Abstract
Background
Strain JAM1T and strain GP59 of the methylotrophic, bacterial species Methylophaga nitratireducenticrescens were isolated from a microbial community of the biofilm that developed in a fluidized-bed, methanol-fed, marine denitrification system. Despite of their common origin, both strains showed distinct physiological characters towards the dynamics of nitrate () reduction. Strain JAM1T can reduce to nitrite () but not to nitric oxide (NO) as it lacks a NO-forming reductase. Strain GP59 on the other hand can carry the complete reduction of to N2. Strain GP59 cultured under anoxic conditions shows a 24-48h lag phase before reduction occurs. In strain JAM1T cultures, reduction begins immediately with accumulation of . Furthermore, is reduced under oxic conditions in strain JAM1T cultures, which does not appear in strain GP59 cultures. These distinct characters suggest differences in the regulation pathways impacting the expression of denitrification genes, and ultimately growth.
Methods
Both strains were cultured under oxic conditions either with or without , or under anoxic conditions with . Transcript levels of selected denitrification genes (nar1 and nar2 encoding reductases, nirK encoding reductase, narK12f encoding /transporter) and regulatory genes (narXL and fnr) were determined by quantitative reverse transcription polymerase chain reaction. We also derived the transcriptomes of these cultures and determined their relative gene expression profiles.
Results
The transcript levels of nar1 were very low in strain GP59 cultured under oxic conditions without . These levels were 37 times higher in strain JAM1T cultured under the same conditions, suggesting that Nar1 was expressed at sufficient levels in strain JAM1T before the inoculation of the oxic and anoxic cultures to carry reduction with no lag phase. Transcriptomic analysis revealed that each strain had distinct relative gene expression profiles, and oxygen had high impact on these profiles. Among denitrification genes and regulatory genes, the nnrS3 gene encoding factor involved in NO-response function had its relative gene transcript levels 5 to 10 times higher in strain GP59 cultured under oxic conditions with than those in both strains cultured under oxic conditions without . Since NnrS senses NO, these results suggest that strain GP59 reduced to NO under oxic conditions, but because of the oxic environment, NO is oxidized back to by flavohemoproteins (NO dioxygenase; Hmp), explaining why reduction is not observed in strain GP59 cultured under oxic conditions.
Conclusions
Understanding how these two strains manage the regulation of the denitrification pathway provided some clues on how they response to environmental changes in the original biofilm community, and, by extension, how this community adapts in providing efficient denitrifying activities.
Keywords: Denitrification, Methylophaga, Gene expression, Transcriptome, RT-qPCR, Nitrate reduction dynamics, Species sub-population
Introduction
Members of the genus Methylophaga are gammaproteobacteria that are common in marine or brackish water. As methylotrophic bacteria, they only use one-carbon compounds, for instance methanol, methylamine or dimethyl sulfide, as carbon and energy sources for methylotrophic growth (Boden, 2012; Boden, 2019). From a naturally occurring biofilm that developed in a fluidized-bed, methanol-fed denitrification system operating in continuous mode, and treating seawater tank at the natural science museum of the Montreal Biodome (Labbé et al., 2003; Labbé, Parent & Villemur, 2003; Parent & Morin, 2000; Labbé et al., 2007; Laurin et al., 2008), two strains belonging to the species Methylophaga nitratireducenticrescens were isolated: strain JAM1T and strain GP59 (Auclair et al., 2010; Villeneuve et al., 2013; Mauffrey, Martineau & Villemur, 2015; Mauffrey et al., 2017; Geoffroy et al., 2018). These strains are the only known Methylophaga species capable to grow under anoxic conditions with nitrate () as terminal electron acceptor. Strain GP59 possesses the complete denitrification pathway, whereas strain JAM1T lacks a NO-forming nitrite () reductase activity. Their genomes have about 90% identity in nucleic acid sequences, and share the same denitrification island, a 66.5 kb region containing operons or gene clusters encoding two Nar-type reductases (Nar1 and Nar2 systems; EC 1.7.5.1), two nitric oxide (NO) reductases (Nor1 and Nor2 systems; EC 1.7.2.5) and one nitrous oxide (N2O) reductase (Nos system; EC 1.7.2.4). This region also encodes three transporters (NarK1, NarK2, NarK12f) and regulatory factors such as NarX/NarL, NosR and NorRE, and NnrS involved in NO-response. Whereas no gene encoding a NO-forming reductase (NirK- or NirS-type) is present in strain JAM1T genome, a gene encoding the reductase NirK (EC 1.7.2.1) is found in another region of strain GP59 genome. Based on the repeat pattern found in their respective CRISPR locus, one strain did not evolve recently from the other but rather originate from a common ancestor (Geoffroy et al., 2018).
We showed in previous reports (Geoffroy et al., 2018; Payette et al., 2019; Villemur et al., 2019) that strain GP59 and strain JAM1T have different growth dynamics in the biofilm community upon environmental changes. In the original biofilm in the Biodome denitrification system, the proportion of strain JAM1T was higher than that of strain GP59. However, when this biofilm was cultured under laboratory-scale anoxic conditions (in vials, batch-fed mode instead of continuous mode, with artificial seawater medium instead of the commercial Instant Ocean medium), strain GP59 increased dramatically in proportion in the biofilm, while the level of strain JAM1T stayed the same. From these accumulated data, we believe that those two subpopulations of M. nitratireducenticrescens display distinct physiological characters in response to environmental changes in the biofilm.
Pure cultures of strain JAM1T and strain GP59 also demonstrate different dynamics in their denitrification activities that impact their growth. Under anoxic conditions, strain GP59 requires a lag time of 24 to 48 h before reduction occurs. Such lag time is not apparent in strain JAM1T anoxic cultures and begins to be reduced almost immediately (Geoffroy et al., 2018). Besides, strain GP59 cultures generate higher biomass yield than strain JAM1T under anoxic conditions because of the completeness of reduction to N2, unlike strain JAM1T where toxic accumulates in the medium. Both strains respond differently when cultured under oxic conditions in presence of . Although cultures from both strains generate equivalent growth yield under oxic conditions, strain JAM1T cultures can reduce to (Mauffrey et al., 2017), which does not appear in strain GP59 cultures (Geoffroy et al., 2018). Among possible mechanisms explaining these distinct behaviors, we hypothesized that one of them involves the control of the gene expression of the denitrification pathway that operates differently in both strains.
The control of denitrification is carried out by a complex network at the transcriptional level. This network involves CRP/FNR family transcriptional regulators and specific two-component systems, in response to various signals perceptible in the bacterial environment (Körner, Sofia & Zumft, 2003; Gaimster et al., 2017; Durand & Guillier, 2021). For instance, FNR intervenes in response to low level of oxygen, in E. coli, by controlling hundreds of genes, including the nar operon (narGHJI), by binding in upstream sequence (fnr box) of target genes (Zumft, 1997; Körner, Sofia & Zumft, 2003; Constantinidou et al., 2006; Van Spanning, Richardson & Ferguson, 2007). NarX/NarL is a two-component system that senses the presence of by stimulating the expression of the nar operon. Putative FNR and NarL nucleic acid binding sites upstream of narXL and of both nar operons were found in strain JAM1T genome (Mauffrey, Martineau & Villemur, 2015).
Besides genes encoding NorRE and NosR that regulate the expression of genes encoding the Nor and Nos systems, respectively, open reading frames (ORFs) encoding putative regulators or proteins with NO-response function were found in both genomes, which include NnrS involved in response to NO, the -sensitive transcriptional repressor NsrR (Rrf2 family transcriptional regulator), flavohemoprotein (NO dioxygenase; hmp) (EC 1.14.12.17), and DnrN/YtfE known to be involved in iron-sulfur cluster repair di-iron protein or to be NO-dependent regulator (Körner, Sofia & Zumft, 2003; Spiro, 2011; Stern et al., 2013; Guo & Gao, 2021).
To test our hypothesis, we aimed by different culture conditions to measure the expression levels of key genes that could have impacted the denitrifying activities of these strains. Pure cultures from both strains were performed to get synchronized culture replicates under oxic or anoxic conditions with . The expression levels of selected genes were measured early in culture growth with minimal reduction to assess early regulation of denitrification genes, or halfway through of this reduction to assess the evolution of this regulation. Total RNA was extracted from culture replicates, and by using reverse transcription quantitative polymerase chain reaction (RT-qPCR) and RNA sequencing, we compared the expression levels of genes involved in the denitrification pathway between these types of cultures for both strains. Deciphering the regulation mechanisms of both strains will shed some light on how they response to environmental changes in the original biofilm community. By extension, our results will provide new knowledge on the dynamism of species’ subpopulations in other bioprocesses.
Material and Methods
Culture medium and conditions
M. nitratireducenticrescens strains JAM1T and GP59 were cultured in the Methylophaga medium 1403 (per 970 mL: 24 g NaCl, 3 g MgCl2.6 H2O, 2 g MgSO4.7H2O, 0.5 g KCl, 1 g CaCl2, 0.5 g Bistris, pH 8.0). This medium was autoclaved before the addition of sterilized solutions: methanol (three mL per liter medium), stock solution T (20 mL per liter medium), 0.1 mg mL−1 vitamin B12 (one mL per liter medium), and stock Wolf’s mineral solution (10 mL per liter medium). When needed, the media was supplemented with 21.4 mM sodium (final concentration). Stock solution T was made of (per 100 mL): 0.7 g KH2PO4, 10 g NH4Cl, 10 g Bistris, 0.3 g Ferric ammonium citrate, pH 8. Stock Wolf’s mineral solution was made of (per 1000 mL): 0.5 g EDTA, 3 g MgSO4.7H2O, 0.5 g MnSO4.H2O, 1 g NaCl, 0.1 g FeSO4.7H2O, 0.1 g CoCl2.6H2O, 0.1 g CaCl2, 0.1 g ZnSO4.7H2O, 0.01 g CuSO4.5H2O, 0.01 g AlK(SO4)2.12H2O, 0.01 g H3BO3, 0.01 g Na2MoO4.2H2O [from American Type Culture Collection, Manassas VI, USA]. The final concentration of was 3.8 mM.
Cultures were carried out under three conditions: anoxic with 21.4 mM (here named < <AN > > conditions), oxic with 21.4 mM (here named < <ON > > conditions) and oxic without (here named < <O > > conditions). These conditions were chosen to assess the impact of oxygen (presence or absence) on the gene expression patterns in both strains. We set two different sampling times in the < <AN > > cultures: one with minimal reduction (<10%) here named “Low period” and the other one during high rate of reduction (20 to 50% reduction) here named “High period”.
Anoxic cultures (< <AN > > conditions) were carried out in 30-mL (High period) or 300-mL (Low period) medium in 70-mL or 500-mL serum vials, respectively. Bottles were sealed with rubber stoppers maintained by a metal ring or sterile septum caps, and medium was purged for 10-15 min with pure nitrogen gas, prior to sterilization. Oxic cultures (< <O > > and < <ON > > conditions) were carried out in 30-mL (High period) or 300-mL (Low period) medium in 250-mL or 1000-mL Erlenmeyer flasks, respectively.
The bacterial inoculum was made of a fresh oxic culture without (< <O > > conditions). All cultures were inoculated at a final optic density 600 nm (OD 600) between 0.05 and 0.1, and incubated at 30 °C, under a constant agitation at 150 rpm. Samples were taken to monitor growth by spectrophotometry (OD 600). These samples were homogenized using a potter-Elvehjem homogenizer prior to measurements to disperse the flocs formed during the growth. Preliminary cultures were performed to determine the correspondence between the culture growth (OD 600) and the level of remaining in cultures. The dynamics of growth and of and reductions occurred as reported before (Geoffroy et al., 2018) (lag phase under the < <AN > > conditions and no reduction under the < <ON > > conditions for GP59 cultures; see Data S1).
We then performed independent cultures (from different inocula and different days), from which total biomass (sacrificed cultures) was collected at prescribed times based on preliminary culture assays, and preserved at −70 °C. The residual concentrations of and in the < <ON > > and < <AN > > cultures were measured later from the supernatants by ion chromatography with the 850 Professional IC (Metrohm, Herisau, Switzerland) or by colorimetric assays described by Cucaita, Piochon & Villemur (2021). From these latter measurements, we chose at least three frozen samples that fit our criteria of reduction to perform RNA extraction. The chosen cultures of strain JAM1T cultured under the < <AN > > and < <ON > > conditions, and cultures of strain GP59 cultured under the < <AN > > conditions showed around 6% reduction during the Low period or around 25% reduction during the High period (Table 1). As reduction does not occur in strain GP59 cultured under the < <ON > > conditions, biomass was collected when cultures reached about the same times than strain JAM1T cultures at the Low and High periods. Under the < <O > > conditions, samples for both periods were collected for each strain around the same time as the < <ON > > conditions (Table 1).
Table 1. Concentration of that was reduced during the Low and High periods.
| Culture conditions | Phase | (mM) | Growth (OD600) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| JAM1T | GP59 | Final | |||||||
| Initial | Final | Initial | Final | JAM1T | Time | GP59 | Time | ||
| < <AN > > | Low | 15.7 (0.2) | 14.7 (0.2) | 22.7 (0.5) | 21.3 (0.2) | 0.08 | 0.5 h | 0.09 | 8 h |
| < <ON > > | Low | 21.0 (0.1) | 19.8 (0.5) | 18.8 (0.0) | 18.7 (0.0) | 0.08 | 0.5 h | 0.08 | 1 h |
| < <O > > | Low | NA | NA | NA | NA | 0.07 | 0.5 h | 0.09 | 1 h |
| < <AN > > | High | 16.2 (0.0) | 12.3 (0.0) | 17.7 (0.1) | 12.9 (0.3) | 0.27 | 15 h | 0.34 | 96 h |
| < <ON > > | High | 12.3 (0.0) | 8.9 (0.2) | 16.8 (0.1) | 17.2 (0.1) | 0.88 | 13 h | 0.53 | 14 h |
| < <O > > | High | NA | NA | NA | NA | 0.91 | 15 h | 0.51 | 15 h |
Notes.
Cultures were inoculated at 0.05 to 0.1 OD600, and samples were immediately taken to measure (Initial). After prescribed times (estimated from prior culture tests), the whole cultures were taken (Final) to collect the biomass and to measure and OD600. Values are from triplicate cultures with standard deviation under parentheses.
- < <AN > >
- anoxic conditions
- < <O > >
- oxic conditions, no
- < <ON > >
- oxic conditions with
- NA
- not applicable
RNA extraction and RT-qPCR assay
The biomass of the 300-mL cultures (Low period) was collected by filtration on a 0.22 µm filter. The biomass of the 30-mL cultures (High period) was collected by centrifugation at 8000 × g for 10 min at 4 °C. The pellets or filters were transferred in 2-mL tubes containing 250 mg of 0.2 mm glass beads, then one mL of extraction buffer (50 mM Tris–HCl, 100 mM EDTA, 150 mM NaCl pH 8.0) and one mL of water-saturated phenol (pH 4.3) were added. The samples were flash frozen in liquid nitrogen and stored at −70 °C until extraction. Sample collection of the anoxic cultures was carried out in controlled airtight chamber flushed with nitrogen gas.
The RNA extraction was previously described by Mauffrey, Martineau & Villemur (2015). Because low amount of biomass was produced by the Low period cultures (despite the 300-mL cultures), total RNA extracted from these cultures allowed us to carry all RT-qPCR assays, but not the transcriptomes. The High period cultures generated much higher amount of biomass, and thus RNA, allowing to carry RT-qPCR assays and transcriptomes from replicate cultures. RNA quality was verified by agarose gel electrophoresis (Fig. S1) and by spectrophotometry (Nanodrop) with ratio OD260/OD280 >1.8. The absence of residual DNA in the RNA samples was verified by end-point PCR using the same primers chosen for the RT-qPCR assays (Table 2). cDNA was synthetized with the Reverse Transcription System according to the manufacturer (Promega, Madison, WI, USA), using 800 ng of RNA and hexameric primers. A reaction with no template was made as negative control. cDNA samples were preserved at −70 °C.
Table 2. Primers used for RT-qPCR assays.
| Name | Sequence (5′–3′) | Hybridization Temp (°C) | Length nt | References |
|---|---|---|---|---|
| Standard genes | ||||
| rpoD (10F) | CAGCAATCACGCGTTAAAGA | 60 | 144 | Mauffrey, Martineau & Villemur (2015) |
| rpoD (153R) | ACCCAGGTCGCTGAACATAC | |||
| rpob (3861F) | TGAGATGGAGGTTTGGGCAC | 60 | 146 | Mauffrey, Martineau & Villemur (2015) |
| rpob (4006R) | GCATACCTGCATCCATCCGA | |||
| dnaG (774F) | CATCCTGATCGTGGAAGGTT | 60 | 121 | Mauffrey, Martineau & Villemur (2015) |
| dnaG (894R) | GCTGCGAATCAACTGACGTA | |||
| Regulatory genes | ||||
| narX1 (1403F) | TGCTGAAGCCCTACAAGTGG | 60 | 133 | This study |
| narX1 (1535R) | TGCGTTAGCGATAGCACCTT | |||
| narL1 (174F) | ATGCCGGGAATAGGAGGAGT | 60 | 136 | This study |
| narL1 (309R) | AATAACCGCGGGCACCATTA | |||
| fnr2 (121F) | ACCGGCTATGTCTACCGTTG | 60 | 149 | This study |
| fnr2 (269R) | CGAGCCTGAGCGAACAACAA | |||
| Selected denitrification genes | ||||
| qnarG1-F | AGCCCACATCGTATCAAGCA | 61 | 149 | Geoffroy et al. (2018) |
| qnarG1-R | CCACGCACCGCAGTATATTG | |||
| narK12 (257F) | TTCTGATCTGCCCGAACTCT | 60 | 106 | Mauffrey, Martineau & Villemur (2015) |
| narK12 (362R) | GCGCCTAGCAATGCTTTTAC | |||
| narG2 (597F) | TTACGCTGCAGGATCACGTT | 60 | 127 | Mauffrey, Martineau & Villemur (2015) |
| narG2 (723R) | TGACTCGGGTACATCGGTCT | |||
| qnirK-F | AAGTCGGTAAAGTAGCCGTTGA | 55 | 138 | Geoffroy et al. (2018) |
| qnirK-R | TCTCCATCGTCATTTGAACAAC |
Quantification of amplified PCR products was performed with the PerfeCTa® SYBR® Green SuperMix ROXTM (Quanta BioSciences) in the C1000 Touch Thermal cycler real-time PCR machine (Bio-Rad Laboratories, Hercules, CA, USA). The mix for one reaction was composed of 10 µL PerfeCTa® SYBR® Green SuperMix, 40 pmoles of each primer (Table 2), 25 ng cDNA and RNA-free water in a final volume of 20 µL. Primer sequences (Table 2) for narX (Q7A_444), narL (Q7A_445), fnr (Q7A_307), narG1 (Q7A_446), narG2 (Q7A_484), narK12f (Q7A_479) and nirK (CDW43_15165, strain GP59) genes were retrieved from the strain JAM1T genome (GenBank accession number CP003390.3) and strain GP59 genome (CP021973.1). Except for nirK, the amplified region of respective genes has identical sequences in both genomes. Three reference genes (Rocha, Santos & Pacheco, 2015) were selected and used for the data normalization: rpoD (sigma factor, GenBank accession number Q7A_343), rpoB (RNA polymerase β subunit, Q7A_2329), dnaG (primase, Q7A_342). The program run 5 min at 95 °C, then 40 cycles: hybridization temperature (Table 2) for 15 s, 20 s at 72 °C and 10 s at 95 °C. The specificity of the reaction was verified by collecting the fluorescence through a melting curve by raising the temperature between 65 and 95 °C. The respective genome was used as template to derive the standard curve. Standard curves were performed with 10-fold serial dilutions in PCR grade water, resulting in a concentration gradient of 107 to 100 copies per reaction. The experiment was validated with efficiency values >85% and r2 values between 0.90 and 1.1. No template controls were performed, with results ranging from 0 to 2 gene copies per reaction. Inhibition was verified by amplification of the normalization genes.
For both strains in each period and culture conditions, RNA was extracted from at least three cultures. RT-qPCR assays were performed in duplicate for each sample. The number of copies per reaction was normalized with the levels of each normalization gene. The transcript levels were expressed as the number of copies per 100 copies of normalized genes. The results of narX and narL were combined and averaged.
Sequences analysis
Sequences of the chromosomic region that corresponds to the denitrification island from both strains were aligned (pairwise local alignment) by Bioedit (7.2.3). Searches for DNA binding sites of (i) potential NarL, (ii) factor for inversion stimulation (FIS), (iii) integration host factor (IHF) and (iv) fumarate and reductase regulon (FNR) were carried out with the Virtual Footprint software of PRODORIC (https://bio.tools/prodoric) (Dudek & Jahn, 2022).
Transcriptome analysis
RNA samples retrieved from the three independent cultures at the High period were sent for sequencing using the Illumina Method (NovaSeq 6000 S4 PE100). Library preparation and sequencing were performed by the Centre d’expertise et de services Génome Québec (Montréal, QC, Canada). Ribosomal RNA (rRNA) were depleted using the Ribo-ZeroTM rRNA Removal Kit (Meta-Bacteria; Epicentre, Madison, WI, USA). The number of reads per replicate ranged from 6.9 to 78.7 millions. RNAseq reads from strain JAM1T and strain GP59 cultured under the < <AN > > conditions were deposited in Sequence Read Archive (SRA; National Center for Biotechnology Information [NCBI]: https://www.ncbi.nlm.nih.gov/) under the bioproject number PRJNA525230 (SRX5461036, SRX5461037, SRX5461044, SRX5461045, SRX5461047, SRX5461048). For those cultured under the < <O > > and < <ON > > conditions, RNAseq reads were deposited in SRA under the bioproject number PRJNA1072961 (SAMN39755713 to SAMN39755725).
Raw sequencing reads were trimmed using fastq_quality_filter (1.0.2) to remove low quality reads (score < 20) (Gordon & Hannon, 2010). The paired reads were then merged and aligned with the reference genome of M. nitratireducenticrescens strain JAM1T or GP59 using Bowtie2 (v 2.5.0) (Langmead & Salzberg, 2012), and annotated with Bedtools (v 2.30.0) (Quinlan & Hall, 2010). The power analysis calculation (alpha = 0.05, effect = 2) were carried out on all the genes of the triplicate cultures of each condition, using the transcript per million (TPM) values as sequencing depth (online at https://rodrigo-arcoverde.shinyapps.io/rnaseq_power_calc/). The power ranged from 0.96 to 1. Genes that were significantly differentially expressed were identified by EdgeR (v 3.36.0) (Robinson, McCarthy & Smyth, 2010) with trimmed mean of M values (TMM) method to normalize library sizes (robust = TRUE; P-value adjusted threshold = 0.05; P-value adjusted method = Benjamini and Hochberg). All these analyses were performed on the Galaxy server (https://usegalaxy.org/). Genes were considered differentially expressed when the false discovery rate (FDR) was ≤ 0.05.
High proportion of reads that did not align with the strain GP59 genome were found in the RNA samples. RNA preparation from strain GP59 cultures were sequenced at the same time than those of strain JAM1T cultures that generated <1% unaligned reads to strain JAM1T genome. In addition, RNA from replicate #3 of strain GP59 cultured under the < <O > > conditions was resequenced and showed again high proportion of unaligned reads. All these results rule out sequence contamination. These “unaligned” reads were assembled de novo by Trinity (v. 2.15.1) or Megahit (1.2.9) at the Galaxy server. Estimation of the transcript abundance of the de novo assembled sequences was performed (Galaxy server) using RSEM as abundance estimation method (Li & Dewey, 2011). Three long transcripts with the highest transcript abundance were examined for putative ORFs with ORF finder (NCBI). ORFs were compared to databases by Blastp at NCBI.
Statistical analysis
Two-way ANOVA were performed on RT-qPCR with log10-transformed transcript levels with Tukey posttest (GraphPad Prism version 10.2.1). Outliners were identified and removed from the analysis (GraphPad Prism). Relative expression profiles of genes common to strain JAM1T and strain GP59 were analyzed by Principal coordinate analysis (PCoA) (Canoco version 5.15; Ter Braak & Šmilauer, 2018), with Log (1 * X + 1) calculating matrix of distances, using percentage difference (Bray-Curtis distance), and PERMANOVA were performed for significance with 999 permutations (Anderson, 2017).
Results and Discussion
Transcript levels of denitrification and regulatory genes
The transcript levels of key genes involved in the denitrification metabolism were measured by RT-qPCR: narG1 and narG2 (nar1 and nar2 operons; reductases), narK12f (/ transporter), nirK ( reductase; only strain GP59), narXL and a gene encoding a CRP/FNR family transcriptional regulator here named fnr (43% identity/66% similarity with E. coli FNR in deduced amino acid sequence). The nar1 polycistronic operon includes genes encoding Nar(1)GHJI, and the NarK1 and NarK2 transporters, as demonstrated by Mauffrey, Martineau & Villemur (2015). The transcript levels of nor1, nor2 and nos were not determined because strain JAM1T cannot carry out the reduction of to NO, making comparisons between strains of transcript levels for those genes inconclusive.
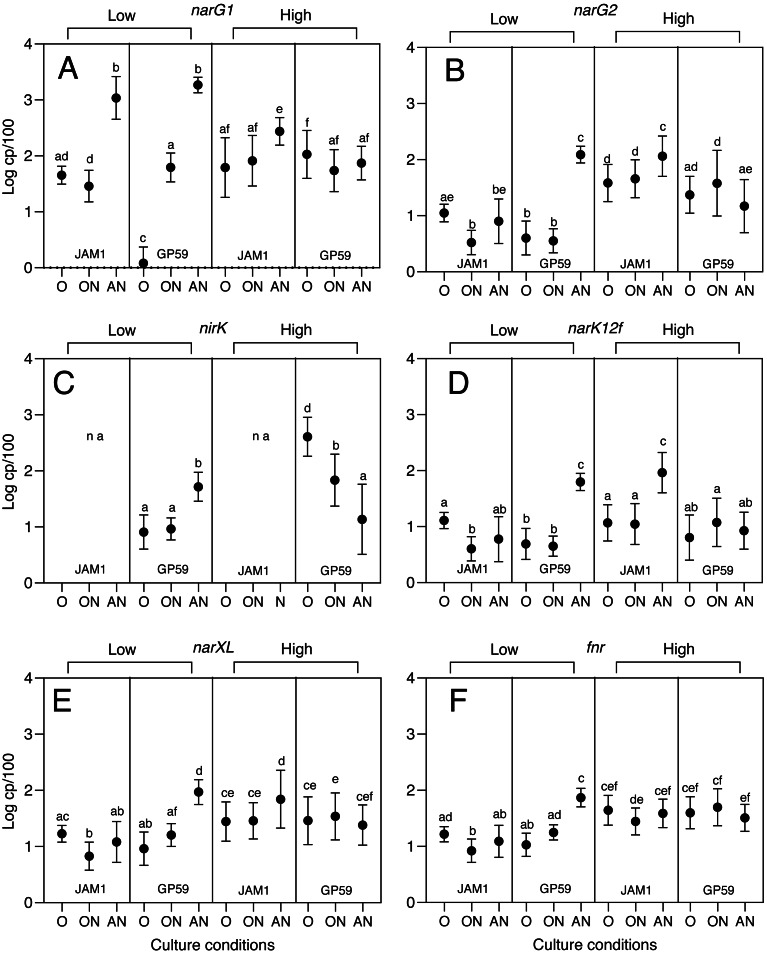
During the Low period (minimum reduction), narG1 had a very low transcript levels at 1.2 copies per 100 normalized genes (cp/100) in strain GP59 cultured under the < <O > > conditions, compared to 45 cp/100 for strain JAM1T (p < 0.0001; Fig. 1). The anoxic conditions (< <AN > >) stimulated the narG1 expression in both strains where they reached similar levels (1090 vs 1850 cp/100; p = 0.082). Such gene expression stimulation was apparent at a lesser extent (62 cp/100) in the < <ON > > cultures for strain GP59, which reached similar levels than those in strain JAM1T < <ON > > cultures (80 cp/100). Contrary to GP59 cultures, the narG1 transcript levels were not different between the < <O > > and < <ON > > conditions (45 vs 80 cp/100; p = 0.128) in strain JAM1T cultures.
Figure 1. Transcript levels of selected denitrification genes and regulatory genes in cultures of strain JAM1T and strain GP59.
Strains JAM1T and strain GP59 were cultured under oxic (< <O > >), oxic with (< <ON > >) and anoxic (, no oxygen; < <AN > >) conditions. Total RNA was extracted from replicate cultures and RT-qPCR were performed. The transcript levels are expressed as the number of gene copies per 100 copies of normalized genes (cp/100). Two-way ANOVA tests with Tukey post hoc tests were performed on log10-transformed transcript levels. Values represented by different letters are highly significantly different (p < 0.0001) across culture conditions and strains. Values represented by the same letter are considered not highly significantly different. For instance, in panel A, Low period: the < <AN > > conditions between strain JAM1T and strain GP59 showed no difference (same letter “b”). Strain JAM1T under the < <O > > conditions (letters “a and d”) showed no difference with strain JAM1T under the < <ON > > conditions (letter “d”) and with strain GP59 under the < <ON > > conditions (letter “a”). However, strain JAM1T under the < <ON > > conditions (letter “d”) was different then strain GP59 under the < <ON > > conditions (letter “a”). Data represent mean log10 values ± SD. na, not applicable.
narG2, narK12f, narXL and fnr had similar transcript levels (3.3 to 17 cp/100) in strain JAM1T cultured under the three conditions (Fig. 1). Contrary to strain JAM1T cultures, these five genes had higher transcript levels (63 to 123 cp/100) in strain GP59 cultured under the < <AN > > conditions than under the < <O > > and < <ON > > conditions (3.6 to 18 cp/100; Fig. 1). For nirK in strain GP59 cultures, its transcript levels were 6 times higher under the < <AN > > conditions (52 cp/100) than under the other conditions (average 8.6 cp/100) (Fig. 1).
During the High period (25% reduction), narG1, narG2, narK12f and narXL had higher transcript levels (2.5 to 8 times) under the < <AN > > conditions compared to the other conditions in strain JAM1T cultures (Fig. 1). For the fnr transcript levels, strain JAM1T showed no significant differences between the three conditions. Surprisingly, the transcript levels of nirK were 30 and 5 times higher in strain GP59 cultured under the < <O > > and < <ON > > conditions, respectively, than under the < <AN > > conditions. For the other genes, their transcript levels were at similar levels in strain GP59 cultured under the three conditions (Fig. 1).
The regulation of the expression of the Nar1 system appears to be one of the key elements of the different dynamics of reduction between strain JAM1T and strain GP59 during the Low period. The < <O > > conditions affected more the expression of the nar1 operon in strain GP59 cultures, in which its transcript levels were 37.5 times lower than those in strain JAM1T cultures. As the inocula of both strains were cultured under the < <O > > conditions, the Nar1 system, including the two transporters (NarK1 and NarK2), was probably already expressed in the inocula of strain JAM1T, which explains no latency in reduction under the < <AN > > and < <ON > > conditions. Because of the very low expression levels of the Nar1 and Nar2 systems under the < <O > > conditions in the strain GP59 inocula, both nar operons (or at least one) have to be induced and the reductase(s) and transporters produced under the < <ON > > and < <AN > > conditions for reduction to occur. However, this does not explain why reduction was not observed in strain GP59 cultured under the < <ON > > conditions as it does in strain JAM1T cultures, because their respective cultures reached the same nar1 transcript levels under these conditions during the Low period.
We did not observe increases of the fnr transcript levels under the < <AN > > conditions for the strain JAM1T cultures compared to the < <O > > and < <ON > > conditions either during the Low or the High period. This lack of stimulation during the Low period correlates with the absence of stimulation of the expression of narXL, narG2 and narK12f, but not with the narG1 expression pattern, where strong stimulation of its expression occurred under the < <AN > > conditions. During the High period, although the fnr transcript levels did not raise in strain JAM1T cultured under the < <AN > > conditions, narXL did, which in turn stimulated the expression of narG1, narG2 and narK12f.
The opposite behavior occurred in strain GP59 cultures where increases of the fnr transcript levels under the < <AN > > conditions during the Low period concur with increases of those of narXL, and the stimulation of the expression of the other genes under these conditions. In addition, during the High period, fnr did not increase its transcript levels under the < <AN > > conditions, which again concurs with the lack of stimulation of narG1, narG2, narXL and narK12f (nirK will be discussed later). Our results suggest that the regulation of denitrification genes in strain GP59 follows the expected control by FNR and NarXL observed in other bacterial species.
The level of the Fnr protein in cells does not have to change when the cultures switch from oxic to anoxic conditions as FNR shifts from an inactive (monomer) to active (dimer) configuration (Mettert & Kiley, 2018). Deduced amino acid sequences of fnr from both strains showed only one amino acid substitution (leucine for isoleucine), and no nucleotide substitution upstream of the fnr start codon, which suggests that differences in the expression pattern of the denitrification genes between both strains do not lie on the function of FNR.
Differences in genome sequences
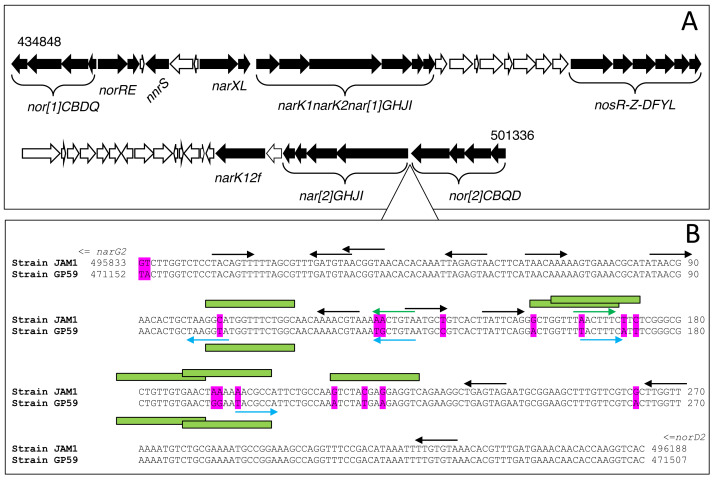
We compared the nucleic sequences of the denitrification island (66 591 nt; Fig. 2A) between each strain looking for substitutions that may provide some indications explaining the differences in gene expression profiles. We found only 44 nt substitutions (Data S2). None of these substitutions were located upstream of narXL, which contains two putative FNR DNA binding sites, and none in the intergenic region between narXL and the nar1 operon. Like fnr, differences in the gene expression pattern between both strains do not lie either on the function of NarX/NarL.
Figure 2. Chromosomic arrangement of the denitrification island.
(A) Black arrows, Denitrification and regulatory genes. (B) Intergenic sequence (354 nt) between the nar2 and nor2 operons. Differences between strain JAM1T and strain GP59 sequences are highlighted in magenta. Arrows pointing right represent putative NarL binding sites in the forward sequence; arrows pointing left are sites in the reverse sequence. Blue arrows are putative NarL binding sites specific to strain GP59; green arrows specific to strain JAM1T. Black arrows are NarL binding sites common to both strains. Green boxes are putative FIS binding sites (only illustrated in the 100–230 region). Coordinates are from GenBank accession number CP003390.3 for strain JAM1T genome and CP021973.1 for strain GP59 genome.
The most noticeable changes were found in the intergenic region (354 nt) between the nor2 and nar2 operons, where the promotor of the nar2 operon might be located, with 17 nt substitutions (Fig. 2B). No putative FNR binding site was found, but several potential NarL binding sites were. Among these NarL sites, five of them were affected by one or two substitutions. In addition, several of these substitutions are located in putative FIS (Fig. 2B) and IHF (not illustrated) binding sites. These substitutions may have influenced the interaction between the binding site and NarL (Maris et al., 2002; Maris et al., 2005). Therefore, higher transcript levels of narXL and fnr in strain GP59 cultured under the < <AN > > conditions combined with proper NarL binding sites may explain the different nar2 expression profiles between both strains during the Low period.
We showed in a previous work by Mauffrey, Martineau & Villemur (2015) that knocking out the narG2 sequence (Nar2 system; not the upstream non-coding sequence) in strain JAM1T impacted the expression of the nar1 operon. Contrary to the wild-type strain, this strain JAM1T mutant, only expressing the Nar1 system, presented a lag phase (as strain GP59) under the < <AN > > conditions, both for growth and reduction. Furthermore, this mutant cultured under the < <O > > conditions showed the narG1 transcript levels decreased by 27 times compared to wild type, levels comparable to those of strain GP59 cultured under the same conditions. All these results point out the complexity of the regulation of the nar1 operon that involves an unknown mechanism linked to the regulation of the nar2 operon. Part of the puzzle might include the differences in the upstream sequences of nar2 affecting the affinity of NarL. Other factors that could be involved are FIS and IHF factors, which are well-known DNA binding proteins. There roles comprise compaction, bending of DNA and often bind in regulatory sequences (Anuchin et al., 2011). Substitutions observed in the nar2-nor2 intergenic sequences would affect some of these putative binding sites. It was shown in E. coli that NarL and FIS compete on promoters, thus affecting gene expression (Squire et al., 2009; Browning, Butala & Busby, 2019). However, in our case, how these factors affect the expression of the nar1 operon 30 kb distant remains unclear.
Even though the control of denitrification happens mostly at the transcriptional level, post transcriptional or translational regulation might play a significant role in the overall process. The involvement of small regulatory bacterial RNA (sRNAs) has been explored in the denitrification pathway. It has been suggested that a sRNA plays a role in modulation the denitrification pathway in Paraccocus denitrificans (Gaimster et al., 2019), and a potential candidate has been isolated in Pseudomonas aerugonisa (Tata et al., 2017).
Influence of culture conditions on the transcriptomes
Transcriptomes were derived from replicate cultures of both strains cultured under the < <O > >, < <ON > > and < <AN > > conditions and sampled during the High period, and their relative gene expression profiles were compared. Strains JAM1T and GP59 share 2802 coding sequences and 11 riboswitches, having highly, if not 100%, identity in their nucleic acid sequences (Data S3). Among these genes and riboswitches, the lowest changes in relative expression profiles were found between the < <O > > and the < <ON > > conditions in strain JAM1T cultures (JO/JON, 2.6%; Fig. S2), and between strain JAM1T and strain GP59 cultured under the < <O > > conditions (JO/GO, 2.7%; Fig. S2), whereas the highest changes were found between the strain JAM1T cultured under the < <AN > > conditions and strain GP59 cultured under the < <O > > and < <ON > > conditions (JAN/GO, JAN/GON, 30%; Fig. S2).
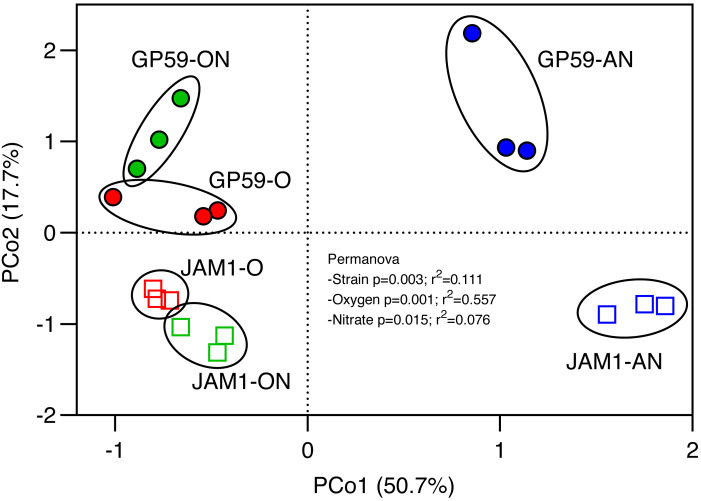
Principal coordinate analysis (PCoA) was performed with the relative transcript profiles of the common genes for the three culture conditions (Fig. 3). PCoA revealed distinct relative transcript profiles between the < <O > > and < <ON > > cultures and the < <AN > > cultures for both strains (explaining by 50.7% variations; p = 0.001). PCoA also revealed distinct profiles between strain GP59 and strain JAM1T (second axis, explained by 17.7% variations; p = 0.003). These results showed the relative expression profiles were strain specific, and that the presence of oxygen had a deep impact on these profiles in both strains.
Figure 3. Principal coordinate analysis of the relative transcript profiles of the culture replicates.
The relative transcript levels of genes and riboswitches (2813 sequences) common in both genomes were derived from the transcriptomes of culture replicates of strain JAM1T and strain GP59 cultured under the < <O > >, < <AN > > and < <ON > > conditions. Principal coordinates analysis was carried out using Bray-Curtis distance calculation, and permutation test (PERMANOVA) were performed with 999 permutations.
In addition to common genes between strain GP59 and strain JAM1T, strain GP59 has nucleic sequences of its own. It contains two plasmids (96 genes) and a 90.6-kb chromosomic region (119 genes) where nirK is located (Geoffroy et al., 2018). The culture conditions did not significantly affect the relative transcript levels of all plasmidic genes. However, genes in the 90.6-kb region were 2.5 times (p < 0.01) higher in the overall relative transcript levels under the < <O > > and < <ON > > conditions compared to the < <AN > > conditions (Data S3), which may have influenced the expression pattern of nirK (see below).
Examining the transcript reads associated with RNA extracted from strain GP59 cultures, we found high proportion of reads not associated with its genome. Transcript reads that did not align with strain GP59 genome and plasmids consisted between 8 to 83% of total reads derived from strain GP59 cultures. De novo assembly of these unaligned reads showed three long transcripts that composed 57 to 94% of the unaligned reads, with homology to bacteriophage affiliated to Cystovirus (Table 3, Data S4). These phages are double stranded RNA that were found in bacteria (mainly in Pseudomonas species) from diverse environments (Mäntynen, Sundberg & Poranen, 2018; Gottlieb & Alimova, 2023). No such reads were found in the transcriptomes of strain JAM1T cultures. These transcripts probably correspond to the unusual long transcripts apparent on agarose gel electrophoresis of our RNA preparation of strain GP59 (Fig. S1). Presence of these transcripts strongly suggest that strain GP59 releases this phage. Such phage was probably present when we originally isolated strain GP59, as these long transcripts were always present in our RNA extracts (resistant to DNAse treatments), despite inocula were made of a single colony. We never encountered apparent lysis in our cultures, neither seen significant impact on strain GP59 growth. Cystoviruses use the translational machinery of the host for the synthesis of their proteins directly from the viral genome, and possess their own RNA-directed RNA polymerase for genome replication (Alphonse & Ghose, 2017). These features suggest that the Cystovirus present in strain GP59 would not have affected the host transcriptional machinery but could have impacted the level of host proteins.
Table 3. Sequence similarity between the metagenomic assembled transcripts and the RNA genome of the Cystovirus Pseudomonas phage phi6.
| Gene names | Phage phi-6 | MAT | ||
|---|---|---|---|---|
| Length (a.a.) | Protein names | Length (a.a.) | Similarity | |
| Segment L (6.4 kb) | (6.9 kb) | |||
| P14 | 62 | Protein | P14 | nsf |
| P7 | 161 | Assembly protein P7 | 162 | 72% |
| P2 | 665 | RNA-directed RNA polymerase | 661 | 72% |
| P4 | 332 | Packaging enzyme P4 | 330 | 62% |
| P1 | 769 | Major inner protein P1 | 800 | 53% |
| Segment M (4.0 kb) | (3.2 kb) | |||
| P8 | 149 | Major outer capsid protein | nsf | |
| P12 | 195 | Morphogenetic protein | nsf | |
| P9 | 90 | Major envelope protein | nsf | |
| P5, P11 | 220 | Peptidoglycan hydrolase gp5 | 241 | 51% |
| Segment S (2.9 kb) | (3.4 kb) | |||
| P10 | 42 | Envelope protein P10 | nsf | |
| P6 | 168 | Fusion protein P6 | 179 | 69% |
| P3 | 648 | Spike protein P3 | 617 | 49% |
| P13 | 72 | Protein P13 | nsf | |
Notes.
Reads that did not align with strain GP59 genome and plasmids were assembled, generating three long metagenomic assembled transcripts (MAT), as indicated by the number of kilobase (kb). Deduced amino acid sequences of open reading frames (ORFs) were examined by BlastP.
- Length
- number of amino acid (a.a.) deduced from the ORFs
- nsf
- no similarity found
Data for the Cystovirus Pseudomonas phage phi6 were retrieved from https://viralzone.expasy.org/586.
Influence of the culture conditions on the relative expression of denitrification genes
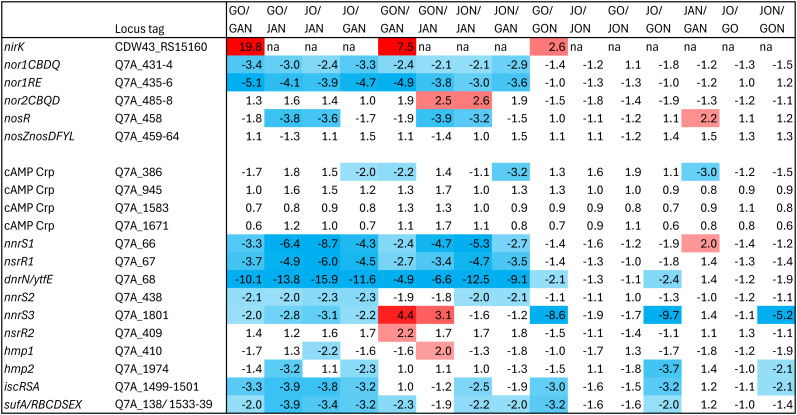
Figure 4 illustrates fold changes (FC) in the relative transcript levels of nirK, nor1, nor2 and nos, and other regulatory genes, of all pairwise comparisons (15) between the three conditions respective to each strain (e.g., strain GP59 versus < <AN > >, < <ON > >, < <O > > conditions), and between strains respective to the three conditions (e.g., strain JAM1T < <AN > > conditions versus strain GP59 < <O > > conditions).
Figure 4. Fold changes in the relative transcript levels of denitrification genes and regulatory genes.
Genes that were significantly differentially expressed were identified by EdgeR (v 3.36.0). Locus tags are from strain JAM1T GenBank annotation (CP003390.3) except nirK that is from strain GP59 GenBank annotations (CP021974.1). Values correspond to fold changes (FC) between one condition to the other. In GO/GAN, for example, positive values refer to genes with relative transcript levels higher in the < <O > > conditions than those in the < <AN > > conditions, and negative values refer to the opposite. FC > 2 (red) or < −2 (blue) that have FDR < 0.05 are highlighted by colors. G, strain GP59; J, strain JAM1T. O, AN, ON: < <O > >, < <AN > > and < <ON > > conditions, respectively.
Genes expressing the Nor1 system and its regulator NorRE had their relative transcript levels between 2 to 5 times higher under the < <AN > > conditions relative to the < <O > > and < <ON > > conditions in both strains. nor2 did not show substantial changes in its relative transcript levels in all culture conditions. Despite higher relative transcript levels of nosR in strain JAM1T cultured under the < <AN > > conditions, no changes were observed with the nos gene cluster for both strains in all culture conditions (Fig. 4). nirK will be discussed below.
Influence of the culture conditions on the relative expression of regulatory genes
In addition to fnr, four ORFs encoding protein with cAMP-binding domain of CRP or a regulatory subunit of cAMP-dependent protein kinases were found, which may be related to function associated with NnrR or DnrD (Körner, Sofia & Zumft, 2003), regulators involved for expression of key denitrification genes (Honisch & Zumft, 2003; Mesa et al., 2003). No substantial changes (if any) in their relative transcript levels were observed in all conditions between strains for the four cAMP CRP genes (Fig. 4).
Several genes encoding factors involved in NO-response were found in both genomes. This includes (Fig. 4): three nnrS genes (nnrS1 to nnrS3), two hmp genes (hmp1 and hmp2), two nsrR genes (nsrR1 and nsrR2) and the dnrN/YtfE gene. Complex and multifaceted response to NO is coordinated by the NO sensitive repressor NsrR (Volbeda et al., 2017). The intrinsic reactivity of iron-sulfur (Fe-S) clusters toward NO by NsrR functions as sensor-regulators (Kennedy, Antholine & Beinert, 1997; Spiro, 2006; Crack et al., 2014). NnrS is a heme-containing protein, expressed under denitrifying conditions and under the control of DnrD or NnrR (Glockner & Zumft, 1996; Bartnikas et al., 2002). NnrS would be one factor that protects cellular iron pool from the formation of dinitrosyl iron complex, and thus avoid NO to inhibit iron-sulfur protein function (Stern et al., 2013). In E. coli, ytfE is under the control of the regulator NsrR and has been shown to protect iron-sulfur cluster-containing proteins (Justino et al., 2006; Justino et al., 2007; Overton et al., 2008). Results from Crack et al. (2022) showed that in E. coli, YtfE act as NO-forming reductase which allow NO to be detected by NsrR for stimulating other genes such as hmp encoding a NO dioxygenase (flavohemoprotein) that oxidizes NO to under oxic conditions (Poole, 2020), or the genes clusters encoding Isc and Suf systems involved in production of Fe-S cluster under stress conditions (Blanc, Gerez & Choudens, 2015; Blahut et al., 2020).
A gene cluster containing nnrS1, nsrR1 and dnrN/YtfE had their relative transcript levels 2.4 to 16 times higher in both strains cultured under the < <AN > > conditions than under the < <O > > and < <ON > > conditions. No substantial differences in the relative transcript levels were observed with nnrS2, nsrR2 and the two hmp genes between all conditions in both strains. Higher relative transcript levels were observed with the Suf and Isc systems in some cultures for both strains cultured under the < <AN > > and < <ON > > conditions (Fig. 4). All these results suggest that both strains sense the presence of NO in the < <AN > > cultures. Although this was expected in strain GP59 cultures, it remains unclear in strain JAM1T cultures because of the lack of NO-forming reductase. It may be related to the anoxic conditions and the presence of in the expectation of NO production.
The most striking results were found with the relative transcript pattern of nnrS3 between strain GP59 and strain JAM1T. This gene had relative transcript levels in strain GP59 cultured under the < <ON > > conditions 9 to 10 times higher than those in strain GP59 and strain JAM1T cultured under the < <O > > conditions (Fig. 4: JO/GON, GO/GON), and it was 5 times higher than those in strain JAM1T cultured also under the < <ON > > conditions (Fig. 4: JON/GON). Even compared to the < <AN > > conditions, these levels were 4 times higher (Fig. 4: GON/GAN) in favor of the < <ON > > conditions in strain GP59. No difference was observed in strain JAM1T between the < <O > > and the < <ON > > conditions (Fig. 4: JO/JON). As NnrS senses NO, our results suggest that NO is generated in strain GP59 cultured under the < <ON > > conditions but because the presence of O2, NO would be transformed back to by the flavohemoproteins Hmp (NO dioxygenase). This reaction could explain why reduction was not observed under the < <ON > > conditions in strain GP59 cultures, even though nar1 and nirK showed significant transcript levels. We therefore hypothesize that the reduction of to NO is concomitant with the oxidation of NO to by Hmp in strain GP59 under oxic conditions.
Relative expression of nirK
The relative transcript levels of nirK in strain GP59 cultures were 7.5 and 20 times higher in the < <ON > > and < <O > > conditions, respectively, relative to the < <AN > > conditions, which concurs with the RT-qPCR assays performed with RNA extracted during the High period. This expression pattern was unexpected. Upstream of nirK are potential FNR and NarL DNA binding sites (Data S2). Therefore, its expression should be regulated like the other denitrification genes. During the Low period, this was the case with higher transcript levels under the < <AN > > conditions compared to the other conditions. During the High period and under the < <AN > > conditions, the denitrification pathway is probably expressed enough and does not require further gene stimulation of nirK. Under the < <ON > > conditions, as we hypothesized before, and would be reduced in NO then oxidized back to , generating a sort of loop, which may explain why gene stimulation of nirK is still operating. However, under the < <O > > conditions, the expression pattern of nirK is puzzling, because of the absence of . What may cause the higher expression of nirK during the High period under the < <O > > condition remains unclear. Strain GP59 cultures may have encountered nitrosative-stresses under the < <O > > conditions. Presence of in the Methylophaga 1403 medium (3.8 mM) is the only source of inorganic N in these conditions. However, genes involved in the transformation of to or are absent in the genome. Another possibility is the abiotic transformation of or organic N compounds generating , or NO (Doane, 2017). However, the relative expressions of most of the /NO reductase regulators (nsrR, nnrS, dnrN/ytfE) were higher under the < <AN > > conditions than under the < <O > > conditions. nirK is located in a particular chromosomic region, between two prophages, and probably acquired by horizontal transfer as suggested by Geoffroy et al. (2018). Transcriptomes revealed that the relative transcript levels of genes in this region are overall higher under the < <O > > conditions than under the < <AN > > conditions during the High period (Data S3), which could have impacted the nirK expression.
Conclusions
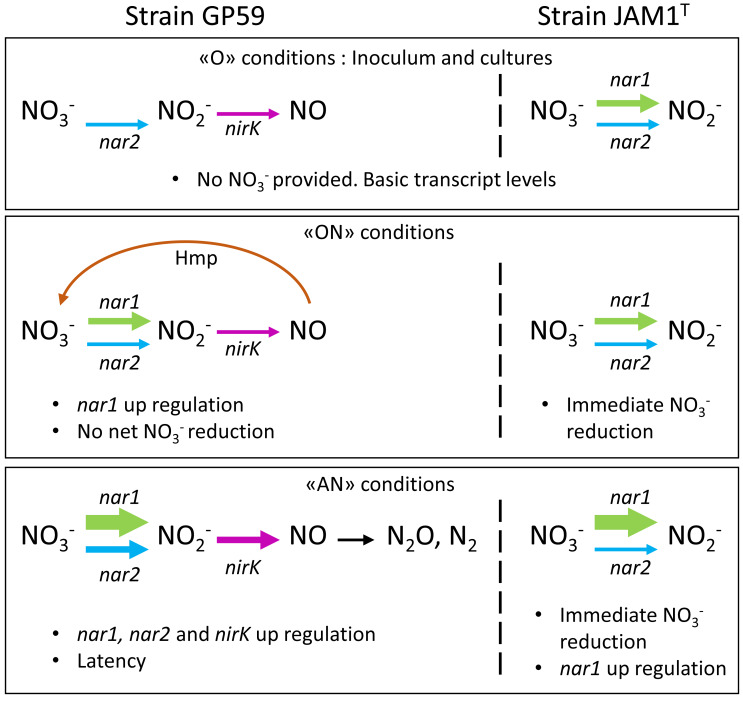
By exploring genes expression, this study shows how two strains of the same species could respond differently and adjust their denitrification pathway according to the environmental conditions. Figure 5 illustrates the proposed mechanisms deduced from our results, in conjunction with the literature, that would explain the denitrification dynamics of each strain. Under the < <O > > conditions that prevailed in the inocula, the Nar1 and Nar2 systems, including the transporters, in strain GP59 cultures are expressed at very low levels. Under the < <AN > > conditions, induction of both systems occurred, which requires some time (latency) before the denitrification pathway is operational. Under the < <ON > > conditions, Nar1 is induced at a low level, similar of what was observed in strain JAM1T cultured under the same conditions. These low levels of the Nar systems and NirK are sufficient to generate NO (as indicated by nnrS3). Instead to proceed through the NO reductase (Nor systems) that may not be operational because of the oxic conditions, the flavohemoprotein (Hmp) transforms NO back to . For strain JAM1T, the Nar1 system is already expressed in the inocula that allows immediate action on and the accumulation of .
Figure 5. Proposed mechanism of denitrification by strain JAM1T and strain GP59 during the Low period.
Thickness of the arrows represents the gene transcript levels as determined in Fig. 1.
The distinct physiological characters of strain JAM1T and strain GP59 could be linked to the biofilm environment in the Biodome denitrification system where they were isolated. Constitutive expression of the Nar1 system allows strain JAM1T to thrive immediately on , which is an advantage against other “typical” denitrifiers such as strain GP59 or Hyphomicrobium nitrativorans (another major strain isolated from the biofilm), especially as strain JAM1T does not reach high level of growth under anoxic conditions.
These different physiological behaviors of strains JAM1T and GP59 that belong to the same species are intriguing as both strains originated from the same microbial community of one denitrification reactor. We used to see complex microbial community as an amalgam of different species, but subpopulations of these species could have important impact on the evolution of this community. Globally, the present study and our previous reports on these strains suggest that studying microbial community to identify and isolate the main species in a bioprocess (or any environment) would also have to be extended at the subpopulation species’ level. Understanding the mechanisms underlying these differences would provide indication on the dynamics of these subpopulations in the microbial community upon environmental changes and thus how this community evolved in providing efficient denitrifying activities.
Supplemental Information
RNA extracts from replicate cultures were segregated by agarose electrophoresis and revealed by ethidium bromide and UV. DNAse treatment did not impact the high molecular weight transcripts of strain GP59 RNA, which may represent the Cystovirus genome detected in the transcriptomes of strain GP59 cultures.
Percentages of genes and riboswitches where the fold changes were >2 with FDR < 0.05 in the relative transcript levels between culture conditions and between strains are illustrated. In GO/GAN, for example, “Up” refers to genes with relative transcript levels higher in the < <O > > conditions than those in the < <AN > > conditions, and “Down” refers to the opposite. *: Comparisons between strain JAM1T and strain GP59 involved the 2813 common genes and riboswitches. G: strain GP59. J: JAM1T. O: < <O > > conditions; ON: < <ON > > conditions; AN: < <AN > > conditions.
Acknowledgments
We would like to thank Philippe Constant from INRS Centre Armand-Frappier Santé Biotechnologie to carry out PERMANOVA.
Funding Statement
This research was supported by a grant to Richard Villemur from the Natural Sciences and Engineering Research Council of Canada # RGPIN-2016-06061. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Livie Lestin conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Richard Villemur conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
DNA Deposition
The following information was supplied regarding the deposition of DNA sequences:
The RNAseq reads from strain JAM1T and strain GP59 cultured under the ¡¡N¿¿ conditions are available at NCBI SRA: PRJNA525230; SRX5461036, SRX5461037, SRX5461044, SRX5461045, SRX5461047, SRX5461048.
For those cultured under the ¡¡O¿¿ and ¡¡ON¿¿ conditions, RNAseq reads are available at: PRJNA1072961; SAMN39755713 to SAMN39755725.
Data Availability
The following information was supplied regarding data availability:
The data is available in the Supplemental Files.
References
- Alphonse & Ghose (2017).Alphonse S, Ghose R. Cystoviral RNA-directed RNA polymerases: regulation of RNA synthesis on multiple time and length scales. Virus Research. 2017;234:135–152. doi: 10.1016/j.virusres.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson (2017).Anderson MJ. Permutational Multivariate Analysis of Variance (PERMANOVA) Wiley StatsRef: Statistics reference online; Hoboken: 2017. [DOI] [Google Scholar]
- Anuchin et al. (2011).Anuchin AM, Goncharenko AV, Demidenok OI, Kaprelyants AS. Histone-like proteins of bacteria. Applied Biochemistry and Microbiology. 2011;47:580–585. doi: 10.1134/S0003683811060020. [DOI] [Google Scholar]
- Auclair et al. (2010).Auclair J, Lepine F, Parent S, Villemur R. Dissimilatory reduction of nitrate in seawater by a Methylophaga strain containing two highly divergent narG sequences. The ISME Journal. 2010;4:1302–1313. doi: 10.1038/ismej.2010.47. [DOI] [PubMed] [Google Scholar]
- Bartnikas et al. (2002).Bartnikas TB, Wang Y, Bobo T, Veselov A, Scholes CP, Shapleigh JP. Characterization of a member of the NnrR regulon in Rhodobacter sphaeroides 2.4.3 encoding a haem-copper protein. Microbiology-SGM. 2002;148:825–833. doi: 10.1099/00221287-148-3-825. [DOI] [PubMed] [Google Scholar]
- Blahut et al. (2020).Blahut M, Sanchez E, Fisher CE, Outten FW. Fe-S cluster biogenesis by the bacterial Suf pathway. Biochimica et Biophysica Acta—Molecular Cell Research. 2020;1867(11):118829. doi: 10.1016/j.bbamcr.2020.118829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc, Gerez & Choudens (2015).Blanc B, Gerez C, Choudens SADe. Assembly of Fe/S proteins in bacterial systems Biochemistry of the bacterial ISC system. Biochimica et Biophysica Acta—Molecular Cell Research. 2015;1853:1436–1447. doi: 10.1016/j.bbamcr.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Boden (2012).Boden R. Emended description of the genus Methylophaga Janvier et al. 1985. International Journal of Systematic and Evolutionary Microbiology. 2012;62:1644–1646. doi: 10.1099/ijs.0.033639-0. [DOI] [PubMed] [Google Scholar]
- Boden (2019).Boden R. Bergey’s manual of systematics of Archeae and Bacteria Online. John Wiley & Sons, Inc in association with Bergey’s manual trust; Hoboken: 2019. Methylophaga. [DOI] [Google Scholar]
- Browning, Butala & Busby (2019).Browning DF, Butala M, Busby SJW. Bacterial transcription factors: gegulation by Pick N Mix. Journal of Molecular Biology. 2019;431:4067–4077. doi: 10.1016/j.jmb.2019.04.011. [DOI] [PubMed] [Google Scholar]
- Constantinidou et al. (2006).Constantinidou C, Hobman JL, Griffiths L, Patel MD, Penn CW, Cole JA, Overton TW. A reassessment of the FNR regulon and transcriptomic analysis of the effects of nitrate, nitrite, NarXL, and NarQP as Escherichia coli K12 adapts from aerobic to anaerobic growth. Journal of Biological Chemistry. 2006;281:4802–4815. doi: 10.1074/jbc.M512312200. [DOI] [PubMed] [Google Scholar]
- Crack et al. (2022).Crack JC, Balasiny BK, Bennett SP, Rolfe MD, Froes A, MacMillan F, Green J, Cole JA, Le Brun NE. The di-iron protein YtfE is a nitric oxide-generating nitrite reductase involved in the management of nitrosative stress. Journal of the American Chemical Society. 2022;144:7129–7145. doi: 10.1021/jacs.1c12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack et al. (2014).Crack JC, Green J, Thomson AJ, Le Brun NE. Iron-sulfur clusters as biological sensors: the chemistry of reactions with molecular oxygen and nitric oxide. Accounts of Chemical Research. 2014;47:3196–3205. doi: 10.1021/ar5002507. [DOI] [PubMed] [Google Scholar]
- Cucaita, Piochon & Villemur (2021).Cucaita A, Piochon M, Villemur R. Co-culturing Hyphomicrobium nitrativorans strain NL23 and Methylophaga nitratireducenticrescens strain JAM1 allows sustainable denitrifying activities under marine conditions. PeerJ. 2021;9:e12424. doi: 10.7717/peerj.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doane (2017).Doane TA. The abiotic nitrogen cycle. ACS Earth and Space Chemistry. 2017;1:411–421. doi: 10.1021/acsearthspacechem.7b00059. [DOI] [Google Scholar]
- Dudek & Jahn (2022).Dudek CA, Jahn D. PRODORIC: state-of-the-art database of prokaryotic gene regulation. Nucleic Acids Research. 2022;50:D295–D302. doi: 10.1093/nar/gkab1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand & Guillier (2021).Durand S, Guillier M. Transcriptional and post-transcriptional control of the nitrate respiration in bacteria. Frontiers in Molecular Biosciences. 2021;8:667758. doi: 10.3389/fmolb.2021.667758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaimster et al. (2017).Gaimster H, Alston M, Richardson DJ, Gates AJ, Rowley G. Transcriptional and environmental control of bacterial denitrification and N2O emissions. FEMS Microbiology Letters. 2017;365:fnx277. doi: 10.1093/femsle/fnx277. [DOI] [PubMed] [Google Scholar]
- Gaimster et al. (2019).Gaimster H, Hews CL, Griffiths R, Soriano-Laguna MJ, Alston M, Richardson DJ, Gates AJ, Rowley G. A central small RNA regulatory circuit controlling bacterial denitrification and N2O emissions. mBio. 2019;10:e01165–01119. doi: 10.1128/mBio.01165-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy et al. (2018).Geoffroy V, Payette G, Mauffrey F, Lestin L, Constant P, Villemur R. Strain-level genetic diversity of Methylophaga nitratireducenticrescens confers plasticity to denitrification capacity in a methylotrophic marine denitrifying biofilm. PeerJ. 2018;6:e4679. doi: 10.7717/peerj.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glockner & Zumft (1996).Glockner AB, Zumft WG. Sequence analysis of an internal 9.72-kb segment from the 30-kb denitrification gene cluster of Pseudomonas stutzeri. Biochimica et Biophysica Acta (BBA)—Bioenergetics. 1996;1277:6–12. doi: 10.1016/S0005-2728(96)00108-9. [DOI] [PubMed] [Google Scholar]
- Gordon & Hannon (2010).Gordon A, Hannon G. FASTQ/A short-reads pre-processing tools. 2010. http://hannonlab.cshl.edu/fastx_toolkit http://hannonlab.cshl.edu/fastx_toolkit
- Gottlieb & Alimova (2023).Gottlieb P, Alimova A. Discovery and classification of the φ6 bacteriophage: an historical review. Viruses. 2023;15(6):1308. doi: 10.3390/v15061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo & Gao (2021).Guo K, Gao H. Physiological roles of nitrite and nitric oxide in bacteria: similar consequences from distinct cell targets, protection, and sensing systems. Advanced Biology. 2021;5:2100773. doi: 10.1002/adbi.202100773. [DOI] [PubMed] [Google Scholar]
- Honisch & Zumft (2003).Honisch U, Zumft WG. Operon structure and regulation of the nos gene region of Pseudomonas stutzeri, encoding an ABC-Type ATPase for maturation of nitrous oxide reductase. Journal of Bacteriology. 2003;185:1895–1902. doi: 10.1128/JB.185.6.1895-1902.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justino et al. (2006).Justino MC, Almeida CC, Gonçalves VL, Teixeira M, Saraiva LM. Escherichia coli YtfE is a di-iron protein with an important function in assembly of iron-sulphur clusters. FEMS Microbiology Letters. 2006;257:278–284. doi: 10.1111/j.1574-6968.2006.00179.x. [DOI] [PubMed] [Google Scholar]
- Justino et al. (2007).Justino MC, Almeida CC, Teixeira M, Saraiva LM. Escherichia coli di-iron YtfE protein is necessary for the repair of stress-damaged iron-sulfur clusters. Journal of Biological Chemistry. 2007;282:10352–10359. doi: 10.1074/jbc.M610656200. [DOI] [PubMed] [Google Scholar]
- Kennedy, Antholine & Beinert (1997).Kennedy MC, Antholine WE, Beinert H. An EPR investigation of the products of the reaction of cytosolic and mitochondrial aconitases with nitric oxide. Journal of Biological Chemistry. 1997;272:20340–20347. doi: 10.1074/jbc.272.33.20340. [DOI] [PubMed] [Google Scholar]
- Körner, Sofia & Zumft (2003).Körner H, Sofia HJ, Zumft WG. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiology Reviews. 2003;27:559–592. doi: 10.1016/S0168-6445(03)00066-4. [DOI] [PubMed] [Google Scholar]
- Labbé et al. (2003).Labbé N, Juteau P, Parent S, Villemur R. Bacterial diversity in a marine methanol-fed denitrification reactor at the Montreal biodome, Canada. Microbial Ecology. 2003;46:12–21. doi: 10.1007/s00248-002-1056-6. [DOI] [PubMed] [Google Scholar]
- Labbé et al. (2007).Labbé N, Laurin V, Juteau P, Parent S, Villemur R. Microbiological community structure of the biofilm of a methanol-fed, marine denitrification system, and identification of the methanol-utilizing microorganisms. Microbial Ecology. 2007;53:621–630. doi: 10.1007/s00248-006-9168-z. [DOI] [PubMed] [Google Scholar]
- Labbé, Parent & Villemur (2003).Labbé N, Parent S, Villemur R. Addition of trace metals increases denitrification rate in closed marine systems. Water Research. 2003;37:914–920. doi: 10.1016/S0043-1354(02)00383-4. [DOI] [PubMed] [Google Scholar]
- Langmead & Salzberg (2012).Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nature Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurin et al. (2008).Laurin V, Labbé N, Parent S, Juteau P, Villemur R. Microeukaryote diversity in a marine methanol-fed fluidized denitrification system. Microbial Ecology. 2008;56:637–648. doi: 10.1007/s00248-008-9383-x. [DOI] [PubMed] [Google Scholar]
- Li & Dewey (2011).Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäntynen, Sundberg & Poranen (2018).Mäntynen S, Sundberg LR, Poranen MM. Recognition of six additional cystoviruses: Pseudomonas virus phi6 is no longer the sole species of the family Cystoviridae. Archives of Virology. 2018;163:1117–1124. doi: 10.1007/s00705-017-3679-4. [DOI] [PubMed] [Google Scholar]
- Maris et al. (2005).Maris AE, Kaczor-Grzeskowiak M, Ma Z, Kopka ML, Gunsalus RP, Dickerson RE. Primary and secondary modes of DNA recognition by the NarL two-component response regulator. Biochemistry-US. 2005;44:14538–14552. doi: 10.1021/bi050734u. [DOI] [PubMed] [Google Scholar]
- Maris et al. (2002).Maris AE, Sawaya MR, Kaczor-Grzeskowiak M, Jarvis MR, Bearson SMD, Kopka ML, Schroder I, Gunsalus RP, Dickerson RE. Dimerization allows DNA target site recognition by the NarL response regulator. Nature Structural & Molecular Biology. 2002;9:771–778. doi: 10.1038/nsb845. [DOI] [PubMed] [Google Scholar]
- Mauffrey et al. (2017).Mauffrey F, Cucaita A, Constant P, Villemur R. Denitrifying metabolism of the methylotrophic marine bacterium Methylophaga nitratireducenticrescens strain JAM1. PeerJ. 2017;5:e4098. doi: 10.7717/peerj.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauffrey, Martineau & Villemur (2015).Mauffrey F, Martineau C, Villemur R. Importance of the two dissimilatory (Nar) nitrate reductases in the growth and nitrate reduction of the methylotrophic marine bacterium Methylophaga nitratireducenticrescens JAM1. Frontiers in Microbiology. 2015;6:1475. doi: 10.3389/fmicb.2015.01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesa et al. (2003).Mesa S, Bedmar EJ, Chanfon A, Hennecke H, Fischer HM. Bradyrhizobium japonicum NnrR, a denitrification regulator, expands the FixLJ-FixK2 regulatory cascade. Journal of Bacteriology. 2003;185:3978–3982. doi: 10.1128/JB.185.13.3978-3982.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettert & Kiley (2018).Mettert EL, Kiley PJ. Reassessing the structure and function relationship of the O2 sensing transcription factor FNR. Antioxidants & Redox Signaling. 2018;29:1830–1840. doi: 10.1089/ars.2017.7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton et al. (2008).Overton TW, Justino MC, Li Y, Baptista JM, Melo AMP, Cole JA, Saraiva LM. Widespread distribution in pathogenic bacteria of di-iron proteins that repair oxidative and nitrosative damage to iron-sulfur centers. Journal of Bacteriology. 2008;190:2004–2013. doi: 10.1128/JB.01733-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent & Morin (2000).Parent S, Morin A. N budget as water quality management tool in closed aquatic mesocosms. Water Research. 2000;34:1846–1856. doi: 10.1016/S0043-1354(99)00343-7. [DOI] [PubMed] [Google Scholar]
- Payette et al. (2019).Payette G, Geoffroy V, Martineau C, Villemur R. Dynamics of a methanol-fed marine denitrifying biofilm: 1-impact of environmental changes on the denitrification and the co-occurrence of Methylophaga nitratireducenticrescens and Hyphomicrobium nitrativorans. PeerJ. 2019;7:e7497. doi: 10.7717/peerj.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole (2020).Poole RK. Flavohaemoglobin: the pre-eminent nitric oxide-detoxifying machine of microorganisms. F1000Research. 2020;9:F1000. doi: 10.12688/f1000research.20563.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan & Hall (2010).Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, McCarthy & Smyth (2010).Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha, Santos & Pacheco (2015).Rocha DJP, Santos CS, Pacheco LGC. Bacterial reference genes for gene expression studies by RT-qPCR: survey and analysis. Antonie Van Leeuwenhoek International Journal of General. 2015;108:685–693. doi: 10.1007/s10482-015-0524-1. [DOI] [PubMed] [Google Scholar]
- Spiro (2006).Spiro S. Nitric oxide-sensing mechanisms in Escherichia coli. Biochemical Society Transactions. 2006;34:200–202. doi: 10.1042/BST0340200. [DOI] [PubMed] [Google Scholar]
- Spiro (2011).Spiro S. Nitric oxide metabolism; physiology and regulatory mecahnisms. In: Moir JWB, editor. Nitrogen cycling in bacteria Molecular analysis. Caister Academic Press; Norfolk: 2011. pp. 177–196. [Google Scholar]
- Squire et al. (2009).Squire DJP, Xu M, Cole JA, Busby SJW, Browning DF. Competition between NarL-dependent activation and Fis-dependent repression controls expression from the Escherichia coli yeaR and ogt promoters. Biochemical Journal. 2009;420:249–257. doi: 10.1042/BJ20090183. [DOI] [PubMed] [Google Scholar]
- Stern et al. (2013).Stern AM, Liu B, Bakken LR, Shapleigh JP, Zhu J. A novel protein protects bacterial iron-dependent metabolism from nitric oxide. Journal of Bacteriology. 2013;195:4702–4708. doi: 10.1128/JB.00836-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata et al. (2017).Tata M, Amman F, Pawar V, Wolfinger MT, Weiss S, Häussler S, Bläsi U. The anaerobically induced sRNA paiI affects denitrification in Pseudomonas aeruginosa PA14. Frontiers in Microbiology. 2017;8:02312. doi: 10.3389/fmicb.2017.02312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ter Braak & Šmilauer (2018).Ter Braak CJF, Šmilauer P. Canoco reference manual and user’s guide: software for ordination (Version 5.10). Microcomputer Power (Ithaca, Ny, USA), 536 2018.
- Van Spanning, Richardson & Ferguson (2007).Van Spanning RJM, Richardson DJ, Ferguson SJ. Introduction to the biochemistry and molecular biology of denitrification. In: Bothe HNSJ, Ferguson WE, editors. Biology of the nitrogen cycle. Elsevier; Amsterdam: 2007. pp. 3–20. [Google Scholar]
- Villemur et al. (2019).Villemur R, Payette G, Geoffroy V, Mauffrey F, Martineau C. Dynamics of a methanol-fed marine denitrifying biofilm: 2-impact of environmental changes on the microbial community. PeerJ. 2019;7:e7467. doi: 10.7717/peerj.7467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve et al. (2013).Villeneuve C, Martineau C, Mauffrey F, Villemur R. Methylophaga nitratireducenticrescens sp. nov. and Methylophaga frappieri sp. nov. isolated from the biofilm of the methanol-fed denitrification system treating the seawater at the Montreal Biodome. International Journal of Systematic and Evolutionary Microbiology. 2013;63:2216–2222. doi: 10.1099/ijs.0.044545-0. [DOI] [PubMed] [Google Scholar]
- Volbeda et al. (2017).Volbeda A, Dodd EL, Darnault C, Crack JC, Renoux O, Hutchings MI, Le Brun NE, Fontecilla-Camps JC. Crystal structures of the NO sensor NsrR reveal how its iron-sulfur cluster modulates DNA binding. Nature Communications. 2017;8:15052. doi: 10.1038/ncomms15052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumft (1997).Zumft WG. Cell biology and molecular basis of denitrification. Microbiology and Molecular Biology Reviews. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA extracts from replicate cultures were segregated by agarose electrophoresis and revealed by ethidium bromide and UV. DNAse treatment did not impact the high molecular weight transcripts of strain GP59 RNA, which may represent the Cystovirus genome detected in the transcriptomes of strain GP59 cultures.
Percentages of genes and riboswitches where the fold changes were >2 with FDR < 0.05 in the relative transcript levels between culture conditions and between strains are illustrated. In GO/GAN, for example, “Up” refers to genes with relative transcript levels higher in the < <O > > conditions than those in the < <AN > > conditions, and “Down” refers to the opposite. *: Comparisons between strain JAM1T and strain GP59 involved the 2813 common genes and riboswitches. G: strain GP59. J: JAM1T. O: < <O > > conditions; ON: < <ON > > conditions; AN: < <AN > > conditions.
Data Availability Statement
The following information was supplied regarding data availability:
The data is available in the Supplemental Files.