; (3) conference abstracts, study protocols, or review articles; (4) lack of data on smoking status in COPD patients, or unutilizable data for evaluation; and (5) ecological studies. An review of the title, abstract, and the full text of the study was conducted by two reviewers independently. Any disagreements regarding study selection were resolved through consensus.
Data extraction and quality evaluation
Two authors, YZ and HLC, conducted separate reviews of full-text articles that were potentially relevant. There were no attempts to contact the study authors in case of missing data, and the aforementioned articles were later omitted. The extraction of data was conducted using standardized data collection forms. Discrepancies were resolved through the process of consulting with the corresponding author, YCS. Following a comprehensive and thorough deliberation on divergent viewpoints, the authors reached a unanimous consensus regarding the ultimate categorization and incorporation of all research studies. The data were extracted as follows:
(1) Study information: first author, publication year, study design, province or city of China, and patient age.
(2) Participants: Definition of COPD, number of COPD patients, number of never-smokers with COPD, sex, and overall and sex-specific percentage of never-smokers with COPD.
(3) Risk factors: biomass use, education level, history of tuberculosis, body mass index (BMI), sex, residence, and passive smoking.
(4) Effect sizes: summary proportion, odds ratio (OR), and relative risk (RR), each accompanied by their respective 95% confidence intervals (95%CI).
Employing the Newcastle-Ottawa Scale [7] and the cross-sectional investigation quality methodology checklist [8], two researchers evaluated the quality of the case-control, cohort, and cross-sectional investigations. The framework comprises three distinct domains, namely population selection, comparability, and exposure or result evaluation, encompassing a cumulative sum of nine points distributed across eight items. The scoring system classified scores from 7 to 9 as “high,” those from 4 to 6 as “medium,” and those from 1 to 3 as “poor.” Inconsistencies in quality assessments were resolved through communication [9].
Statistical analysis
To ascertain the proportion of never-smokers within the COPD population, we conducted a single-proportion meta-analysis using the metan command in STATA 14.0 (StataCorp, College Station, TX, USA) as outlined by DerSimonian and Laird [10]. A forest plot was used to estimate the overall incidence and effect of each study with their respective 95% CIs using a random-effects model. The same statistical methods were used to evaluate the sex-specific percentage of never-smokers with COPD. We employed the inverse variance index (I2) as a means of evaluating the level of heterogeneity present within the articles that were included (low, < 25%; moderate, 25–50%; and high, > 50%) [11]. Sensitivity analyses were conducted by separately eliminating one investigation at a time from each analysis to evaluate the effect on the overall percentage and robustness of the results. To identify any potential publication biases in the combined findings, we conducted funnel plots and Egger’s tests, employing a significance threshold of P < 0.05 [12].
Given the inconsistent findings regarding the COPD risk factors in never-smokers, as reported by various studies, and the presence of overlapping risk factors across multiple studies, we carried out a meta-analysis to systematically measure the risk factors associated with COPD in never-smokers. Risk factors were estimated using ORs with 95% CIs, and I2 > 50% was defined as high heterogeneity [12]. Considering the variability in clinical settings across the studies, it was assumed that there would be heterogeneity. Therefore, a random-effects model was used for all subsequent analyses. The utilization of this methodology resulted in more cautious conclusions, as it considered variations both within and between studies when determining the error terms employed in the analysis. The combined OR was considered statistically significant if the 95% CI did not encompass 1.00. We did not assess sensitivity analyses and publication bias for articles that evaluated the likelihood factors for COPD in never-smokers, as there were fewer than ten studies in this section.
Results
Search results and study characteristics
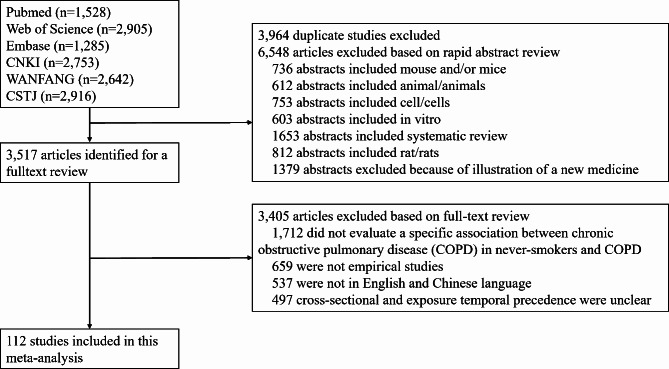
The systematic search and selection process is illustrated in Fig. 1, showcasing the various steps involved. In total, 14,029 articles were analyzed. After removing duplicate entries and screening titles and abstracts, 3,517 articles were retained. Following a comprehensive review of the available literature, 112 investigations satisfied the predetermined criteria for inclusion and were subsequently incorporated into the present study [1, 13–123].
Fig. 1.
Flow diagram of study selection
Table 1 displays a comprehensive overview of the key attributes of the investigations that have been incorporated into this analysis. The published studies encompassed a time frame spanning from 2003 to 2024, comprising a total of 3 retrospective investigations, 14 cohort investigations, 17 case-control investigations, and 78 cross-sectional investigations. All the studies (n = 112) were conducted in China. Regarding study quality, 75 were categorized as high quality, and the remaining were categorized as medium quality (Additional file 2). The current systematic review included a sample size ranging from 75 to 317,000, with a total of 491,812 participants. All studies examined the associations between non-smoking status and COPD or COPD risk factors in never-smokers.
Table 1.
Characteristics of the included studies
| Study | Study design | Age | Area | Definition of COPD | The number of COPD patients (n) | The number of never-smokers with COPD (n) | Prevalence of never-smokers among people with COPD | Quality | |
|---|---|---|---|---|---|---|---|---|---|
| Liu et al. (2024) [13] | Cross-Sectional | Aged 65 years or older | A university hospital in southwestern China | Post-bronchodilator FEV1/FVC < 0.7 | 319 | 130 | 40.8% | High | |
| Tan et al. (2024) [14] | Cross-Sectional | Aged 20 years or older | First Affiliated Hospital of Guangzhou Medical University | Post-bronchodilator FEV1/FVC < 0.7 | 1,282 | 411 | 32.1% | High | |
| Fan et al. (2024) [15] | Cohort | Aged 40 years or older | 10 medical centers in China | Post-bronchodilator FEV1/FVC < 0.7 | 235 | 115 | 48.9% | High | |
| Zhang et al. (2024) [16] | Cohort | Aged 35–70 years | 12 communities in different districts in Eastern China | Post-bronchodilator FEV1/FVC < 0.7 | 1,102 | 832 | 75.5% | High | |
| Zhao et al. (2024) [17] | Cross-Sectional | Aged 60 years or older | 5 public hospitals in Ningxia | Post-bronchodilator FEV1/FVC < 0.7 | 627 | 366 | 58.3% | Medium | |
| Zhao et al. (2024) [18] | Cohort | Aged 60 years or older | Affiliated Hospital of Guangdong Medical University | Post-bronchodilator FEV1/FVC < 0.7 | 579 | 124 | 21.4% | High | |
| Cui et al. (2023) [19] | Cohort | Aged 40 years or older | 12 tertiary hospitals in Hunan and Guangxi provinces | Post-bronchodilator FEV1/FVC < 0.7 | 845 | 120 | 14.2% | High | |
| Liu et al. (2023) [20] | Cross-Sectional | Not defined | Three tertiary hospitals from Anhui Province | Post-bronchodilator FEV1/FVC < 0.7 | 761 | 334 | 43.9% | Medium | |
| Liu et al. (2023) [21] | Cross-Sectional | Aged 40 years or older | Beijing Chao-Yang Hospital | Post-bronchodilator FEV1/FVC < 0.7 | 226 | 32 | 14.2% | High | |
| Zhu et al. (2023) [22] | Retrospective study | Aged 40–90 years | Southern and central districts of Beijing | Post-bronchodilator FEV1/FVC < 0.7 | 1,348 | 552 | 41.0% | High | |
| Song et al. (2023) [23] | Cross-Sectional | Not defined | 6 hospitals in Hunan province | Post-bronchodilator FEV1/FVC < 0.7 | 910 | 233 | 25.6% | High | |
| Lin et al. (2023) [24] | Cohort | Aged 18 years or older | The Second Xiangya Hospital of Central South University | Post-bronchodilator FEV1/FVC < 0.7 | 461 | 72 | 15.6% | Medium | |
| Lin et al. (2023) [25] | Cross-Sectional | Aged 40 years or older | Longnan City, Jiuquan City, Qingyang City, and Gannan City in Gansu | Post-bronchodilator FEV1/FVC < 0.7 | 508 | 266 | 52.4% | Medium | |
| Zou et al. (2023) [26] | Retrospective study | Not defined | The First Affiliated Hospital of Hebei North University | Post-bronchodilator FEV1/FVC < 0.7 | 174 | 44 | 25.3% | Medium | |
| Lv et al. (2023) [27] | Cohort | Not defined | Hefei, Bozhou, and Fuyang City | Post-bronchodilator FEV1/FVC < 0.7 | 789 | 352 | 44.6% | High | |
| Zhan et al. (2023) [28] | Cohort | Aged 35–80 years | 12 hospitals nationwide | Post-bronchodilator FEV1/FVC < 0.7 | 404 | 109 | 27.0% | High | |
| Tang et al. (2022) [29] | Retrospective study | Aged 40–80 years | Zhejiang hospital in Zhejiang Province | Post-bronchodilator FEV1/FVC < 0.7 | 375 | 202 | 53.9% | High | |
| Wu et al. (2022) [30] | Case-Control | Not defined | Hainan Provincial People's Hospital | Post-bronchodilator FEV1/FVC < 0.7 | 498 | 280 | 56.2% | Medium | |
| Li et al. (2022) [31] | Cohort | Not defined | Tangshan City of Hebei Province | Post-bronchodilator FEV1/FVC < 0.7 | 474 | 295 | 62.2% | High | |
| Wen et al. (2022) [32] | Cross-Sectional | Aged 40–80 years | Guangzhou, Shao Guan, and He Yuan communities of Guangdong Province | Post-bronchodilator FEV1/FVC < 0.7 | 895 | 102 | 11.4% | High | |
| Zhang et al. (2022) [33] | Cohort | Not defined | People’s Hospital of Xinjiang Uygur Autonomous Region | Post-bronchodilator FEV1/FVC < 0.7 | 174 | 149 | 85.6% | Medium | |
| Tang et al. (2022) [34] | Cross-Sectional | Aged 50–92 years | Nanjing City in Jiangsu Provence | Post-bronchodilator FEV1/FVC < 0.7 | 170 | 35 | 20.6% | High | |
| Yang et al. (2022) [35] | Cohort | Aged 40 years or older | 50 hospitals across six geographical regions | Post-bronchodilator FEV1/FVC < 0.7 | 4,978 | 1,280 | 25.7% | High | |
| Pan et al. (2022) [36] | Case-Control | Aged 18 years or older | Xiangya Second Hospital of Central South University and the 1st People′s Hospital of Huaihua | Post-bronchodilator FEV1/FVC < 0.7 | 959 | 112 | 11.7% | High | |
| Dai et al. (2022) [37] | Cross-Sectional | Aged 60 years or older | Several hospitals in Yunnan Province | Post-bronchodilator FEV1/FVC < 0.7 | 624 | 478 | 76.7% | Medium | |
| Wu et al. (2022) [38] | Cross-Sectional | Aged 60 years or older | Dancheng Branch of Xiangshan First People’s Hospital Medical and Health Group | Post-bronchodilator FEV1/FVC < 0.7 | 219 | 53 | 24.2% | Medium | |
| Chen et al. (2022) [39] | Cross-Sectional | Not defined | Three tertiary hospitals in Chongqing city | Post-bronchodilator FEV1/FVC < 0.7 | 335 | 100 | 29.9% | Medium | |
| Wang et al. (2021) [40] | Cross-Sectional | Aged 35 years or older | Northwest China | Post-bronchodilator FEV1/FVC < 0.7 | 394 | 320 | 81.2% | High | |
| Wang et al. (2021) [41] | Cross-Sectional | Aged 20 years or older | Shanxi Province | Post-bronchodilator FEV1/FVC < 0.7 | 363 | 160 | 44.0% | Medium | |
| Liu et al. (2021) [42] | Cross-Sectional | Aged 40 to 80 years | Chinese Epidemiological Survey of COPD (CESCOPD) study | Post-bronchodilator FEV1/FVC < 0.7 | 1,582 | 608 | 38.4% | High | |
| Li et al. (2021) [43] | Cross-Sectional | Aged 40 years or older | The Uyghur population in the Kashi region | Post-bronchodilator FEV1/FVC < 0.7 | 504 | 388 | 77.0% | Medium | |
| Li et al. (2021) [44] | Case-Control | Not defined | Hainan General Hospital | Post-bronchodilator FEV1/FVC < 0.7 and FEV1 < 80% predicted | 315 | 166 | 52.7% | Medium | |
| Abudureheman et al. (2021) [45] | Case-Control | Not defined | Kashi in XinJiang | Post-bronchodilator FEV1/FVC < 0.7 and FEV1 < 80% predicted | 541 | 417 | 77.1% | High | |
| Zhao et al. (2021) [46] | Cross-Sectional | Aged 40 years or older | 10 communities of Shijingshan District in Beijing | Post-bronchodilator FEV1/FVC < 0.7 | 416 | 192 | 46.2% | High | |
| Dong et al. (2021) [47] | Cross-Sectional | Not defined | Dali Region in Yunnan Provence | Post-bronchodilator FEV1/FVC < 0.7 | 113 | 39 | 34.5% | Medium | |
| Chen et al. (2021) [48] | Cross-Sectional | Not defined | Guiyang public health treatment center | Post-bronchodilator FEV1/FVC < 0.7 | 315 | 184 | 58.4% | Medium | |
| Li et al. (2021) [49] | Cross-Sectional | Aged 18 years or older | Outpatient or inpatient department of 3 hospitals in Fujian province | Post-bronchodilator FEV1/FVC < 0.7 | 248 | 51 | 20.6% | Medium | |
| Zhou et al. (2020) [50] | Cross-Sectional | Aged 40 years or older | Outpatient department of the Second Xiangya Hospital | Post-bronchodilator FEV1/FVC < 0.7 | 1,241 | 263 | 21.2% | High | |
| Shi et al. (2020) [51] | Cross-Sectional | Not defined | Hainan Affiliated Hospital of Hainan Medical University | Post-bronchodilator FEV1/FVC < 0.7 | 313 | 164 | 52.4% | High | |
| Dong et al. (2020) [52] | Cross-Sectional | Not defined | Eight hospitals in Jilin Province | Post-bronchodilator FEV1/FVC < 0.7 | 306 | 87 | 28.4% | High | |
| Xiong et al. (2020) [53] | Case-Control | Aged 40 years or older | Southern part of China | Post-bronchodilator FEV1/FVC < 0.7 | 513 | 126 | 24.6% | High | |
| Ding et al. (2020) [54] | Case-Control | Not defined | Hainan Han population | Post-bronchodilator FEV1/FVC < 0.7 | 313 | 164 | 52.4% | High | |
| Zhang et al. (2020) [55] | Case-Control | Not defined | Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital | Post-bronchodilator FEV1/FVC < 0.7 | 480 | 197 | 41.0% | Medium | |
|
Zhu et al. (2020) (part of a national key research and development program about COPD) [56] |
Cohort | Aged 40 years or older | Four Chinese tertiary hospitals | Post-bronchodilator FEV1/FVC < 0.7 | 1,644 | 325 | 19.8% | High | |
| Yan et al. (2020) [57] | Cross-Sectional | Aged 40 years or older | Suzhou City in Jiangsu Province | Post-bronchodilator FEV1/FVC < 0.7 | 586 | 397 | 67.8% | High | |
| Jia et al. (2020) [58] | Cohort | Aged 18 years or older | Outpatient department of West China Hospital | Post-bronchodilator FEV1/FVC < 0.7 | 75 | 15 | 20.0% | High | |
| Cheng et al. (2020) [59] | Cross-Sectional | Aged 20 years or older | 4 districts in Shanghai | Post-bronchodilator FEV1/FVC < 0.7 | 591 | 339 | 57.4% | High | |
| Duan et al. (2020) [60] | Cross-Sectional | Aged 40 years or older | 12 Grade-A hospitals in Hunan Province and Guangxi Zhuang Autonomous Prefecture | Post-bronchodilator FEV1/FVC < 0.7 | 5,183 | 1,495 | 28.8% | High | |
| Wang et al. (2020) [61] | Case-Control | Aged 35 years or older | Permanent residents in Hulunbuir city | Post-bronchodilator FEV1/FVC < 0.7 | 2,320 | 184 | 7.9% | Medium | |
| Ma et al. (2020) [62] | Cross-Sectional | Not defined | A tertiary general hospital in Qinghai | Post-bronchodilator FEV1/FVC < 0.7 | 3,264 | 2,085 | 63.9% | Medium | |
| Sheng et al. (2019) [63] | Cross-Sectional | Aged 60 years or older | Island area of Ningbo | Post-bronchodilator FEV1/FVC < 0.7 | 254 | 66 | 26.0% | High | |
| Zha et al. (2019) [64] | Cross-Sectional | Aged 40 years or older | Anhui Province | Post-bronchodilator FEV1/FVC < 0.7 | 272 | 92 | 33.8% | High | |
| Jia et al. (2018) [65] | Cross-Sectional | Not defined | Eleven tertiary hospitals | Post-bronchodilator FEV1/FVC < 0.7 | 1,698 | 497 | 29.3% | High | |
| Zhang et al. (2018) [66] | Case-Control | Aged 40 years or older | Ningxia Hui Autonomous Region | Post-bronchodilator FEV1/FVC < 0.7 | 491 | 219 | 44.6% | High | |
| Wang et al. (2018) [1] | Cross-Sectional | Aged 20 years or older | Ten provinces, autonomous regions, and municipalities | Post-bronchodilator FEV1/FVC < 0.7 | 4,908 | 2,479 | 50.5% | High | |
| Wu et al. (2018) [67] | Cross-Sectional | Aged 40 years or older |
Shandong Province and Shanghai City |
Post-bronchodilator FEV1/FVC < 0.7 | 744 | 316 | 42.5% | High | |
| Fang et al. (2018) [68] | Cross-Sectional | Aged 40 years or older | Seven major regions of China | Post-bronchodilator FEV1/FVC < 0.7 | 9,134 | 3,326 | 36.4% | High | |
| Hu et al. (2018) [69] | Cross-Sectional | Aged 40 years or older | Respiratory Medicine Clinic of Zhujiang Hospital | Post-bronchodilator FEV1/FVC < 0.7 | 631 | 197 | 31.2% | High | |
| Ma et al. (2018) [70] | Cross-Sectional | Aged 40 years or older | Linxia prefecture | Post-bronchodilator FEV1/FVC < 0.7 | 403 | 203 | 50.4% | Medium | |
| Shi et al. (2018) [71] | Cross-Sectional | Not defined | Hebei Province | Post-bronchodilator FEV1/FVC < 0.7 | 116 | 45 | 38.8% | Medium | |
| Zhang et al. (2017) [72] | Case-Control | Aged 40–80 years | Guangdong and Hubei Province | Post-bronchodilator FEV1/FVC < 0.7 | 989 | 167 | 16.9% | High | |
| Deng et al. (2017) [73] | Case-Control | Not defined | The Armed Police Corps Hospital | Post-bronchodilator FEV1/FVC < 0.7 | 120 | 55 | 45.8% | High | |
| Xiao et al. (2017) [74] | Cross-Sectional | Not defined | Beijing, Shanghai, Chengdu and Guangzhou City | Post-bronchodilator FEV1/FVC < 0.7 | 678 | 218 | 32.2% | High | |
| Yan et al. (2017) [75] | Cross-Sectional | Aged 35–70 years | 12 provinces of China | Post-bronchodilator FEV1/FVC < 0.7 | 3,690 | 2,614 | 70.8% | High | |
| Li et al. (2017) [76] | Cross-Sectional | Aged 43–94 years | 10 Beijing suburb hospitals | Post-bronchodilator FEV1/FVC < 0.7 | 447 | 141 | 31.5% | Medium | |
| Duan et al. (2017) [77] | Cross-Sectional | Aged 40 years or older | Wuwei City in Gansu Province | Post-bronchodilator FEV1/FVC < 0.7 | 190 | 76 | 40.0% | Medium | |
| Gong et al. (2017) [78] | Cross-Sectional | Aged 30–94 years | 3 third-grade class-A hospitals in Shenyang City | Post-bronchodilator FEV1/FVC < 0.7 | 2,400 | 858 | 35.8% | High | |
| Lv et al. (2017) [79] | Cross-Sectional | Aged 40 years or older | Maoming City in Gangdong Province | Post-bronchodilator FEV1/FVC < 0.7 | 203 | 61 | 30.0% | High | |
| Zhang et al. (2017) [80] | Cross-Sectional | Aged 40 years or older | Changchun urban area in Jilin Province | Post-bronchodilator FEV1/FVC < 0.7 | 176 | 83 | 47.2% | High | |
| Zhu et al. (2017) [81] | Cross-Sectional | Aged 60–89 years | Zhejiang Province | Post-bronchodilator FEV1/FVC < 0.7 | 248 | 70 | 28.2% | High | |
| Qi et al. (2017) [82] | Cross-Sectional | Aged 40 years or older | Xinjiang Province | Post-bronchodilator FEV1/FVC < 0.7 | 500 | 110 | 22.0% | Medium | |
| Xiao et al. (2017) [83] | Cross-Sectional | Not defined | Beijing, Shanghai, Chengdu and Guangzhou city | Post-bronchodilator FEV1/FVC < 0.7 | 678 | 218 | 32.2% | High | |
| Cai et al. (2017) [84] | Cross-Sectional | Not defined | Chaozhou-Shantou Region of Guangdong Province | Post-bronchodilator FEV1/FVC < 0.7 | 876 | 260 | 29.7% | Medium | |
| Cui et al. (2017) [85] | Cross-Sectional | Not defined | Luoyang City in Henan Provence | Lung function test record | 1,356 | 436 | 32.2% | Medium | |
| Ding et al. (2016) [86] | Case-Control | Aged 40 years or older | Hlai (the Li) ethnicity, Hainan Province | Post-bronchodilator FEV1/FVC < 0.7 | 212 | 154 | 72.6% | Medium | |
| Lu et al. (2016) [87] | Case-Control | Aged 40–80 years | Guangzhou City | FEV1/FVC < the lower limit of normal (LLN) | 855 | 213 | 24.9% | High | |
| Liao et al. (2015) [88] | Cross-Sectional | Aged 40–70 years | Chengdu City in Sichuan Provence | Post-bronchodilator FEV1/FVC < 0.7 | 151 | 100 | 66.2% | High | |
| Han et al. (2015) [89] | Cross-Sectional | Aged 40 years or older | Heilongjiang Provence | Post-bronchodilator FEV1/FVC < 0.7 | 297 | 157 | 52.9% | High | |
| Yang et al. (2015) [90] | Case-Control | Not defined | Guangzhou City in Guangdong Provence and Suzhou city in Jiangsu Provence | Post-bronchodilator FEV1/FVC < 0.7 | 1,791 | 888 | 49.6% | Medium | |
| Feng et al. (2015) [91] | Cross-Sectional | Not defined | Wuhan cohort study | Post-bronchodilator FEV1/FVC < 0.7 | 228 | 169 | 74.1% | High | |
| Li et al. (2015) [92] | Cross-Sectional | Aged 40 years or older | Ningbo City in Zhejiang Provence | Post-bronchodilator FEV1/FVC < 0.7 | 396 | 172 | 43.4% | Medium | |
| Liu et al. (2015) [93] | Cross-Sectional | Aged 40–75 years | Jilin Provence | Post-bronchodilator FEV1/FVC < 0.7 | 77 | 41 | 53.2% | Medium | |
| Xiao et al. (2015) [94] | Cross-Sectional | Not defined | Linxia Hui Autonomous Prefecture | Post-bronchodilator FEV1/FVC < 0.7 | 269 | 60 | 22.3% | Medium | |
| Yu et al. (2014) [95] | Cross-Sectional | Aged 40 years or older | Jiading Districts of Shanghai | Post-bronchodilator FEV1/FVC < 0.7 | 165 | 63 | 38.2% | High | |
| Qiu et al. (2013) [96] | Cross-Sectional | Aged 40 years or older | Ningxia Hui Autonomous Region | Post-bronchodilator FEV1/FVC < 0.7 | 360 | 180 | 50.0% | High | |
| Jiang et al. (2013) [97] | Cross-Sectional | Not defined | Guangzhou City in Guangdong Provence | Post-bronchodilator FEV1/FVC < 0.7 | 911 | 230 | 25.2% | High | |
| Miao et al. (2013) [98] | Prospective cohort | Not defined | Zhengzhou City in Henan Provence | Post-bronchodilator FEV1/FVC < 0.7 | 1,166 | 507 | 43.5% | High | |
| Zou et al. (2013) [99] | Cross-Sectional | Aged 40 years or older | Haiyang City in Shandong Province | Post-bronchodilator FEV1/FVC < 0.7 | 159 | 67 | 42.1% | High | |
| Zhang et al. (2013) [100] | Cross-Sectional | Aged 40 years or older | Kunming City in Yunnan Province | Post-bronchodilator FEV1/FVC < 0.7 | 92 | 26 | 28.3% | Medium | |
| Lou et al. (2012) [101] | Cross-Sectional | Aged 40–75 years | Rural area of Xuzhou City | Post-bronchodilator FEV1/FVC < 0.7 | 5,650 | 3,136 | 55.5% | High | |
| Zhou et al. (2012) [102] | Case-Control | Aged 40 years or older |
Eight hospitals in Beijing City |
Post-bronchodilator FEV1/FVC < 0.7 | 193 | 81 | 42.0% | High | |
| Wang et al. (2012) [103] | Cross-Sectional | Aged 40–91 years | Chengdu City in Sichuan Province | Post-bronchodilator FEV1/FVC < 0.7 | 1,335 | 307 | 23.0% | Medium | |
| Hu et al. (2012) [104] | Cross-Sectional | Aged 15 years or older | Cixi City in Zhejiang Province | Post-bronchodilator FEV1/FVC < 0.7 | 1,650 | 472 | 28.6% | Medium | |
| Wang et al. (2011) [105] | Case-Control | Not defined | Qilu Hospital and the Second Affiliated Hospital of Shandong University of Traditional Chinese Medicine | Post-bronchodilator FEV1/FVC < 0.7 | 409 | 190 | 46.5% | High | |
| Ling et al. (2011) [106] | Cross-Sectional | Aged 15–92 years | Xinjiang rural areas | Post-bronchodilator FEV1/FVC < 0.7 | 138 | 101 | 73.2% | High | |
| Du et al. (2011) [107] | Cross-Sectional | Aged 40 years or older | Jilin City in Jilin Province | Post-bronchodilator FEV1/FVC < 0.7 | 403 | 80 | 19.9% | Medium | |
| Luo et al. (2011) [108] | Cross-Sectional | Not defined | Beijing City | Post-bronchodilator FEV1/FVC < 0.7 | 134 | 38 | 28.4% | Medium | |
| Weng et al. (2011) [109] | Cross-Sectional | Aged 40 years or older | Chongqing City | Post-bronchodilator FEV1/FVC < 0.7 | 160 | 42 | 26.3% | High | |
| Lam et al. (2010) [110] | Cross-Sectional | Aged 50 years or older | The Guangzhou Biobank Cohort Study | Post-bronchodilator FEV1/FVC < 0.7 | 522 | 364 | 69.7% | High | |
| Zhang et al. (2009) [111] | Cross-Sectional | Aged 40 years or older | Rural areas of Liaoning province | Post-bronchodilator FEV1/FVC < 0.7 | 172 | 61 | 35.5% | High | |
| Zhou et al. (2009) [112] | Cross-Sectional | Aged 40 years or older | Rural area in 7 provinces or cities of China | Post-bronchodilator FEV1/FVC < 0.7 | 830 | 321 | 38.7% | High | |
| Wang et al. (2009) [113] | Case-Control | Aged 30 years or older | Eight hospitals in Beijing City | Post-bronchodilator FEV1/FVC < 0.7 | 306 | 105 | 34.3% | High | |
| Ko et al. (2008) [114] | Cohort | Aged 60 years or older | Hong Kong City | Post-bronchodilator FEV1/FVC < 0.7 | 261 | 172 | 65.9% | High | |
| Zhong et al. (2007) [115] | Cross-Sectional | Aged 40 years or older | Seven provinces/cities | Post-bronchodilator FEV1/FVC < 0.7 | 1,668 | 644 | 38.6% | High | |
| Liu et al. (2007) [116] | Cross-Sectional | Aged 40 years or older |
Guangdong Province |
Post-bronchodilator FEV1/FVC < 0.7 | 310 | 114 | 36.8% | High | |
| Xu et al. (2007) [117] | Cross-Sectional | Aged 35 years or older | Nanjing City in Jiangsu Provence | Post-bronchodilator FEV1/FVC < 0.7 | 1,743 | 1,097 | 62.9% | High | |
| Wang et al. (2005) [118] | Cross-Sectional | Aged 40 years or older |
Northern part of Guangdong province |
Post-bronchodilator FEV1/FVC < 0.7 | 176 | 70 | 39.8% | High | |
| Liu et al. (2005) [119] | Cross-Sectional | Aged 40 years or older |
Guangdong Province |
Post-bronchodilator FEV1/FVC < 0.7 | 310 | 128 | 41.3% | High | |
| Li et al. (2003) [120] | Cross-Sectional | Not defined | First Affiliated Hospital of Zhongshan University | Post-bronchodilator FEV1/FVC < 0.7 | 713 | 378 | 53.0% | Medium | |
Abbreviations: COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; FVC, forced vital capacity
Percentage of never-smokers in the COPD population
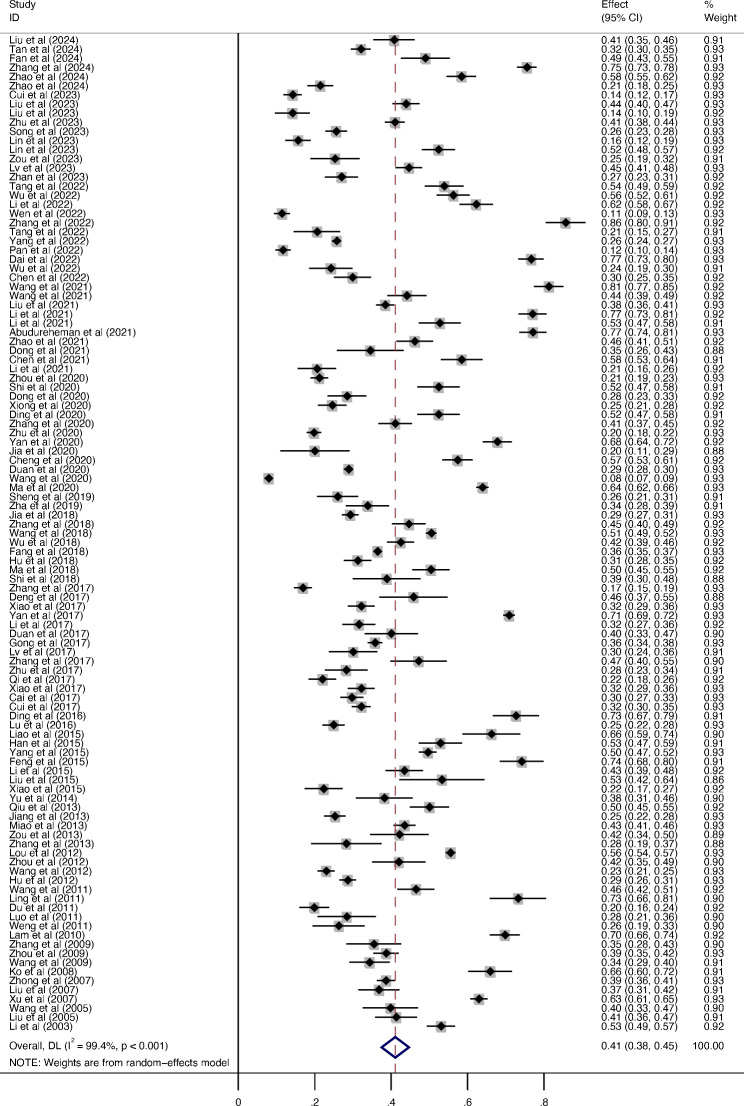
One hundred and nine studies (72 high-quality studies) reported the percentage of never-smokers among individuals with COPD. The outcomes showed that the percentage of never-smokers in the COPD population was 41.1% (95%CI: 37.5–44.6%, I2 = 99.4%) (Fig. 2).
Fig. 2.
The percentage of never-smokers in COPD patients
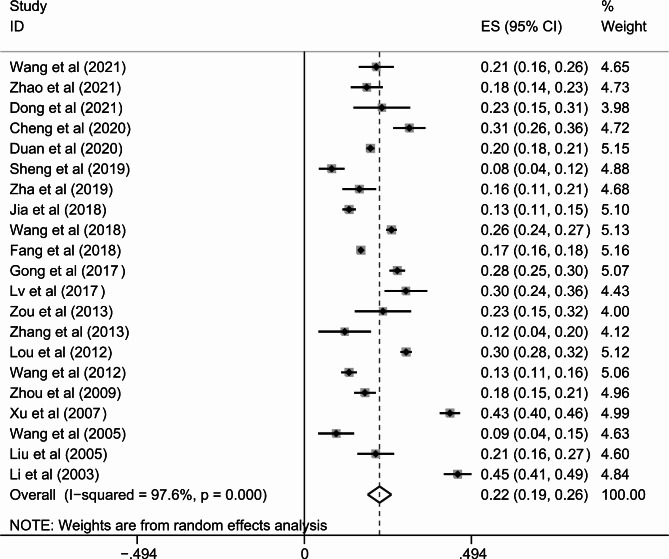
Meta-analysis of the 21 studies [1, 41, 46, 47, 59, 60, 63–65, 68, 78, 79, 99–101, 103, 112, 117–120] reported the prevalence of never-smokers among COPD men. Our study indicated that the percentage of male never-smokers with COPD was 22.3% (95%CI: 18.8–25.7%, I2 = 97.6%) (Fig. 3 and Additional file 3).
Fig. 3.
The percentage of never-smokers in male COPD patients
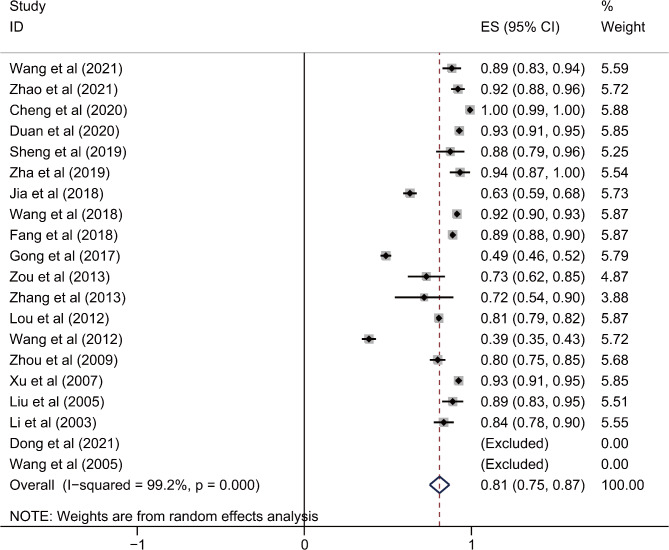
Twenty studies [1, 41, 46, 47, 59, 60, 63–65, 68, 78, 99–101, 103, 112, 117–120] reported the percentage of female non-smokers with COPD. The investigation revealed that the proportion of COPD female never-smokers was 81.3% (95%CI: 75.3–87.2%, I2 = 99.2%) (Fig. 4 and Additional file 3).
Fig. 4.
The percentage of never-smokers in female COPD patients
COPD risk factors in never-smokers
Five studies have reported the risk factors of COPD in never-smokers (Additional file 4). High-quality evidence based on the results from two studies of biomass exposure [1, 121] showed an association between biomass fuel and COPD in never-smokers (OR: 1.25, 95% CI: 1.06–1.44) (Additional file 5). Correspondingly, evidence from four investigations [1, 121–123] showed a positive association between being underweight (BMI < 18.5 kg/m2) and COPD in never-smokers (OR: 1.89, 95% CI 1.78–2.00) (Additional file 6). A significant association between the history of tuberculosis [1, 110, 122, 123] and COPD was observed in never-smokers (OR: 1.71, 95% CI 1.53–1.88) (Additional file 7). No significant associations were identified between passive smoking [1, 121–123] and COPD in never-smokers (OR: 0.90, 95% CI 0.87–0.94) (Additional file 8). Moreover, never-smokers who resided in rural regions [1, 122, 123] were more susceptible to COPD (OR: 1.20; 95% CI 1.16–1.25) than those who did not (Additional file 9). Four additional investigations [1, 121–123] showed that never-smokers with low educational status (primary school and lower) were more prone to developing COPD (OR: 1.17, 95% CI 1.12–1.22) than those with a high educational status (Additional file 10).
Sensitivity analysis and publication bias
Sensitivity analysis suggested that there was no substantial alteration in the percentage of never-smokers among patients with COPD after systematically eliminating one study at a time; we have verified the robustness and consistency of our findings (Additional file 11). As for the prevalence of never-smokers among male or female COPD patients, the results of sensitivity analysis remain stable after leave-one-out analysis (Additional file 12 and Additional file 13).
As most of the included studies were small-scale, the asymmetrical funnel plot indicated a potential publication bias. However, after performing Egger’s test, no significant publication bias was observed in relation to the percentage of never-smokers among COPD patients (P = 0.069) (Additional file 14).
Discussion
The high incidence, associated disability, and mortality rates of COPD in China present a significant public health concern [1]. Cigarette smoking is identified as the primary risk factor associated with COPD [124]. Nevertheless, other environmental factors and host susceptibility are also involved. This systematic review and meta-analysis encompassed a total of 112 investigations involving a substantial participant pool of 491,812 individuals. The investigation outcomes reveal that the incidence of non-smokers among COPD patients in China was as high as 41.1% (95%CI: 37.5–44.6%). In the investigations encompassed in the meta-analysis, the sex ratio of COPD patients was not completely consistent, which could influence the prevalence of non-smokers within the population of individuals with COPD. To further explore the differences in sex-specific proportions, we summarized 21 articles that contained relevant sex-based data separately. We found that the number of non-smokers among COPD patients greatly varied between men and women. Specifically, the proportion of non-smokers among male patients was 22.3% (95%CI: 18.8–25.7%), while that in female patients was as high as 81.3% (95%CI: 75.3–87.2%).
The implications of the study’s findings are significant in terms of comprehending the risk factors linked to COPD among non-smokers. The outcomes of this meta-analysis indicate that the utilization of biomass fuel is associated with the onset of COPD among non-smokers. In contrast to developed nations, indoor air pollution, encompassing coal and biomass combustion, has been considered as a significant factor in COPD development in China and other developing countries [116, 125, 126], particularly among non-smokers. Biomass encompasses wood, grass, charcoal, crop stems, and animal dung, which are utilized as fuel sources for cooking and heating [127]. The primary constituents of biomass smoke that pose a significant risk to human health consist of nitrogen oxides, oxysulfides, carbonic oxides, polycyclic organic compounds, and hydrocarbon compounds. These substances are generated as a result of the incomplete combustion of biomass [3]. According to data derived from China, in rural areas, it was found that approximately 60% of households utilized biomass, while approximately 31% of households relied on coal for cooking or heating [128]. Hence, the combustion of biomass fuel emits smoke that constitutes a significant risk factor contributing to COPD development within the Chinese population. The significance of these findings lies in the fact that a considerable proportion of non-smokers in China, particularly those residing in rural areas, are exposed to biomass smoke on a regular basis through cooking or heating activities. Hence, it is imperative to consider the contribution of biomass smoke in the progression of COPD.
China is confronted with a significant burden of pulmonary tuberculosis. The findings indicated that the occurrence of COPD in individuals without a smoking history who have tuberculosis was greater than that in the overall population, supporting the notion that pulmonary tuberculosis is an independent COPD risk factor in non-smokers. However, the mechanisms underlying airflow obstruction after tuberculosis remain largely unknown. The presence of bronchostenosis, characterized by inflammation and lesions, has been demonstrated to be a consequence of tuberculosis affecting the endobronchial region, in addition to contributing to the development of secondary tuberculous lymphadenopathy [129]. Moreover, tuberculosis can cause the dysregulation of matrix metalloproteinases and widespread parenchymal lung destruction [130]. Thus, it is possible that a combination of airway and lung parenchyma impairment leads to the development of airflow obstruction and abnormalities in ventilation, resulting in reduced lung function. In addition, cumulative effects of life-course exposure may also impact on the association [5].
We found that COPD risk increased in non-smoking patients with lower educational attainment and in those residing in rural regions, which may be attributable to occupational exposure to dust, smoke, biomass smoke, unfavorable living conditions, malnutrition, and further risk factors associated with the lifestyle of these patients. The meta-analysis revealed a correlation between low socioeconomic status, as indicated by educational attainment, and the occurrence of COPD in non-smokers. However, this association could potentially be influenced by various established risk factors, including occupational exposure, respiratory disease throughout childhood, malnutrition, and limited access to healthcare [121].
Tobacco smoke exposure is common in China. Moreover, secondhand smoke has been linked to an increased level of bronchial responsiveness and a progressive decrease in pulmonary function [125, 131]. However, our analysis concluded that passive smoking was not a significant COPD risk factor in non-smokers in China. Wang et al. [1] assessed the prevalence of cohabitating smokers among individuals diagnosed with COPD. Furthermore, passive smoking, as defined by Long et al. [122], refers to the act of inhaling smoke by never-smokers who may or may not reside with smokers. Conversely, passive smoking, as defined by Smith et al. [123] is characterized by the quantification of exposure through the assessment of frequency and duration of hours per week. The Guangzhou Biobank Cohort investigation [132] revealed that the duration of passive smoking experienced at home or in the workplace was significantly associated with the development of COPD. This association was found to be more influential than the one involving the mere presence of smokers within the same household or indoor setting. Hence, whether passive smoking is a risk factor for non-smoker COPD, the duration of exposure to passive smoking, and the prevalence of smokers in the surrounding environment are crucial factors to consider.
Moreover, we identified a strong association between low BMI (BMI < 18.5 kg/m2) and COPD risk. A diet with a greater consumption of vegetables, fruits, and fish could decrease COPD risk [133, 134]. Previous research has revealed that a low BMI is a significant and independent indicator of the risk of mortality [135]. In addition, it has been observed that COPD is associated with a gradual decline in skeletal muscle mass [4]. The outcomes of this investigation indicate that COPD is related to dietary patterns and weight.
In this meta-analysis, the proportion of non-smokers was found to be greater among female COPD patients than among their male counterparts. This may be explained by the fact that females are more likely to be exposed to biomass fuel for cooking in China [121]. Current evidence suggests a plausible sex-specific effect on COPD development related to exposure to different risk factors [136, 137].
In this meta-analysis, we summarized 112 articles on the smoking status of COPD patients. In total, 78 study designs were cross-sectional, with 491,812 participants. To the best of our knowledge, this is the first report investigating the disparities between sexes regarding the prevalence of non-smokers diagnosed with COPD within the Chinese population. Furthermore, this meta-analysis is the first to evaluate the COPD risk factors in non-smokers in China.
Limitations
Our investigation has several limitations. First, the records of smoking history and risk factor exposures relied solely on questionnaires; therefore, recall and responder biases may be unavoidable. Second, heterogeneity in the combined proportions was primarily caused by different study designs, procedures, and measurements. The heterogeneity presented in the summary estimates was primarily owing to potential confounders; hence, it is advisable to exercise caution when interpreting the outcomes of the analysis.
Conclusion
In conclusion, this meta-analysis elucidated the prevalence of and potential risk factors for COPD in non-smokers. The results suggested that lower BMI, less education, living in a rural residence, biomass use, and history of pulmonary tuberculosis were associated with a greater COPD risk among never-smokers. Compared with that in smokers, COPD in non-smokers may be caused by different mechanisms, and alternative preventive or therapeutic modalities are needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- COPD
Chronic obstructive pulmonary disease
- CPH
China pulmonary health
- I2
Inverse variance index
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RR
Relative risk
Author contributions
YCS designed the study. YZ, XYG, HLC, JGQ, LL and YCS analyzed and interpreted the data. YZ, XYG and YCS conducted the literature screening. YZ and YCS participated in the statistical analysis. YCS and YZ wrote the manuscript, and YCS revised the manuscript. All the authors read and accepted this paper.
Funding
The research described in this investigation received financial support from the National Natural Science Foundation of China (grant number 81970041), the Capital’s Funds for Health Improvement and Research (grant number 2022-2G-40910), and the Key Clinical Projects of Peking University Third Hospital (grant number BYSYZD2022014).
Data availability
The entirety of the data utilized or investigated in the course of this investigation has been incorporated within this published article, along with its accompanying Supplementary Material.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang C, Xu J, Yang L, Xu Y, Zhang X, Bai C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–17. [DOI] [PubMed] [Google Scholar]
- 2.Burney P, Jithoo A, Kato B, Janson C, Mannino D, Nizankowska-Mogilnicka E, et al. Chronic obstructive pulmonary disease mortality and prevalence: the associations with smoking and poverty–a BOLD analysis. Thorax. 2014;69(5):465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet. 2009;374(9691):733–43. [DOI] [PubMed] [Google Scholar]
- 4.Lamprecht B, McBurnie MA, Vollmer WM, Gudmundsson G, Welte T, Nizankowska-Mogilnicka E, et al. COPD in never smokers: results from the population-based burden of obstructive lung disease study. Chest. 2011;139(4):752–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melén E, Faner R, Allinson JP, Bui D, Bush A, Custovic A, et al. Lung-function trajectories: relevance and implementation in clinical practice. Lancet. 2024;403(10435):1494–503. [DOI] [PubMed] [Google Scholar]
- 6.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. w264. [DOI] [PubMed] [Google Scholar]
- 7.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. [DOI] [PubMed] [Google Scholar]
- 8.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brozek JL, Akl EA, Alonso-Coello P, Lang D, Jaeschke R, Williams JW, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy. 2009;64(5):669–77. [DOI] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 12.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ (Clinical Res ed). 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Song J, Wang Z, Wu S, Qiu S, Chen B, et al. Investigation of nutrition status and analysis of 180-day readmission factors in elderly hospitalized patients with COPD. Aging Clin Exp Res. 2024;36(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan L, Li Y, Wang Z, Wang Z, Liu S, Lin J, et al. Comprehensive appraisal of lung function in young COPD patients: a single center observational study. BMC Pulm Med. 2024;24(1):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fan J, Fang L, Cong S, Zhang Y, Jiang X, Wang N, et al. Potential pre-COPD indicators in association with COPD development and COPD prediction models in Chinese: a prospective cohort study. Lancet Reg Health West Pac. 2024;44:100984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Peng J, Liu L, Cui H, Zang D, Wu Z, et al. Prevalence, characteristics and significant predictors for cardiovascular disease of patients with preserved ratio impaired spirometry: a 10-year prospective cohort study in China. Respir Med. 2024;222:107523. [DOI] [PubMed] [Google Scholar]
- 17.Zhao J, Zhang X, Li X, Zhang R, Chang Y, Li Y, et al. Unraveling the mediation role of frailty and depression in the relationship between social support and self-management among Chinese elderly COPD patients: a cross-sectional study. BMC Pulm Med. 2024;24(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Liu G, Liu D, Zou L, Huang Q, Chen M, et al. Clinical and economic burden of anxiety/depression among older adult COPD patients: evidence from the COPD-AD China Registry study. Front Psychiatry. 2023;14:1221767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui Y, Ma Y, Dai Z, Long Y, Chen Y. Does the 2017 global initiative for chronic obstructive lung disease revision really improve the assessment of Chinese chronic obstructive pulmonary disease patients? A multicenter prospective study for more than 5 years. Chin Med J (Engl). 2023;136(21):2587–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu YF, Tang MM, Sun J, Li JF, Jiang YL, Zhao H, et al. Arsenic exposure and lung function decline in chronic obstructive pulmonary disease patients: the mediating influence of systematic inflammation and oxidative stress. Food Chem Toxicol. 2023;181:114044. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, Wang D, Fang J, Chang Y, Hu Y, Huang K. Validation of the generalized anxiety Disorder-7 in patients with COPD: a cross-sectional study. BMC Psychiatry. 2023;23(1):593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu RX, Nie XH, Liu XF, Zhang YX, Chen J, Liu XJ, et al. Short-term effect of particulate matter on lung function and impulse oscillometry system (IOS) parameters of chronic obstructive pulmonary disease (COPD) in Beijing, China. BMC Public Health. 2023;23(1):1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Q, Zhou A, Lin L, Li X, Cheng W, Liu C, et al. The clinical characteristics and treatment response of patients with chronic obstructive pulmonary disease with low body mass index. Front Pharmacol. 2023;14:1131614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin L, Song Q, Duan J, Liu C, Cheng W, Zhou A, et al. The impact of impaired sleep quality on symptom change and future exacerbation of chronic obstructive pulmonary disease. Respir Res. 2023;24(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin A, Mao C, Rao B, Zhao H, Wang Y, Yang G, et al. Development and validation of nomogram including high altitude as a risk factor for COPD: a cross-sectional study based on Gansu population. Front Public Health. 2023;11:1127566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zou D, Zhu X. Association of CT phenotype with pulmonary function in patients with chronic obstructive pulmonary disease and influencing factors of prognosis. Am J Transl Res. 2023;15(3):2164–74. [PMC free article] [PubMed] [Google Scholar]
- 27.Lv BB, Yang CL, Tan ZX, Zheng L, Li MD, Jiang YL, et al. Association between cadmium exposure and pulmonary function reduction: potential mediating role of telomere attrition in chronic obstructive pulmonary disease patients. Ecotoxicol Environ Saf. 2023;251:114548. [DOI] [PubMed] [Google Scholar]
- 28.Zhan Z, Ma Y, Chen Y, Zhang J, Li W, He Z, et al. Validation of COPD Population Screener Questionnaire in Chinese Population: A National Multicenter Study. Respiration. 2023;102(12):995–1002. [DOI] [PubMed] [Google Scholar]
- 29.Tang T, Li Z, Lu X, Du J. Development and validation of a risk prediction model for anxiety or depression among patients with chronic obstructive pulmonary disease between 2018 and 2020. Ann Med. 2022;54(1):2181–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu H, He C, Fu Y, Li X, Zheng Y, Mo R, et al. IL6R gene polymorphisms and their relation to chronic obstructive pulmonary disease susceptibility in the Chinese population. Biomark Med. 2022;16(17):1229–37. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Zhang P, An Z, Yue C, Wang Y, Liu Y, et al. Effectiveness of influenza and pneumococcal vaccines on chronic obstructive pulmonary disease exacerbations. Respirology. 2022;27(10):844–53. [DOI] [PubMed] [Google Scholar]
- 32.Wen X, Peng J, Zheng Y, Liu J, Tian H, Wu F, et al. Predictors of high Sputum eosinophils in Chronic Obstructive Pulmonary Disease. Chronic Obstr Pulm Dis. 2022;9(3):413–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Maitinuer A, Lian Z, Li Y, Ding W, Wang W, et al. Home based pulmonary tele-rehabilitation under telemedicine system for COPD: a cohort study. BMC Pulm Med. 2022;2(1):284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang T, Dou B, Zha HX, Tao LS, Gu ZJ, Liu KY, et al. Factors related to activation in Chinese patients with Chronic Obstructive Pulmonary Disease: a cross-sectional survey study. J Nurs Research: JNR. 2022;30(3):e209. [DOI] [PubMed] [Google Scholar]
- 35.Yang T, Cai B, Cao B, Kang J, Wen F, Chen Y, et al. Severity distribution and treatment of chronic obstructive pulmonary disease in China: baseline results of an observational study. Respir Res. 2022;23(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan J, Zeng Y, Xiang M, Mi X, Chen P, Xiang Z. The level of fractional exhaled nitric oxide and its influencing factors in chronic obstructive pulmonary disease. J Chin Physician. 2022;24(7):965–9. [Google Scholar]
- 37.Dai J, Li J, He X, Li Y, Li Y. Analysis of the prevalence and influencing factors of Chronic Obstructive Pulmonary Disease in Elderly hospitalized patients: a study based on a Comprehensive Geriatric Assessment System in Yunnan Province. Chin Gen Pract. 2022;25(11):1320–6. [Google Scholar]
- 38.Wu H, Ye M, Lou W, Xu C, Dong X, Ma H, et al. Screening of COPD and analysis of its risk factors in a community-based elderly population. China Med Pharm. 2022;12(13):196–200. [Google Scholar]
- 39.Chen B, Chen G, Li Q, Li Q. Model of factors influencing cognitive dysfunction in patients with COPD. J Mod Med Health. 2022;38(10):1639–44. [Google Scholar]
- 40.Wang Y, Li Z, Li FS. Development and Assessment of Prediction models for the development of COPD in a typical rural area in Northwest China. Int J Chron Obstruct Pulmon Dis. 2021;16:477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang R, Xu J, Wang Y. A population-based survey of the prevalence and risk factors of chronic obstructive pulmonary disease in Shanxi Province, China. Rev Clin Esp. 2022;222(4):218–28. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Zhou Y, Zou W, Tan X, Ran P. Prevalence and characteristics of chronic obstructive pulmonary disease in China with a diagnostic criterion of FEV(1)/FVC less than the lower limit of normal-a reanalysis of Chinese epidemiological survey of COPD (CESCOPD) study. J Thorac Disease. 2021;13(7):4043–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Zhong X, Zheng A, JianKun C, Budukadeer AA, Aini P, et al. Prevalence and risk factors of Chronic Obstructive Pulmonary Disease in Kashi Region, Northwestern China. Int J Chron Obstruct Pulmon Dis. 2021;16:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X, Zhou G, Tian X, Chen F, Li G, Ding Y. The polymorphisms of FGFR2 and MGAT5 affect the susceptibility to COPD in the Chinese people. BMC Pulm Med. 2021;21(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abudureheman Z, Li L, Zhong X, Xu J, Gong H, Yilamujiang S, et al. The rs74794265 SNP of the SREK1 gene is Associated with COPD in Kashi, China. Int J Chron Obstruct Pulmon Dis. 2021;16:2631–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao C, Sun P, Zhang E, An X, Ma S, Liu L, et al. Association of smoking and second-hand smoking with chronic obstructive pulmonary disease among residents aged 40 years or above in 10 communities of Beijing City. Chin J Clin (Electronic Edition). 2021;15(06):450–8. (in Chinese). [Google Scholar]
- 47.Dong J, Yang S, Lu H, Zhao Y, Duan C. Analysis of demography characteristics and risk factors of COPD patients in a hospital in Dali, Yunnan. J Mod Med Health. 2021;37(09):1537–41. (in Chinese). [Google Scholar]
- 48.Chen J, Bai J. Relationship between physical activity and complications in patients with chronic obstructive pulmonary disease: a hospital-based survey. Chin J Public Health. 2021;37(11):1687–90. (in Chinese). [Google Scholar]
- 49.Li M, Li H, Liu M, Lin Q, Sun H, Liu S. The current situation and influential factor of frailty on patients with chronic obstructive pulmonary disease. J Nurses Train. 2021;36(14):1249–54. (in Chinese). [Google Scholar]
- 50.Zhou Z, Zhou A, Peng Y, Duan J, Zeng Y, Zhao Y, et al. Determinants of clinical COPD questionnaire in patients with COPD: a cross-sectional observational study. Respir Int Rev Thorac Dis. 2020;99(7):606–16. [DOI] [PubMed] [Google Scholar]
- 51.Shi H, Xu J, Feng Q, Sun J, Yang Y, Zhao J, et al. The effect of CYP3A4 genetic variants on the susceptibility to chronic obstructive pulmonary disease in the Hainan Han population. Genomics. 2020;112(6):4399–405. [DOI] [PubMed] [Google Scholar]
- 52.Dong H, Hao Y, Li D, Su Z, Li W, Shi B, et al. Risk factors for Acute Exacerbation of Chronic Obstructive Pulmonary Disease in Industrial regions of China: a Multicenter cross-sectional study. Int J Chron Obstruct Pulmon Dis. 2020;15:2249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiong M, Guo M, Huang D, Li J, Zhou Y. TRPV1 genetic polymorphisms and risk of COPD or COPD combined with PH in the Han Chinese population. Cell Cycle (Georgetown Tex). 2020;19(22):3066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ding Y, Yang Y, Li Q, Feng Q, Xu D, Wu C, et al. The correlation between CYP4F2 variants and chronic obstructive pulmonary disease risk in Hainan Han population. Respir Res. 2020;21(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Y, Li XH, Zhou YT, Xiang L, Xiao M, Guo JS, et al. The association study of apolipoprotein E polymorphisms and chronic obstructive pulmonary disease in the Chinese population: a case-control study. Medicine. 2020;99(49):e23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu J, Zhao Z, Wu B, Shi Z, Nie Q, Fu Z, et al. Effect of body Mass Index on lung function in Chinese patients with chronic obstructive Pulmonary Disease: a Multicenter cross-sectional study. Int J Chron Obstruct Pulmon Dis. 2020;15:2477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan X, Xu L, Shi B, Wang H, Xu X, Xu G. Epidemiology and risk factors of chronic obstructive pulmonary disease in Suzhou: a population-based cross-sectional study. J Thorac Disease. 2020;12(10):5347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia HZ, Wu YQ, Qin JY, Shen YC, Wen FQ. [Association of plasma roundabout 4 concentration with pulmonary ventilation function decline in COPD patients]. Zhonghua Yi Xue Za Zhi. 2020;100(2):116–20. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 59.Cheng M, Li L, Hou D, Yang Y, Chen S, Wang G, et al. Prevalence and risk factors of adult chronic obstructive pulmonary disease in Shanghai. Shanghai Med J. 2020;43(11):651–8. (in Chinese). [Google Scholar]
- 60.Duan J, Xiang Z, Li X, Cheng W, Zeng Y, Chen Y, et al. Differences of clinical features between smokers and non-smokers with chronic obstructive pulmonary disease. J Chin Physician. 2020;22(10):1452–6. (in Chinese). [Google Scholar]
- 61.Wang X, Shi R, Siqintana, Wang Y. Investigation and analysis of the causes of Chronic Obstructive Pulmonary Disease in A City. 2020;18(13):149–50. (in Chinese).
- 62.Ma X, Li Z, Xie Q, Yang D, Zhang H, Chan L, et al. Clinical analysis of complications of different stages of COPD in high altitude areas. Gansu Med J. 2020;39(05):385–8. (in Chinese). [Google Scholar]
- 63.Sheng W, Huang Y, Deng Z, Ma H. Investigation of the prevalence and diagnosis of Chronic Obstructive Pulmonary Disease in a Group of Elderly individuals residing in an Island Area of Ningbo. Can Respir J. 2019;2019:6918340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zha Z, Leng R, Xu W, Bao H, Chen Y, Fang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in Anhui Province, China: a population-based survey. BMC Pulm Med. 2019;19(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jia G, Lu M, Wu R, Chen Y, Yao W. Gender difference on the knowledge, attitude, and practice of COPD diagnosis and treatment: a national, multicenter, cross-sectional survey in China. Int J Chron Obstruct Pulmon Dis. 2018;13:3269–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Qiu J, Zhang P, Zhang J, Jiang M, Ma Z. Genetic variants in FAM13A and IREB2 are associated with the susceptibility to COPD in a Chinese rural population: a case-control study. Int J Chron Obstruct Pulmon Dis. 2018;13:1735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Z, Yang D, Ge Z, Yan M, Wu N, Liu Y. Body mass index of patients with chronic obstructive pulmonary disease is associated with pulmonary function and exacerbations: a retrospective real world research. J Thorac Disease. 2018;10(8):5086–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang L, Gao P, Bao H, Tang X, Wang B, Feng Y, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respiratory Med. 2018;6(6):421–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu YH, Liang ZY, Xu LM, Xu WH, Liao H, Li R, et al. Comparison of the clinical characteristics and comprehensive assessments of the 2011 and 2017 GOLD classifications for patients with COPD in China. Int J Chron Obstruct Pulmon Dis. 2018;13:3011–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma B, Ma F, Qin Y. Chronic obstructive pulmonary disease factors in Linxia area Research and prevention measures. Foreign Med Sci (Section Medgeography). 2018;39(04):296–9. (in Chinese). [Google Scholar]
- 71.Shi W, Liu L, Zhang F, Liu Y, Zhang X, Gao J, et al. Correlation analysis between CAT score and COPD disease evaluation index in patients with COPD. Geriatr Health Care. 2018;24(03):257–61. (in Chinese). [Google Scholar]
- 72.Zhang Z, Wang J, Zheng Z, Chen X, Zeng X, Zhang Y, et al. Genetic variants in the Hedgehog Interacting Protein Gene Are Associated with the FEV1/FVC ratio in Southern Han Chinese subjects with chronic obstructive Pulmonary Disease. Biomed Res Int. 2017;2017:2756726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deng X, Yuan CH, Chang D. Interactions between single nucleotide polymorphism of SERPINA1 gene and smoking in association with COPD: a case-control study. Int J Chron Obstruct Pulmon Dis. 2017;12:259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiao T, Chen XY, Wang N, Zhao Q, Fu CW, Xu B. [Study on the situation of drug use in patients with chronic obstructive pulmonary diseases in the Chinese communities of large cities]. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38(2):142–6. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 75.Yan R, Wang Y, Bo J, Li W. Healthy lifestyle behaviors among individuals with chronic obstructive pulmonary disease in urban and rural communities in China: a large community-based epidemiological study. Int J Chron Obstruct Pulmon Dis. 2017;12:3311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X, Zhang Y, Yu C, Nie Q, Xu Z, Wang C, et al. Investigation on epidemic factors and complications of patients hospitalized for chronic obstructive pulmonary disease. Soft Sci Health. 2017;31(12):58–61. (in Chinese). [Google Scholar]
- 77.Duan S. An epidemiological investigation and risk factorsanalysis of COPD in a Wuwei population. Foreign Med Sci (Section Medgeography). 2017;38(01):32–4. (in Chinese). [Google Scholar]
- 78.Gong J, Yang C, Chen D, Chen Y, Yu S, Mao Y et al. Clinical features of inpatients with chronic obstructive pulmonary disease in Shenyang. J Southeast Univ (Medical Sci Edition). 2017;36(03). (in Chinese).
- 79.Lv H, Li H, Zhang T, Chen K, Tan S, Song Y, et al. Epidemiological investigation of chronic obstructive pulmonary disease in urban men of Maoming City. J Guangdong Med Coll. 2017;35(03):270–4. (in Chinese). [Google Scholar]
- 80.Zhang X, Ma J, Meng G, Wang Q, Li W, Xu Y, et al. Analysis on prevalence of chronic obstructive pulmonary disease based on pulmonary function screening in Changchun urban area of Jilin Province. J Jilin Univ (Medicine Edition). 2017;43(05):1047–52. (in Chinese). [Google Scholar]
- 81.Zhu J, Lu H, Hu Z, Gao J. The effect of dyspnea belief on the functional status of elderly patients with chronic obstructive pulmonary disease. Chin J Prev Control Chronic Dis. 2017;25(06):453–6. (in Chinese). [Google Scholar]
- 82.Qi Y, Hou S. Epidemiological investigation and influencing factor analysis of chronic obstructive pulmonary disease. Chin J Mod Drug Application. 2017;11(04):77–9. (in Chinese). [Google Scholar]
- 83.Xiao T, Chen X, Wang N, Zhao Q, Fu C, Xu B. Survey on influencing factors related to exacerbation of COPD patients in Chinese urban communities. Chin J Disease Control Prev. 2017;21(02):110–3. (in Chinese). [Google Scholar]
- 84.Cai B, Li S, Zheng X, Chen Y. Diagnosis and treatment status and existing problems of chronic obstructive pulmonary disease patients in rural areas of Chaoshan area. Chin J Clin Res. 2017;30(10):1423–8. (in Chinese). [Google Scholar]
- 85.Cui J, Han Y, Wang Q, Liu Q, Wang W, Song X. Investigation on the factors related to acute exacerbation of chronic obstructive pulmonary disease among community residents. Chin J Public Health Eng. 2017;16(04):471–3. (in Chinese). [Google Scholar]
- 86.Ding Y, Yang D, He P, Yao J, Sun P, Li Q, et al. Prevalence and risk factors of chronic obstructive pulmonary diseases in a Hlai community in Hainan Island of China. Clin Respir J. 2018;12(1):126–33. [DOI] [PubMed] [Google Scholar]
- 87.Lu W, Zheng Z, Chen X, Tan H, Wang J, Zhang Z, et al. Study Design and Interim Outcomes of Guangzhou Institute of Respiratory Disease COPD Biobank. Copd. 2016;13(2):203–13. [DOI] [PubMed] [Google Scholar]
- 88.Liao XY, Peng DQ, Wang WW, Lei Y, Luo FM, Yang ZY, et al. [Risk factors associated with chronic obstructive pulmonary disease: a comparison between urban and rural populations in Chengdu]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2015;46(2):258–62. (in Chinese). [PubMed] [Google Scholar]
- 89.Han R, Zou J, Shen X, Wu C, Guo Y, Feng Z, et al. [The risk factors of chronic obstructive pulmonary disease in Heilongjiang province]. Zhonghua Jie He He Hu Xi Za Zhi. 2015;38(2):93–8. (in Chinese). [PubMed] [Google Scholar]
- 90.Yang L, Qiu F, Fang W, Zhang L, Xie C, Lu X, et al. The Functional Copy Number Variation-67048 in WWOX contributes to increased risk of COPD in Southern and Eastern Chinese. Copd. 2015;12(5):494–501. [DOI] [PubMed] [Google Scholar]
- 91.Feng W, Huang X, Zhang C, Liu C, Cui X, Zhou Y, et al. The dose-response association of urinary metals with altered pulmonary function and risks of restrictive and obstructive lung diseases: a population-based study in China. BMJ Open. 2015;5(5):e007643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li X, Lin H, Zhang T, Cui J. A survey of 396 patients with chronic obstructive pulmonary disease. Zhejiang Prev Med. 2015;27(10):1034–6. (in Chinese). [Google Scholar]
- 93.Liu X, Zhu S, Lu Y. The prevalence of FAW residents of Chronic Obstructive Pulmonary Disease. China Health Ind. 2015;12(11):181–3. (in Chinese). [Google Scholar]
- 94.Xiao C. A survey on the current status of health literacy in patients with chronic obstructive pulmonary disease. Health Vocat Educ. 2015;33(17):118–20. (in Chinese). [Google Scholar]
- 95.Yu Y, Liu J, Liang K, Tu C, Chen Z, Chang W. A community survey of chronic obstructive pulmonary disease in Jiading district of Shanghai. Practical Geriatr. 2014;28(06):478–80. (in Chinese). [Google Scholar]
- 96.Qiu J, Zhang YN, Chen J, Luo T, Yu XH, Wang JC, et al. [Prevalence of chronic obstructive pulmonary disease in Ningxia Hui Autonomous Region of China]. Zhonghua Jie He He Hu Xi Za Zhi. 2013;36(4):265–8. (in Chinese). [PubMed] [Google Scholar]
- 97.Jiang M, Ma JF. [Comprehension and recognition of acute exacerbation among chronic obstructive pulmonary disease patients]. Zhonghua Liu Xing Bing Xue Za Zhi. 2013;34(10):1030–4. (in Chinese). [PubMed] [Google Scholar]
- 98.Miao L, Zheng F, Wang J, Wang H. Association between inflammatory biomarkers and survival in patients with chronic obstructive pulmonary disease: a prospective study. Chin J Geriatr. 2013;32(04):404–7. (in Chinese). [Google Scholar]
- 99.Zou D, Zhang L, Sun W, Wang Y, Gao H, Zhao J, et al. Current status in the prevention and management of chronic obstructive pulmonary disease in residents of Haiyang Shandong Province. Chin J Nautical Med Hyperbaric Med. 2013;20(02):107–10. (in Chinese). [Google Scholar]
- 100.Zhang X, Liu A, li S, Yang W, Fan M, Meng J, NATIONAL DEFENDING FORCES IN SOUTHWEST CHINA. Investigation on the prevalence and related factors of Chronic Obstructive Pulmonary Disease in Kunming City. Volume 23. MEDICAL JOURNAL OF; 2013. pp. 1273–4. 11(in Chinese).
- 101.Lou P, Zhu Y, Chen P, Zhang P, Yu J, Zhang N, et al. Vulnerability of patients with chronic obstructive pulmonary disease according to gender in China. Int J Chron Obstruct Pulmon Dis. 2012;7:825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhou X, Han J, Song Y, Zhang J, Wang Z. Serum levels of 25-hydroxyvitamin D, oral health and chronic obstructive pulmonary disease. J Clin Periodontol. 2012;39(4):350–6. [DOI] [PubMed] [Google Scholar]
- 103.Wang J, Lan Y, Liu X. Study on the pulmonary function and risk factors of the patients with chronic obstructive pulmonary disease in Wenjiang district of Chengdu. Med J West China. 2012;24(01):16–8. (in Chinese). [Google Scholar]
- 104.Hu D. Epidemiological survey of chronic obstructive pulmonary disease in Cixi city: 2009~2012. China Mod Doctor. 2012;50(19):9–10. 13. (in Chinese). [Google Scholar]
- 105.Wang A, Yin Y, Chen P, Liu Q, Yu Q, Xiao W. The association of SERPINE2 gene with COPD in a Chinese Han population. Yonsei Med J. 2011;52(6):953–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ling M, Rong Y, Gou AS, Niu L, Wang H, Abaydulla Z, et al. [The risk factors for chronic obstructive pulmonary disease in Xinjiang rural areas]. Zhonghua Jie He He Hu Xi Za Zhi. 2011;34(9):666–8. (in Chinese). [PubMed] [Google Scholar]
- 107.Du R, Dong L, Hu Z, Jin S. Evaluation of quality of life for some COPD patients in rural areas in Jilin City. Chin RURAL HEALTH SERVICE Adm. 2011;31(08):881–2. (in Chinese). [Google Scholar]
- 108.Luo Z, Ma L, Nie X, Zhang L. The effect of smoking on lung function in COPD patients. J Clin PULMONARY Med. 2011;16(08):1250–1. (in Chinese). [Google Scholar]
- 109.Weng H, Lai F, He Z, Gou Q, Xu X, Tan G, et al. Prevalence of chronic obstructive pulmonary disease in community residents of urban Chongqing. Chin J Public Health. 2011;27(11):1393–6. (in Chinese). [Google Scholar]
- 110.Lam KB, Jiang CQ, Jordan RE, Miller MR, Zhang WS, Cheng KK, et al. Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest. 2010;137(3):593–600. [DOI] [PubMed] [Google Scholar]
- 111.Zhang H, Wang Q, Hou G, Xu W, Li M, Kang J. Relationship between smoking and the prevalence of Chronic Obstructive Pulmonary Disease in Rural areas of Liaoning Province. J CHINA Med Univ. 2009;38(11):855–7. (in Chinese). [Google Scholar]
- 112.Zhou YM, Wang C, Yao WZ, Chen P, Kang J, Huang SG, et al. [Current status of prevention and management of chronic obstructive pulmonary disease in rural area in China]. Zhonghua Nei Ke Za Zhi. 2009;48(5):358–61. (in Chinese). [PubMed] [Google Scholar]
- 113.Wang Z, Zhou X, Zhang J, Zhang L, Song Y, Hu FB, et al. Periodontal health, oral health behaviours, and chronic obstructive pulmonary disease. J Clin Periodontol. 2009;36(9):750–5. [DOI] [PubMed] [Google Scholar]
- 114.Ko FW, Woo J, Tam W, Lai CK, Ngai J, Kwok T, et al. Prevalence and risk factors of airflow obstruction in an elderly Chinese population. Eur Respir J. 2008;32(6):1472–8. [DOI] [PubMed] [Google Scholar]
- 115.Zhong N, Wang C, Yao W, Chen P, Kang J, Huang S, et al. Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. 2007;176(8):753–60. [DOI] [PubMed] [Google Scholar]
- 116.Liu S, Zhou Y, Wang X, Wang D, Lu J, Zheng J, et al. Biomass fuels are the probable risk factor for chronic obstructive pulmonary disease in rural South China. Thorax. 2007;62(10):889–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu F, Yin X, Shen H, Xu Y, Ware RS, Owen N. Better understanding the influence of cigarette smoking and indoor air pollution on chronic obstructive pulmonary disease: a case-control study in Mainland China. Respirology. 2007;12(6):891–7. [DOI] [PubMed] [Google Scholar]
- 118.Wang XP, Zhou YM, Zeng XY, Liu SM, Qiu R, Xie JF, et al. [Study on the prevalence rate of chronic obstructive pulmonary disease in northern part of Guangdong province]. Zhonghua Liu Xing Bing Xue Za Zhi. 2005;26(3):211–3. (in Chinese). [PubMed] [Google Scholar]
- 119.Liu SM, Wang XP, Wang DL, Zhou YM, Lü JC, Zheng JP et al. [Epidemiologic analysis of COPD in Guangdong province]. Zhonghua Yi Xue Za Zhi. 2005;85(11):747–52. (in Chinese). [PubMed]
- 120.Li ZP, Huang JQ, Tang KJ. [Retrospective studies on 713 cases chronic obstructive pulmonary disease]. Zhonghua Liu Xing Bing Xue Za Zhi. 2003;24(8):722–4. (in Chinese). [PubMed] [Google Scholar]
- 121.Zhou Y, Wang C, Yao W, Chen P, Kang J, Huang S, et al. COPD in Chinese nonsmokers. Eur Respir J. 2009;33(3):509–18. [DOI] [PubMed] [Google Scholar]
- 122.Long H, Xing Z, Chai D, Liu W, Tong Y, Wang Y, et al. Solid fuel exposure and chronic obstructive Pulmonary Disease in never-smokers. Front Med (Lausanne). 2021;8:757333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith M, Li L, Augustyn M, Kurmi O, Chen J, Collins R, et al. Prevalence and correlates of airflow obstruction in ∼317,000 never-smokers in China. Eur Respir J. 2014;44(1):66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou Y, Chen R. Risk factors and intervention for chronic obstructive pulmonary disease in China. Respirology. 2013;18(Suppl 3):4–9. [DOI] [PubMed] [Google Scholar]
- 125.David GL, Koh WP, Lee HP, Yu MC, London SJ. Childhood exposure to environmental tobacco smoke and chronic respiratory symptoms in non-smoking adults: the Singapore Chinese Health Study. Thorax. 2005;60(12):1052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ekici A, Ekici M, Kurtipek E, Akin A, Arslan M, Kara T, et al. Obstructive airway diseases in women exposed to biomass smoke. Environ Res. 2005;99(1):93–8. [DOI] [PubMed] [Google Scholar]
- 127.Fullerton DG, Bruce N, Gordon SB. Indoor air pollution from biomass fuel smoke is a major health concern in the developing world. Trans R Soc Trop Med Hyg. 2008;102(9):843–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mestl HE, Aunan K, Seip HM, Wang S, Zhao Y, Zhang D. Urban and rural exposure to indoor air pollution from domestic biomass and coal burning across China. Sci Total Environ. 2007;377(1):12–26. [DOI] [PubMed] [Google Scholar]
- 129.Kim HY, Song KS, Goo JM, Lee JS, Lee KS, Lim TH. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001;21(4):839–58. discussion 859–860. [DOI] [PubMed] [Google Scholar]
- 130.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61(3):259–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax. 2004;59(4):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yin P, Jiang CQ, Cheng KK, Lam TH, Lam KH, Miller MR, et al. Passive smoking exposure and risk of COPD among adults in China: the Guangzhou Biobank Cohort Study. Lancet (London England). 2007;370(9589):751–7. [DOI] [PubMed] [Google Scholar]
- 133.Varraso R, Fung TT, Hu FB, Willett W, Camargo CA. Prospective study of dietary patterns and chronic obstructive pulmonary disease among US men. Thorax. 2007;62(9):786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.McKeever TM, Lewis SA, Cassano PA, Ocké M, Burney P, Britton J, et al. Patterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volume. Am J Clin Nutr. 2010;92(2):408–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(10):1005–12. [DOI] [PubMed] [Google Scholar]
- 136.Leynaert B, Bousquet J, Henry C, Liard R, Neukirch F. Is bronchial hyperresponsiveness more frequent in women than in men? A population-based study. Am J Respir Crit Care Med. 1997;156(5):1413–20. [DOI] [PubMed] [Google Scholar]
- 137.de Torres JP, Campo A, Casanova C, Aguirre-Jaime A, Zulueta J. Gender and chronic obstructive pulmonary disease in high-risk smokers. Respir Int Rev Thorac Dis. 2006;73(3):306–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The entirety of the data utilized or investigated in the course of this investigation has been incorporated within this published article, along with its accompanying Supplementary Material.