Abstract
Purpose
Acute and transient psychotic disorder (ATPD), a psychosis frequently diagnosed, can potentially evolve into chronic conditions like schizophrenia (SCZ) and other mental disorders. This study aimed to develop a predictive model based on clinical data to forecast the transition from ATPD to SCZ and to identify the predictive factors.
Methods
According to the diagnostic criteria issued by the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10), 396 inpatients diagnosed with ATPD were collected in this study. The Cox proportional-hazards regression model was performed using demographic data, clinical characteristics, and inflammatory markers to identify independent predictors for subsequent diagnostic transition (SDT) to SCZ.
Results
During the follow-up period, 43.69% (n = 173) of ATPD patients had their diagnoses revised to SCZ. The multivariate Cox regression analysis identified post-treatment monocyte count, post-treatment monocyte/lymphocyte ratio (MLR), and the presence of schizophreniform symptoms as significant predictors for the diagnostic revision. Time-dependent receiver operating characteristic (TimeROC) analyses were developed. The AUC value at the 5-year follow-up was 0.728 for combined predictors, 0.702 for post-treatment monocyte count, 0.764 for post-treatment MLR, and 0.535 for the presence of schizophreniform symptoms.
Conclusion
The combined predictors had good predictive ability for the diagnostic transition from acute and transient psychotic disorder to schizophrenia.
Keywords: acute and transient psychotic disorder, Schizophrenia, inflammation, subsequent-diagnostic-transition, monocyte count, monocyte–lymphocyte ratio
Introduction
The annual incidence of ATPD varies significantly, with rates ranging from 3.9 per 100,000 people in the UK to 9.6 in Denmark.1 The lifetime prevalence of Brief psychotic disorder (BPD) using the Diagnostic and Statistical Manual of Mental Disorders 5th ed. as the diagnostic criteria was lower than 0.1% in China,2 and nearly two thirds of patients with ATPDs met the criteria for BPD.3 ICD-10 endorses the concept of ATPD within the group of schizophrenia, schizotypal, and delusional disorders, characterized by an acute onset and a remission within 1 to 3 months. DSM-5 defines BPD based on the presence of at least four of the five core symptoms of schizophrenia, and the duration must be at least one day but less than one month.4 A Meta-analysis revealed that 25% of ATPD cases converted into schizophrenia (SCZ) and related disorders within an average follow-up period of 6.3 years, and the risk factors associated with the elevated risk and accelerated progression to SCZ included younger age at onset, male gender, premorbid impairment, longer hospital admissions, first-rank symptoms, and family history.5 Another meta-analysis found that 19% changed their diagnoses to SCZ spectrum psychoses on average follow-up of 47 months.6 López-Díaz et al found that the role of premorbid adjustment and the presence of schizophreniform symptoms at the onset of psychosis were predictors of transition to schizophrenia in patients with first-episode ATPD.7 While Wang et al demonstrated that only olanzapine-equivalent-Dosage of Antipsychotics at the End of the Hospitalization(DAEH) was significantly associated with the subsequent diagnostic transition (SDT) to SCZ.8
Previous research highlighted the presence of persistent, low-grade inflammation in certain patients with SCZ.9 Several studies reported leukocyte abnormalities in SCZ patients, particularly in inflammatory ratios such as the neutrophil/lymphocyte ratio (NLR), monocyte/lymphocyte ratio (MLR), and the ratio of neutrophil×platelet/lymphocyte, also known as the systemic immune inflammation index (SII).10 Blood cell counts are routine items of examination for admitted patients and are reviewed after treatment. Hence, these biological markers are available for pre- and post-treatment assessments. Previous studies primarily focused on baseline or pre-treatment levels of inflammatory markers, with limited attention given to their post-treatment changes. Currently, the diagnostic stability of ATPD is relatively low, and biomarkers for the SDT from ATPD to SCZ have not yet been explored. Our study aimed to fill this gap by investigating pre-treatment and post-treatment inflammatory markers as predictors for the SDT from ATPD to SCZ.
In this retrospective study, we integrated a spectrum of inflammatory markers, demographic data, and clinical characteristics to develop a predictive model using the Cox proportional-hazards regression model. This study was designed to enhance early risk assessment for patients with ATPD at high risk of SDT to SCZ.
Material and Methods
Subjects
The retrospective study consisted of 396 patients with ATPD who were hospitalized at the Affiliated Brain Hospital of Nanjing Medical University, Jiangsu, China, between January 2015 and December 2022. The inclusion criteria for patients involved in this study were as follows: (1) Aged 16–70 years; (2) Met the diagnostic criteria for ATPD as outlined in ICD-10; and (3) Diagnosis was assessed and confirmed by two associate chief physicians or above. Exclusion criteria were as follows: (1) organic mental disorders; (2) mental disorders due to psychoactive substances or non-addictive substances; (3) mood disorders; (4) smoking; (5) history of autoimmune disorders, hematological diseases, tumors, viral hepatitis, or tuberculosis; (6) with ongoing respiratory tract infection or urinary tract infection; and (7) use of oral anti-immune agents.
Clinical Features and Laboratory Results
The variables included sociodemographic factors, clinical characteristics, and inflammatory markers.
The Sociodemographic Characteristics of the Patients Included Age and Gender
The Clinical Characteristics of the Patients
The clinical characteristics of patients included a) length of hospital stay,(b) family history of psychosis, (c) the presence of schizophreniform symptoms, (d) olanzapine-equivalent-DAEH. The length of hospital stay was measured in days from admission to discharge, while the olanzapine-equivalent-DAEH was calculated based on the final antipsychotic regimen at discharge.
The Inflammatory Markers Before and After Treatment
Inflammatory markers, including leukocyte count, neutrophil count, monocyte counts, lymphocyte count, and platelet count, were collected both before and after treatment. Additionally, the neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and systemic immune-inflammation index (SII) were calculated before and after treatment. All blood samples were analyzed in the hospital’s central laboratory following standardized procedures.
Clinical Follow-Up
The follow-up interviews were administered in December 2023, and the information on diagnostic transition from the time of discharge to the follow-up period was collected through telephone follow-ups, review of patients’ outpatient records, and examination of readmission medical records. This collection did not include early intervention programs.
Statistical Analyses
The SPSS version 26 was used for multivariate Cox regression analysis. The Cox proportional-hazards regression model is a statistical technique employed to analyze data pertaining to time-to-event variables, particularly in the presence of censored cases where patients may not have experienced the outcome event by the end of the follow-up period. The model allows for the assessment of multiple covariates simultaneously, providing a robust method to evaluate the impact of various factors on the risk of SDT from ATPD to schizophrenia. The R Programming Language version 4.4.1 was used for generating time-dependent receiver operating characteristic curves (timeROC), and for calculating the sample size. For parameters with continuous data, normally distributed data were expressed as the mean ± standard deviation (SD). Count data were expressed as a rate (%). All statistical tests were two-sided, and a P value < 0.05 was considered significant.
Results
We performed multivariate Cox regression analysis in a cohort of 396 patients who met the enrollment criteria. The cohort consisted of 182 male (45.96%) and 214 female (54.04%) patients, with an age range of 16 to 69 years. The age at onset was 31.76 ± 11.81 years, with an average of 30.36 ± 10.90 years for male patients and 32.94± 12.43 years for female patients. These patients were followed up for a period ranging from 1 to 9 years after their initial treatment and discharge. Of the total, 173 patients (43.69%) were diagnosed with SCZ, as indicated by the development and transition of their symptoms. In addition, 92 patients (23.23%) were diagnosed with mood disorder, 113 patients (28.54%) maintained their ATPD diagnosis, and 18 patients (4.55%) lost to follow-up (Table 1).
Table 1.
Outcome of ATPD
| Diagnostic Category | Number of Cases | Proportion |
|---|---|---|
| SCZ | 173 | 43.69% |
| ATPD | 113 | 28.54% |
| Mood disorder | 92 | 23.23% |
| Lost to follow-up | 18 | 4.55% |
We performed a multivariate Cox regression model incorporating variables such as gender, age, length of hospital stay, family history of psychosis, olanzapine-equivalent-DAEH, and the presence of schizophreniform symptoms. Additionally, to avoid multicollinearity, we finally included six inflammatory markers as variables in the analysis: pre-treatment leukocyte count, pre-treatment MLR, pre-treatment SII, post-treatment monocyte count, post-treatment NLR, and post-treatment MLR. The result of the multivariate regression model was significant (χ2 =48.643, P<0.001).
Table 2 shows the results of multivariate Cox regression analysis, identifying post-treatment monocyte count (HR =4.540, HR 95% CI: 1.305–15.787,P=0.017), post-treatment MLR (HR =18.019, HR 95% CI: 1.569–206.908, P=0.020), and the presence of schizophreniform symptoms (HR =2.127, HR 95% CI: 1.030–4.392,P=0.041), were significant predictors for SDT from ATPD to SCZ.
Table 2.
Summary of Multivariate Cox Regression Analysis (n = 396)
| Regression Coefficient | Standa-rd Error | z value | P value | HR Value | HR Value 95% CI | |
|---|---|---|---|---|---|---|
| Age | −0.010 | 0.007 | −1.317 | 0.188 | 0.991 | 0.977 ~ 1.005 |
| Gender | ||||||
| Male | - | - | - | - | - | - |
| Female | −0.021 | 0.160 | −0.130 | 0.897 | 0.979 | 0.716 ~ 1.341 |
| Length of hospital stay | 0.004 | 0.010 | 0.429 | 0.668 | 1.004 | 0.985 ~ 1.023 |
| Family history of psychosis | ||||||
| No | - | - | - | - | - | - |
| Yes | −0.012 | 0.199 | −0.063 | 0.950 | 0.988 | 0.668 ~ 1.460 |
| The presence of schizophreniform symptoms | ||||||
| No | - | - | - | - | - | - |
| Yes | 0.755 | 0.370 | 2.040 | 0.041* | 2.127 | 1.030 ~ 4.392 |
| Olanzapine-equivalent-DAEH (mg) | 0.009 | 0.015 | 0.611 | 0.541 | 1.009 | 0.981 ~ 1.038 |
| Pre-treatment leukocyte count | 0.035 | 0.037 | 0.953 | 0.341 | 1.036 | 0.963 ~ 1.115 |
| Pre-treatment MLR | −0.098 | 0.278 | −0.354 | 0.723 | 0.906 | 0.526 ~ 1.562 |
| Pre-treatment SII | −0.000 | 0.000 | −0.100 | 0.921 | 1.000 | 1.000 ~ 1.000 |
| Post-treatment monocyte count | 1.513 | 0.636 | 2.379 | 0.017* | 4.540 | 1.305 ~ 15.787 |
| Post-treatment NLR | −0.129 | 0.068 | −1.897 | 0.058 | 0.879 | 0.770 ~ 1.004 |
| Post-treatment MLR | 2.891 | 1.245 | 2.322 | 0.020* | 18.019 | 1.569 ~ 206.908 |
Note: * P < 0.05.
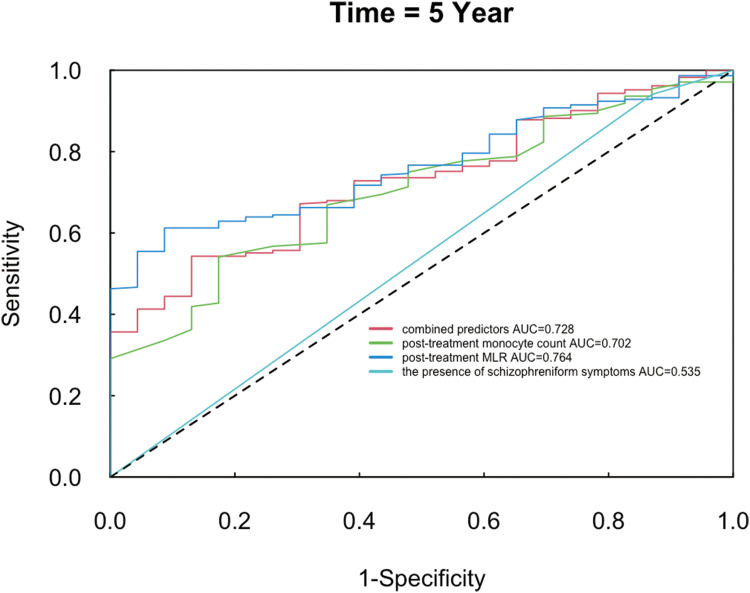
The time-dependent ROC curves at the 5-year follow-up were constructed using combined predictors, the post-treatment monocyte count, post-treatment MLR, and the presence of schizophreniform symptoms. The AUC value at the 5-year follow-up for combined predictors was 0.728 (95% CI: 0.636–0.821), for post-treatment monocyte count was 0.702 (95% CI: 0.604–0.800), for post-treatment MLR was 0.764 (95% CI: 0.686–0.843), for the presence of schizophreniform symptoms was 0.535 (95% CI: 0.463–0.608). (Table 3 and Figure 1).
Table 3.
Results of 5-Year TimeROC Curves
| Variables | AUC | 95% CI | Cut-off value | YI | Sensitivity | Specificity |
|---|---|---|---|---|---|---|
| Combined predictors | 0.728 | 0.636–0.821 | 0.961 | 0.400 | 0.497 | 0.903 |
| Post-treatment monocyte count | 0.702 | 0.604–0.800 | 0.470 | 0.369 | 0.567 | 0.802 |
| Post-treatment MLR | 0.764 | 0.686–0.843 | 0.263 | 0.450 | 0.569 | 0.881 |
| The presence of schizophreniform symptoms | 0.535 | 0.463–0.608 | 1.000 | 0.039 | 0.950 | 0.089 |
Figure 1.
5-Year Time-ROC curves.
The optimum cut-off value of combined predictors was 0.961, with the Youden index, sensitivity and specificity of combined predictors as 0.400, 0.497 and 0.903; The optimum cut-off value of post-treatment monocyte count was 0.470, with the Youden index, sensitivity and specificity as 0.369, 0.567 and 0.802; The optimum cut-off value of post-treatment MLR was 0.263, with the Youden index, sensitivity and specificity as 0.450, 0.569 and 0.881; The optimum cutoff value of the presence of schizophreniform symptoms was 1, with the Youden index, sensitivity and specificity as 0.039, 0.950 and 0.089.(Table 3).
These values indicated good predictive ability for the prognostic index of combined predictors, post-treatment monocyte count, and post-treatment MLR. However, the presence of schizophreniform symptoms had a poorer predictive ability.
Using the pmsampsize package in R 4.4.1 software to calculate the sample size required for establishing a multivariate prediction model with 3 candidate predictive parameters, it was assumed that the incidence rate of the outcome was 46.7%,7 and the AUC statistic of the prediction model was 0.728. Based on the preset parameters, it was calculated that a minimum of 383 cases were needed. Our study included a total of 396 patients, ensuring the reliability of the results.
Discussion
During the long-term follow-up, a considerable number of ATPD patients were diagnosed as schizophrenia.11 In this study, we established a predictive model to predict the SDT from ATPD to SCZ. Although previous studies had investigated the role of demographic and clinical factors in the transition, we developed survival analysis as a predictive model for the transition for the first time, incorporating both pre-and post-treatment inflammatory markers as analysis variables. Our study highlighted post-treatment monocyte count and post-treatment MLR as predictors of SDT from ATPD to SCZ, emphasizing the role of innate immune cells and inflammatory markers in early SCZ development. While a general association between inflammation and SCZ has been established, our study underscores the importance of monitoring changes in immune markers after initial treatment, which has not been fully explored in the literature.
Prior reports suggested a higher prevalence of ATPD in women,1 which was consistent with our finding. The average age of ATPD onset was 32 years across all the patients, with a slight variation between genders: 30 years for males and 33 years for females. According to previous studies, ATPD diagnosis rates began to climb in the early to mid-20s; for females, the peak incidence occurred about a decade later.1 Factors such as gender, age, length of hospital stay, family history of psychosis, the presence of schizophreniform symptoms, and olanzapine-equivalent-DAEH were not correlated with the transition from ATPD to SCZ. These results were partially consistent with previous research,7,8 which may be related to the size of the included sample. Our study found that the presence of schizophreniform symptoms was a significant predictor for the SDT from ATPD to SCZ, but its predictive ability was poorer at the 5-year follow-up. Our findings were not entirely consistent with previous results.7 Therefore, future prospective studies are needed to further validate these findings.
Our study revealed a notable association between post-treatment monocyte count and the SDT from ATPD to SCZ, underscoring the role of the innate immune cells in the early stage of SCZ development. Monocytes are part of the peripheral innate immune system, playing a critical role in the first-line defense against invading pathogens and in the initiation and control of inflammatory responses.12 The brain contains several types of mononuclear cell-derived cells, including perivascular cells, meningeal macrophages, choroid plexus macrophages and microglia.13 The accumulation of monocytes and macrophages were found in the cerebrospinal fluid of patients with SCZ during acute psychotic episodes.14 Postmortem examination revealed a specific increase in the numerical density of HLA-DR1 microglia in the temporal and frontal cortex of chronic schizophrenics.15 A high monocyte count was considered a possible peripheral marker of microglial activation in SCZ patients.13
The count of circulating monocytes is an easily obtainable indirect marker of activation of the mononuclear phagocyte system. A meta-analysis found that, compared to the control group, there was a significant increase in monocytes in patients with first-episode psychosis.16 Another meta-analysis found that patients with SCZ and related psychoses had a higher monocyte count than healthy controls.13 A study comparing patients with SCZ to control groups revealed that monocyte count and the ratio of monocyte to high-density lipoprotein were significantly higher in SCZ patients.17 Gao et al conducted bidirectional two-sample Mendelian randomization analyses and found that SCZ was associated with elevated levels of white blood cells (including neutrophils and monocytes).18 The trend was further evidenced by the finding that neutrophils, monocytes, and C-reactive protein levels were increased in first-episode psychosis(FEP) and SCZ patients vs controls at baseline.19
Research has indicated that psychosocial stressors partially contribute to the pathology of SCZ by promoting neuroinflammation.20 Chronic exposure to stressors leads to persistent low-grade inflammation. This condition is characterized by an upsurge in monocyte production in the bone marrow, as well as elevated levels of monocytes in the circulation, spleen, and brain. Moreover, the influx of monocytes into the brain is facilitated by increased permeability of the blood-brain barrier, a response triggered by chronic stress.21 Inflammation plays a pivotal role in the structural brain alterations associated with neuropsychiatric disorders, primarily through the actions of microglia and astrocytes. This process involves disordered synaptic pruning, significantly impacting the gray matter volume (GMV).22 WBC count was correlated with reductions in total brain volume and GMV.23 Notably, Parker’ s research highlighted that monocyte count had the most substantial genetic overlap with cortical brain structure, suggesting a shared genetic foundation between cortical brain structure and blood immune markers.24 The majority of previous studies concentrated on baseline inflammatory markers, while a minority investigated the alterations in related inflammatory indicators subsequent to treatment. Cui et al revealed a possible correlation between changes in the transcriptional profile of monocytes and cortical thickness following treatment in patients with schizophrenia.25 A study using a rat model demonstrated that clodronate-induced monocyte depletion in peripheral inflammation reduced both inflammatory mediator production and microglial activation.26 Such insights supported the hypothesis that the interplay among immunity, brain activity, and behavior could be a key factor in the pathogenesis of SCZ.
WBCs secrete a spectrum proinflammatory cytokines. The compromised blood-brain barrier (BBB) allows circulating cytokines and leukocytes into the brain, leading to persistent neuroinflammation.27 Cytokines such as IL-1β, IL-6, IL-8, and TNF-αwere increased in SCZ patients.28,29 Meta-analyses consistently identified elevated levels of IL-6 and IL-8 in patients with both acute and chronic SCZ spectrum disorders.30 Notably, these cytokines were predominantly produced by innate immune cells, including monocytes.12 Inflammatory mediators, such as IL-6 and IL-8, were intricately associated with the pathogenesis and progression of psychiatric disorders.31 Specifically, IL-6 was correlated with brain volume changes in the middle temporal gyrus.32 These findings accentuated the potential significance of the mononuclear phagocyte system in the pathology of psychotic disorders.
Inflammatory ratios such as NLR, MLR, and SII are gaining attention in research associated with SCZ. The elevated inflammatory ratios in patients with schizophrenia suggested an imbalance in the immune system, particularly in innate immunity.33 Two studies found that NLR and MLR were significantly higher in patients with SCZ compared with healthy controls.33,34 These findings were corroborated by another study, showing that WBC count, neutrophil count, monocyte count, NLR, and MLR were all significantly higher in SCZ patients than in the control group.35 These findings confirmed the hypothesis that elevated inflammatory markers were characteristic of SCZ, particularly in acute episodes. However, our study did not observe a correlation between inflammatory biomarker levels before the treatment and the SDT from ATPD to SCZ. This finding might reflect the complex nature of the inflammatory response, which was prevalent in the acute phase of mood disorders and SCZ.
One possible mechanism of antipsychotics in treating psychosis was mitigating neuroinflammation, which was supported by a previous study indicating a significant decrease in monocyte levels in patients with SCZ from the baseline to 12 months following antipsychotic treatment.36. However, after 2 years of continuous antipsychotic treatment, the NLR and MLR levels remained higher in patients with SCZ than healthy controls. Given that the inflammation persisted after treatment in the acute stage, our finding supported the hypothesis that inflammation was a pathological mechanism of chronic diseases such as SCZ. Inflammation is a fundamental part of the human body’s immune response to infection and physiological injury. Acute inflammation is crucial for restoring cellular and tissue homeostasis after a pathogenic or physical insult, and chronic inflammation is maladaptive and can result in gradual tissue damage, potentially leading to disease development.37 Low-grade, persistent inflammation was present in a subgroup of SCZ patients.9 Regardless of whether patients with SCZ were in acute exacerbation or remission phase, the NLR, PLR, and MLR in patients tended to be higher than those in healthy individuals.38 Our study highlighted post-treatment monocyte count and post-treatment MLR as predictors of SDT from ATPD to SCZ, illustrating that inflammation was still present in the remission phase of ATPD. This emphasized the role of persistent inflammation in early SCZ development. Our study found that post-treatment NLR was not a predictor of the SDT from ATPD to SCZ, which was consistent with a previous study. Kocak et al found that only MLR values were significantly higher in the schizophrenia remission group than in the healthy controls group. Therefore, they suggested that MLR may be a trait marker for schizophrenia.39
Our study had several limitations that merit consideration. First, our study was retrospective and could not draw any conclusions about the causal relationships between post-treatment inflammatory markers and the SDT from ATPD to schizophrenia. Further prospective studies may better demonstrate their relationship. Secondly, although we controlled for some confounding factors, including smoking associated with elevated monocyte counts,40 we overlooked premorbid adjustment, which was associated with schizophrenia.7 These data need to be investigated in future prospective studies. Thirdly, due to the inconsistent scales used by patients in the retrospective study, investigation of the correlation between inflammatory markers and the severity of psychotic symptoms was not feasible. For instance, one study revealed a weak to moderate positive correlation of NLR and MLR with the scores on the PANSS negative scale.41 Fourthly, some outcomes were relied on telephone follow-ups and outpatient records, potentially introducing recall bias.
Conclusions
The combined predictors had good predictive ability for the diagnostic transition from acute and transient psychotic disorder to schizophrenia.
Acknowledgments
We would like to thank Xuzhao Lu for writing assistance.
Funding Statement
This research was funded by the Key Research and Development Plan in Jiangsu (Social Development, Grant/Award Number BE2022677), the National Natural Science Foundation of China (Grant/Award Numbers: 82172061), and the 16th Batch of Six Talent Peak Projects in Jiangsu (Grant/Award Number WSN-166).
Abbreviations
ATPD, Acute and transient psychotic disorder; SCZ, schizophrenia; SDT, subsequent diagnostic transition; DAEH,Dosage of Antipsychotics at the End of the Hospitalization; MLR, Monocyte-to-Lymphocyte Ratio; AUC, area under the curve; BPD, Brief psychotic disorder; NLR, neutrophil/lymphocyte ratio; SII, systemic immune inflammation index; WBC, white blood cell; GMV, gray matter volume; BBB, blood-brain barrier.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
This study was carried out in strict adherence to the principles of the Helsinki Declaration (revised in 2013) and received approval from the Institutional Ethical Committee for Clinical Research of the Brain Hospital Affiliated to Nanjing Medical University (Approval number: 2024-KY001-01FSAT). Informed consent was waived by our Institutional Review Board because of the retrospective nature of our study.
Consent for Publication
Not applicable.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Malhotra S, Sahoo S, Balachander S. Acute and transient psychotic disorders: newer understanding. Curr Psychiatry Rep. 2019;21(11):113. doi: 10.1007/s11920-019-1099-8 [DOI] [PubMed] [Google Scholar]
- 2.Huang Y, Wang Y, Wang H, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi: 10.1016/s2215-0366(18)30511-x [DOI] [PubMed] [Google Scholar]
- 3.Castagnini A, Galeazzi GM. Acute and transient psychoses: clinical and nosological issues. BJPsych Advances. 2016;22(5):292–300. doi: 10.1192/apt.bp.115.015198 [DOI] [Google Scholar]
- 4.Fusar-Poli P, Salazar de Pablo G, Rajkumar RP, et al. Diagnosis, prognosis, and treatment of brief psychotic episodes: a review and research agenda. Lancet Psychiatry. 2022;9(1):72–83. doi: 10.1016/s2215-0366(21)00121-8 [DOI] [PubMed] [Google Scholar]
- 5.Castagnini A, Foldager L, Caffo E, Berrios GE. The predictive validity and outcome of ICD-10 and DSM-5 short-lived psychotic disorders: a review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2022;272(7):1157–1168. doi: 10.1007/s00406-021-01356-7 [DOI] [PubMed] [Google Scholar]
- 6.López-Díaz Á, Fernández-González JL, Lara I, Ruiz-Veguilla M. Predictors of diagnostic stability in acute and transient psychotic disorders: validation of previous findings and implications for ICD-11. Eur Arch Psychiatry Clin Neurosci. 2020;270(3):291–299. doi: 10.1007/s00406-019-01014-z [DOI] [PubMed] [Google Scholar]
- 7.López-Díaz Á, Fernández-González JL, Lara I, Crespo-Facorro B, Ruiz-Veguilla M. Predictors of transition to schizophrenia and other long-lasting non-affective psychoses in first-episode patients with acute and transient psychotic disorders: a validation study. Eur J Psych. 2024;38(2):100234. doi: 10.1016/j.ejpsy.2023.100234 [DOI] [Google Scholar]
- 8.Wang HY, Guo WJ, Li XJ, et al. Higher required dosage of antipsychotics to relieve the symptoms of first-onset Acute and Transient Psychotic Disorder (ATPD) predicted the subsequent diagnostic transition to schizophrenia: a longitudinal study. Schizophr Res. 2018;193:461–462. doi: 10.1016/j.schres.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 9.Fišar Z. Biological hypotheses, risk factors, and biomarkers of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2023;120:110626. doi: 10.1016/j.pnpbp.2022.110626 [DOI] [PubMed] [Google Scholar]
- 10.Karageorgiou V, Milas GP, Michopoulos I. Neutrophil-to-lymphocyte ratio in schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2019;206:4–12. doi: 10.1016/j.schres.2018.12.017 [DOI] [PubMed] [Google Scholar]
- 11.Guidelines for the Diagnosis and Treatment of Mental Disorders (2020 Edition). Available at: https://ncmhc.org.cn/channel/newsinfo/6339. Accessed February 4, 2022.
- 12.Kübler R, Ormel PR, Sommer IEC, Kahn RS, de Witte LD. Gene expression profiling of monocytes in recent-onset schizophrenia. Brain Behav Immun. 2023;111:334–342. doi: 10.1016/j.bbi.2023.04.019 [DOI] [PubMed] [Google Scholar]
- 13.Mazza MG, Capellazzi M, Lucchi S, Tagliabue I, Rossetti A, Clerici M. Monocyte count in schizophrenia and related disorders: a systematic review and meta-analysis. Acta Neuropsychiatr. 2020;32(5):229–236. doi: 10.1017/neu.2020.12 [DOI] [PubMed] [Google Scholar]
- 14.Nikkilä HV, Müller K, Ahokas A, Miettinen K, Rimón R, Andersson LC. Accumulation of macrophages in the CSF of schizophrenic patients during acute psychotic episodes. Am J Psychiatry. 1999;156(11):1725–1729. doi: 10.1176/ajp.156.11.1725 [DOI] [PubMed] [Google Scholar]
- 15.Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59(2):137–150. doi: 10.1093/jnen/59.2.137 [DOI] [PubMed] [Google Scholar]
- 16.Jackson AJ, Miller BJ. Meta-analysis of total and differential white blood cell counts in schizophrenia. Acta Psychiatr Scand. 2020;142(1):18–26. doi: 10.1111/acps.13140 [DOI] [PubMed] [Google Scholar]
- 17.Sahpolat M, Ayar D, Ari M, Karaman MA. Elevated monocyte to high-density lipoprotein ratios as an inflammation markers for schizophrenia patients. Clin Psychopharmacol Neurosci. 2021;19(1):112–116. doi: 10.9758/cpn.2021.19.1.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao Z, Li B, Guo X, Bai W, Kou C. The association between schizophrenia and white blood cells count: a bidirectional two-sample Mendelian randomization study. BMC Psychiatry. 2023;23(1):271. doi: 10.1186/s12888-023-04760-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steiner J, Frodl T, Schiltz K, et al. Innate immune cells and C-reactive protein in acute first-episode psychosis and schizophrenia: relationship to psychopathology and treatment. Schizophr Bull. 2020;46(2):363–373. doi: 10.1093/schbul/sbz068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Comer AL, Carrier M, Tremblay M, Cruz-Martín A. The inflamed brain in Schizophrenia: the convergence of genetic and environmental risk factors that lead to uncontrolled neuroinflammation. Front Cell Neurosci. 2020;14:274. doi: 10.3389/fncel.2020.00274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Wouw M, Boehme M, Dinan TG, Cryan JF. Monocyte mobilisation, microbiota & mental illness. Brain Behav Immun. 2019;81:74–91. doi: 10.1016/j.bbi.2019.07.019 [DOI] [PubMed] [Google Scholar]
- 22.Gmu K, Jones PB, eds. Neuroinflammation and Schizophrenia. Springer International Publishing. 2020; [Google Scholar]
- 23.Janowitz D, Habes M, Toledo JB, et al. Inflammatory markers and imaging patterns of advanced brain aging in the general population. Brain Imaging Behav. 2020;14(4):1108–1117. doi: 10.1007/s11682-019-00058-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker N, Cheng W, Hindley GFL, et al. Genetic overlap between global cortical brain structure, C-reactive protein, and white blood cell counts. Biol Psychiatry. 2024;95(1):62–71. doi: 10.1016/j.biopsych.2023.06.008 [DOI] [PubMed] [Google Scholar]
- 25.Cui LB, Wang XY, Fu YF, et al. Transcriptional level of inflammation markers associates with short-term brain structural changes in first-episode schizophrenia. BMC Med. 2023;21(1):250. doi: 10.1186/s12916-023-02963-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vidal PM, Pacheco R. The cross-talk between the dopaminergic and the immune system involved in Schizophrenia. Front Pharmacol. 2020;11:394. doi: 10.3389/fphar.2020.00394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong X, Qiang Y, Wang L, et al. Peripheral immunity and risk of incident brain disorders: a prospective cohort study of 161,968 participants. Transl Psychiatry. 2023;13(1):382. doi: 10.1038/s41398-023-02683-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70(7):663–671. doi: 10.1016/j.biopsych.2011.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Notter T, Meyer U. Microglia and schizophrenia: where next? Mol Psychiatry. 2017;22(6):788–789. doi: 10.1038/mp.2017.67 [DOI] [PubMed] [Google Scholar]
- 30.Halstead S, Siskind D, Amft M, et al. Alteration patterns of peripheral concentrations of cytokines and associated inflammatory proteins in acute and chronic stages of schizophrenia: a systematic review and network meta-analysis. Lancet Psychiatry. 2023;10(4):260–271. doi: 10.1016/s2215-0366(23)00025-1 [DOI] [PubMed] [Google Scholar]
- 31.Yuan N, Chen Y, Xia Y, Dai J, Liu C. Inflammation-related biomarkers in major psychiatric disorders: a cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl Psychiatry. 2019;9(1):233. doi: 10.1038/s41398-019-0570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams JA, Burgess S, Suckling J, et al. Inflammation and brain structure in Schizophrenia and other neuropsychiatric disorders: a Mendelian randomization study. JAMA Psychiatry. 2022;79(5):498–507. doi: 10.1001/jamapsychiatry.2022.0407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazza MG, Lucchi S, Rossetti A, Clerici M. Neutrophil-lymphocyte ratio, monocyte-lymphocyte ratio and platelet-lymphocyte ratio in non-affective psychosis: a meta-analysis and systematic review. World J Biol Psychiatry. 2020;21(5):326–338. doi: 10.1080/15622975.2019.1583371 [DOI] [PubMed] [Google Scholar]
- 34.Zhu X, Zhou J, Zhu Y, et al. Neutrophil/lymphocyte, platelet/lymphocyte and monocyte/lymphocyte ratios in schizophrenia. Australas Psychiatry. 2022;30(1):95–99. doi: 10.1177/10398562211022753 [DOI] [PubMed] [Google Scholar]
- 35.Xu H, Wei Y, Zheng L, et al. Relation between unconjugated bilirubin and peripheral biomarkers of inflammation derived from complete blood counts in patients with acute stage of schizophrenia. Front Psychiatry. 2022;13:843985. doi: 10.3389/fpsyt.2022.843985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feng T, McEvoy JP, Miller BJ. Longitudinal study of inflammatory markers and psychopathology in schizophrenia. Schizophr Res. 2020;224:58–66. doi: 10.1016/j.schres.2020.10.003 [DOI] [PubMed] [Google Scholar]
- 37.Furman D, Campisi J, Verdin E, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25(12):1822–1832. doi: 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balcioglu YH, Kirlioglu SS. C-Reactive Protein/Albumin and Neutrophil/Albumin ratios as novel inflammatory markers in patients with Schizophrenia. Psychiatry Invest. 2020;17(9):902–910. doi: 10.30773/pi.2020.0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kocak MB, Atkaya NO, Oruc MA. Evaluation of inflammatory markers obtained from complete blood count in different stages of schizophrenia. Curr. Med. Res. Opin. 2024;40(8):1413–1419. doi: 10.1080/03007995.2024.2378180 [DOI] [PubMed] [Google Scholar]
- 40.Sangani R, Deepak V, Anwar J, Patel Z, Ghio AJ. Cigarette smoking and blood monocyte count correlate with chronic lung injuries and mortality. Int J Chron Obstruct Pulmon Dis. 2023;18:431–446. doi: 10.2147/COPD.S397667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Šagud M, Madžarac Z, Nedic Erjavec G, et al. The associations of neutrophil-lymphocyte, platelet-lymphocyte, monocyte-lymphocyte ratios and immune-inflammation index with negative symptoms in patients with Schizophrenia. Biomolecules. 2023;13(2):297. doi: 10.3390/biom13020297 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.