Abstract
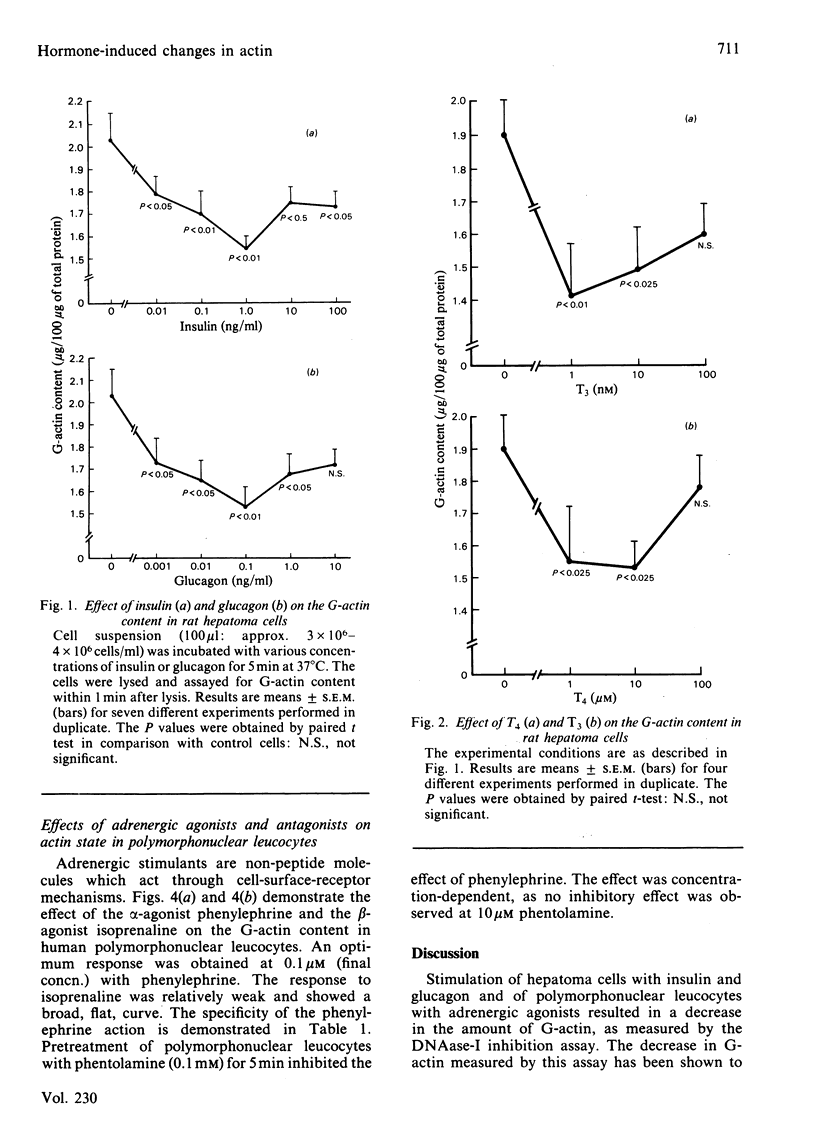
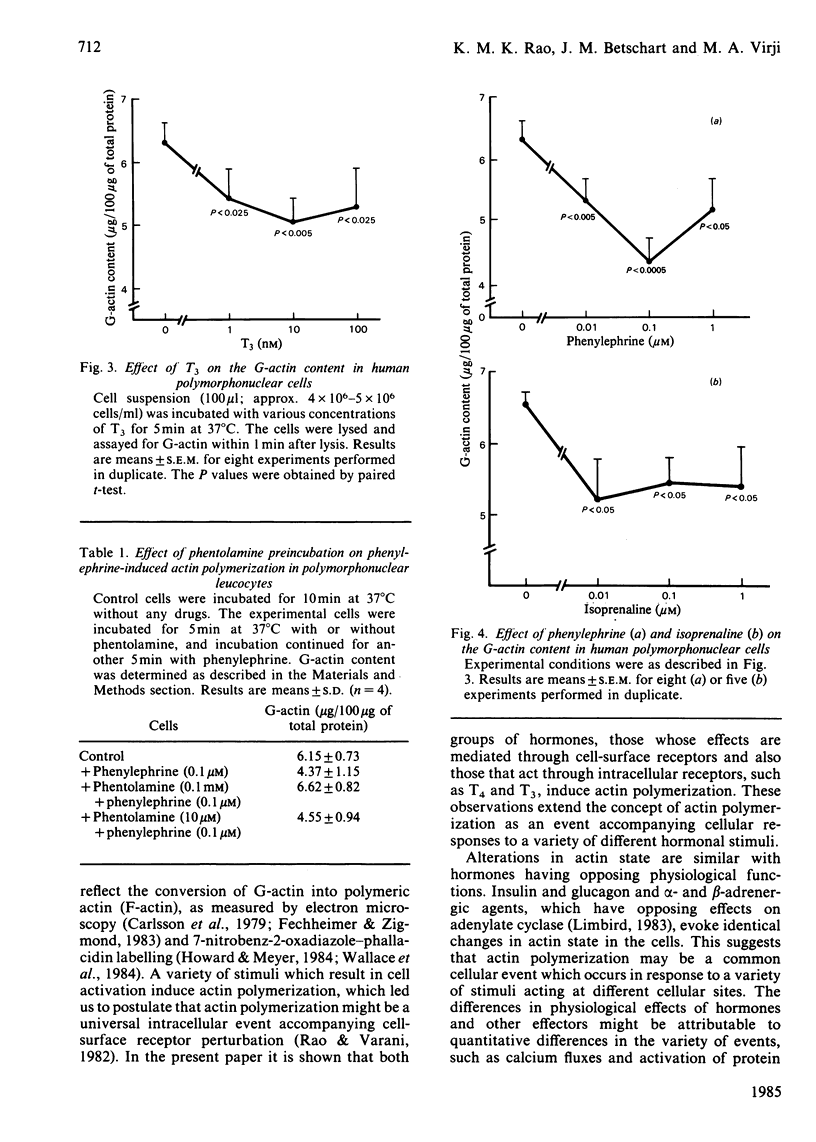
Treatment of rat hepatoma cells with insulin, glucagon, thyroxine (T4) and triiodothyronine (T3) caused a concentration-dependent decrease in the monomeric actin content as measured by the deoxyribonuclease-I inhibition assay. Similarly, human peripheral blood neutrophils responded with a decrease in monomeric actin content when stimulated with T4, T3 and the adrenergic agonists phenylephrine and isoprenaline. The effect of phenylephrine could be blocked by phentolamine, demonstrating the specificity of the interaction. These observations suggest that hormone-induced actin changes might be an important event in response to both cell-surface-reactive hormones, such as insulin, glucagon and adrenergic agents, and those hormones that act through intracellular receptors, such as thyroid hormones. It is suggested that changes in actin state may have a role in metabolic regulation and cell growth.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold H., Pette D. Binding of glycolytic enzymes to structure proteins of the muscle. Eur J Biochem. 1968 Nov;6(2):163–171. doi: 10.1111/j.1432-1033.1968.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Avivi A. On the mechanism of TSH-induced formation of follicle-like structures in primary cultures of thyroid cells. Cell Biol Int Rep. 1982 Dec;6(12):1109–1118. doi: 10.1016/0309-1651(82)90028-5. [DOI] [PubMed] [Google Scholar]
- Balter N. J., Cowden M. W., Schwartz S. L. Induction of membrane alterations by norepinephrine: studies with macrophages and phospholipids monolayers. J Pharmacol Exp Ther. 1977 Jun;201(3):627–635. [PubMed] [Google Scholar]
- Blikstad I., Markey F., Carlsson L., Persson T., Lindberg U. Selective assay of monomeric and filamentous actin in cell extracts, using inhibition of deoxyribonuclease I. Cell. 1978 Nov;15(3):935–943. doi: 10.1016/0092-8674(78)90277-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Markey F., Blikstad I., Persson T., Lindberg U. Reorganization of actin in platelets stimulated by thrombin as measured by the DNase I inhibition assay. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6376–6380. doi: 10.1073/pnas.76.12.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheitlin R., Ramachandran J. Regulation of actin in rat adrenocortical cells by corticotropin. J Biol Chem. 1981 Apr 10;256(7):3156–3158. [PubMed] [Google Scholar]
- De Robertis E. M., Longthorne R. F., Gurdon J. B. Intracellular migration of nuclear proteins in Xenopus oocytes. Nature. 1978 Mar 16;272(5650):254–256. doi: 10.1038/272254a0. [DOI] [PubMed] [Google Scholar]
- Fayet G., Michel-Béchet M., Lissitzky S. Thyrotrophin-induced aggregation and reorganization into follicles of isolated porcine-thyroid cells in culture. 2. Ultrastructural studies. Eur J Biochem. 1971 Dec 22;24(1):100–111. doi: 10.1111/j.1432-1033.1971.tb19659.x. [DOI] [PubMed] [Google Scholar]
- Fechheimer M., Zigmond S. H. Changes in cytoskeletal proteins of polymorphonuclear leukocytes induced by chemotactic peptides. Cell Motil. 1983;3(4):349–361. doi: 10.1002/cm.970030406. [DOI] [PubMed] [Google Scholar]
- Fox J. E., Phillips D. R. Inhibition of actin polymerization in blood platelets by cytochalasins. Nature. 1981 Aug 13;292(5824):650–652. doi: 10.1038/292650a0. [DOI] [PubMed] [Google Scholar]
- González A., Garrido J., Vial J. D. Epidermal growth factor inhibits cytoskeleton-related changes in the surface of parietal cells. J Cell Biol. 1981 Jan;88(1):108–114. doi: 10.1083/jcb.88.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson J. C. The effects of various hormones on the surface morphology of testicular cells in culture. Am J Anat. 1978 Jan;151(1):55–69. doi: 10.1002/aja.1001510106. [DOI] [PubMed] [Google Scholar]
- Laub F., Kaplan M., Gitler C. Actin polymerization accompanies Thy-1-capping on mouse thymocytes. FEBS Lett. 1981 Feb 9;124(1):35–38. doi: 10.1016/0014-5793(81)80048-8. [DOI] [PubMed] [Google Scholar]
- Lawrence T. S., Ginzberg R. D., Gilula N. B., Beers W. H. Hormonally induced cell shape changes in cultured rat ovarian granulosa cells. J Cell Biol. 1979 Jan;80(1):21–36. doi: 10.1083/jcb.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. H., Gould M. K. ATP-dependence of 125I-insulin binding by rat soleus muscle. Biochem Int. 1983 Feb;6(2):163–169. [PubMed] [Google Scholar]
- Limbird L. E. Beta-adrenergic stimulation of adenylate cyclase and alpha-adrenergic inhibition of adenylate cyclase: GTP-binding proteins as macromolecular messengers. Adv Exp Med Biol. 1983;161:91–111. doi: 10.1007/978-1-4684-4472-8_6. [DOI] [PubMed] [Google Scholar]
- Masters C. J. Interactions between soluble enzymes and subcellular structure. CRC Crit Rev Biochem. 1981;11(2):105–143. doi: 10.3109/10409238109108700. [DOI] [PubMed] [Google Scholar]
- Monaco G., Calissano P., Mercanti D. Effect of NGF on in vitro preformed microtubules. Evidence for a protective action against vinblastine. Brain Res. 1977 Jul 1;129(2):265–274. doi: 10.1016/0006-8993(77)90006-3. [DOI] [PubMed] [Google Scholar]
- Oppenheimer J. H. Thyroid hormone action at the cellular level. Science. 1979 Mar 9;203(4384):971–979. doi: 10.1126/science.218285. [DOI] [PubMed] [Google Scholar]
- PETERS R. A. Hormones and the cytoskeleton. Nature. 1956 Mar 3;177(4505):426–426. doi: 10.1038/177426a0. [DOI] [PubMed] [Google Scholar]
- Pribluda V., Laub F., Rotman A. The state of actin in activated human platelets. Eur J Biochem. 1981 May 15;116(2):293–296. doi: 10.1111/j.1432-1033.1981.tb05332.x. [DOI] [PubMed] [Google Scholar]
- Ramachandran J., Suyama A. T. Inhibition of replication of normal adrenocortical cells in culture by adrenocorticotropin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):113–117. doi: 10.1073/pnas.72.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. M., Varani J. Actin polymerization induced by chemotactic peptide and concanavalin A in rat neutrophils. J Immunol. 1982 Oct;129(4):1605–1607. [PubMed] [Google Scholar]
- Rapoport B., Jones A. L. Acute effects of thyroid-stimulating hormone on cultured thyroid cell morphology. Endocrinology. 1978 Jan;102(1):175–181. doi: 10.1210/endo-102-1-175. [DOI] [PubMed] [Google Scholar]
- Scheer U., Hinssen H., Franke W. W., Jockusch B. M. Microinjection of actin-binding proteins and actin antibodies demonstrates involvement of nuclear actin in transcription of lampbrush chromosomes. Cell. 1984 Nov;39(1):111–122. doi: 10.1016/0092-8674(84)90196-x. [DOI] [PubMed] [Google Scholar]
- Schubert D., LaCorbiere M., Whitlock C., Stallcup W. Alterations in the surface properties of cells responsive to nerve growth factor. Nature. 1978 Jun 29;273(5665):718–723. doi: 10.1038/273718a0. [DOI] [PubMed] [Google Scholar]
- Sigel P., Pette D. Intracellular localization of glycogenolytic and glycolytic enzymes in white and red rabbit skeletal muscle: a gel film method for coupled enzyme reactions in histochemistry. J Histochem Cytochem. 1969 Apr;17(4):225–237. doi: 10.1177/17.4.225. [DOI] [PubMed] [Google Scholar]
- Tramontano D., Avivi A., Ambesi-Impiombato F. S., Barak L., Geiger B., Schlessinger J. Thyrotropin induces changes in the morphology and the organization of microfilament structures in cultured thyroid cells. Exp Cell Res. 1982 Feb;137(2):269–275. doi: 10.1016/0014-4827(82)90027-1. [DOI] [PubMed] [Google Scholar]
- Tung P. S., Dorrington J. H., Fritz I. B. Structural changes inducted by follicle-stimulating hormone or dibutyryl cyclic AMP on presumptive Sertoli cells in culture. Proc Natl Acad Sci U S A. 1975 May;72(5):1838–1842. doi: 10.1073/pnas.72.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varani J., Wass J. A., Rao K. M. Actin changes in normal human and rat leukocytes and in transformed human leukocytic cells. J Natl Cancer Inst. 1983 May;70(5):805–809. [PubMed] [Google Scholar]
- Wallace P. J., Wersto R. P., Packman C. H., Lichtman M. A. Chemotactic peptide-induced changes in neutrophil actin conformation. J Cell Biol. 1984 Sep;99(3):1060–1065. doi: 10.1083/jcb.99.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace P. J., Wersto R. P., Packman C. H., Lichtman M. A. Chemotactic peptide-induced changes in neutrophil actin conformation. J Cell Biol. 1984 Sep;99(3):1060–1065. doi: 10.1083/jcb.99.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessells N. K., Spooner B. S., Ash J. F., Bradley M. O., Luduena M. A., Taylor E. L., Wrenn J. T., Yamada K. Microfilaments in cellular and developmental processes. Science. 1971 Jan 15;171(3967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wilson E. J., McMurray W. C. Regulation of malic enzyme and mitochondrial alpha-glycerophosphate dehydrogenase by thyroid hormones, insulin, and glucocorticoids in cultured hepatocytes. J Biol Chem. 1981 Nov 25;256(22):11657–11662. [PubMed] [Google Scholar]
- Zor U. Role of cytoskeletal organization in the regulation of adenylate cyclase-cyclic adenosine monophosphate by hormones. Endocr Rev. 1983 Winter;4(1):1–21. doi: 10.1210/edrv-4-1-1. [DOI] [PubMed] [Google Scholar]


