Abstract
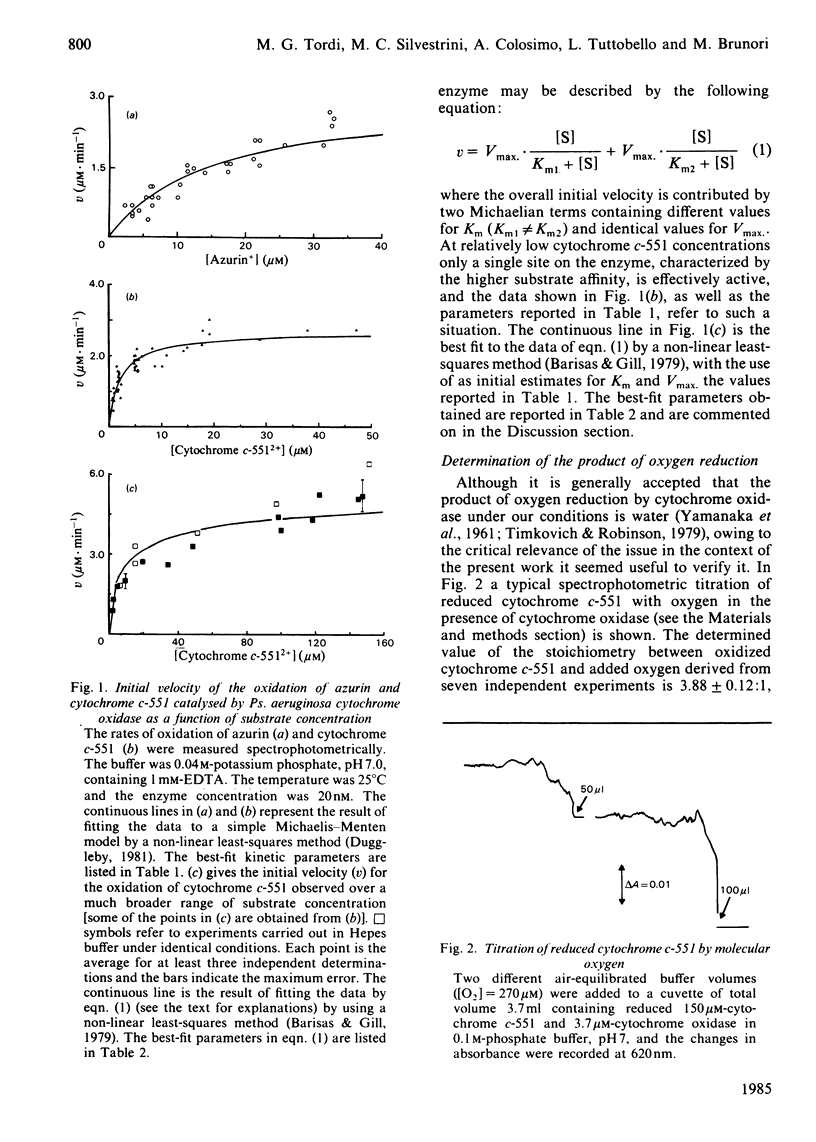
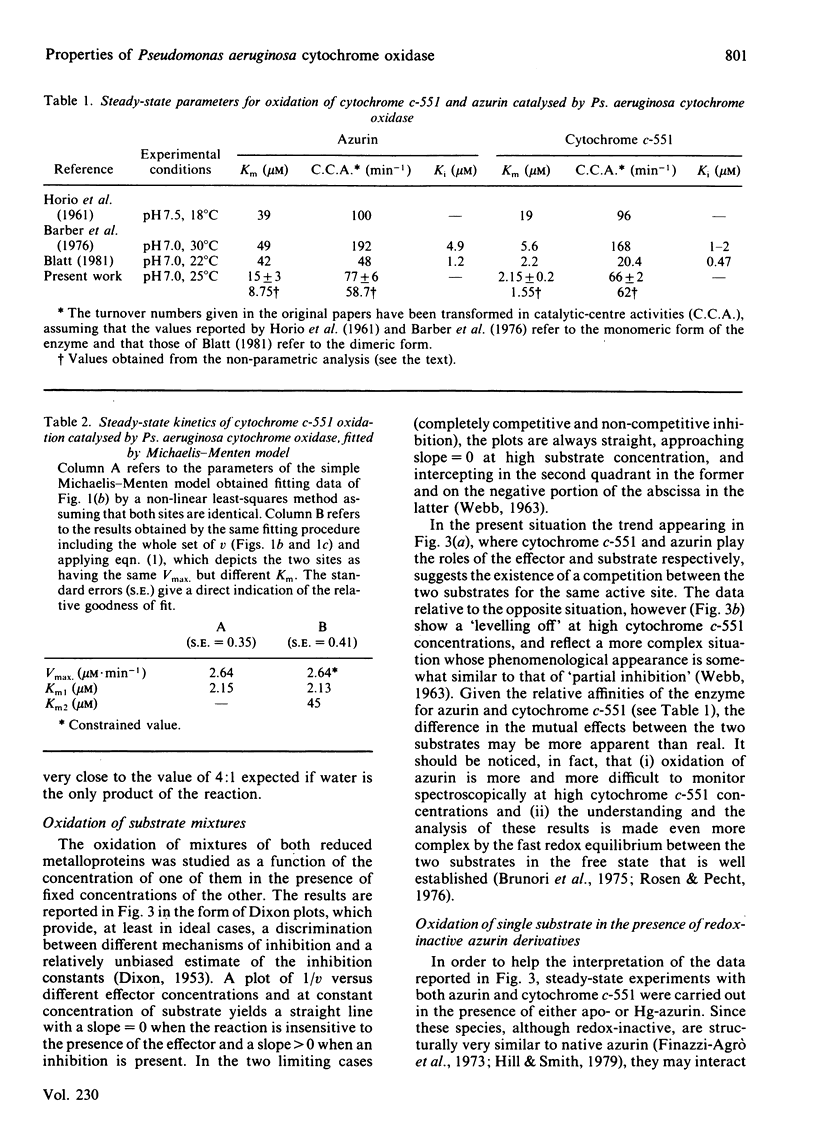
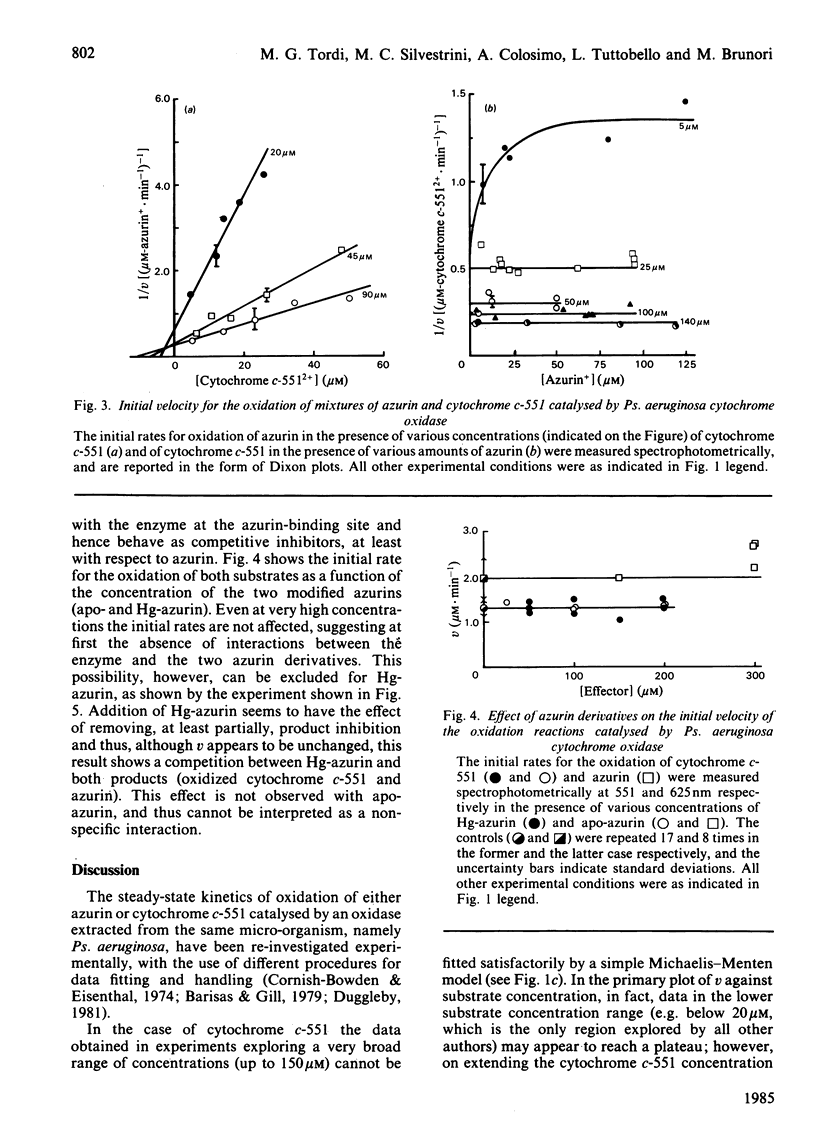
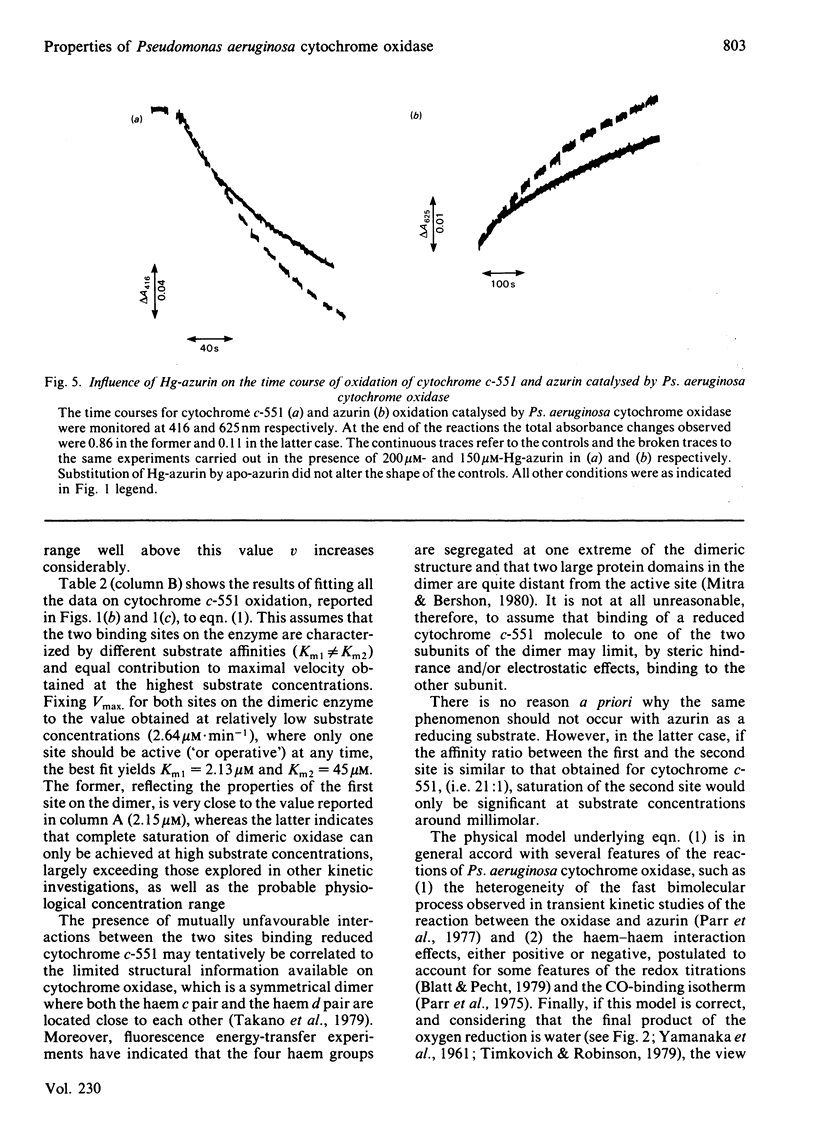
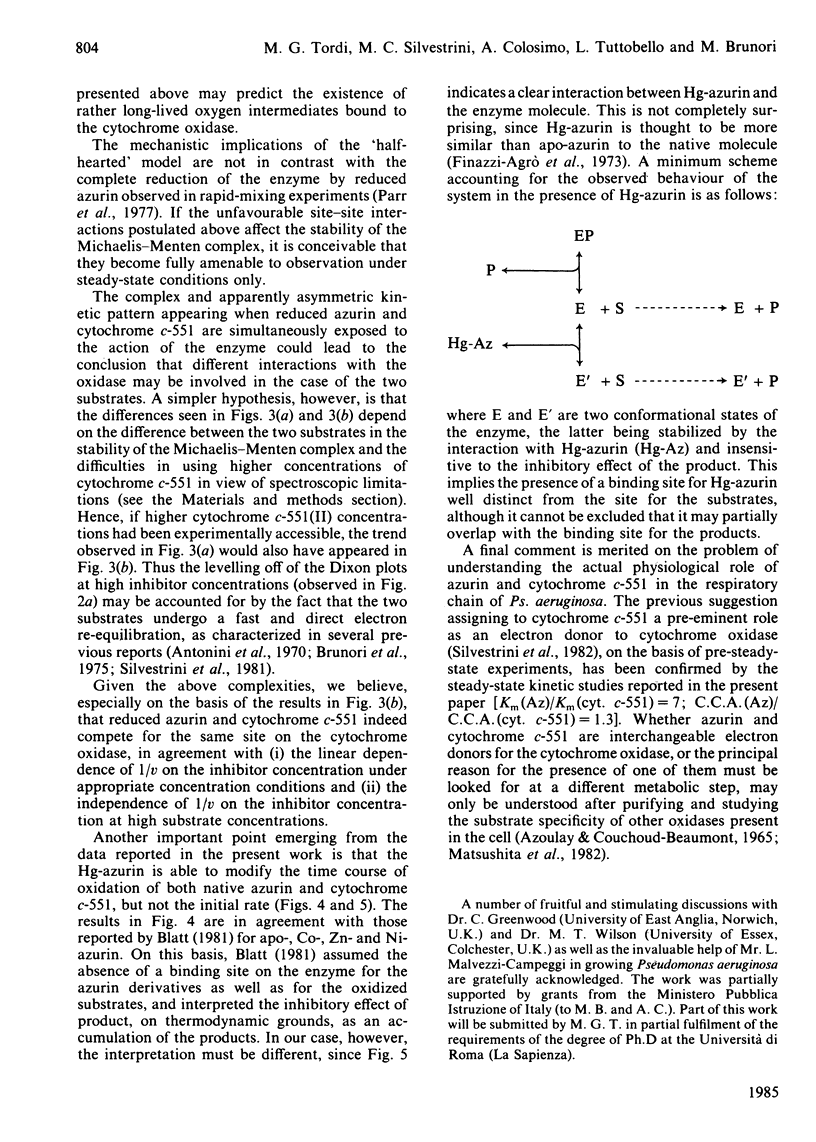
The kinetics of oxidation of azurin and cytochrome c-551 catalysed by Pseudomonas aeruginosa cytochrome oxidase were re-investigated, and the steady-state parameters were evaluated by parametric and non-parametric methods. At low concentrations of substrates (e.g. less than or equal to 50 microM) the values obtained for Km and catalytic-centre activity are respectively 15 +/- 3 microM and 77 +/- 6 min-1 for azurin and 2.15 +/- 0.23 microM and 66 +/- 2 min-1 for cytochrome c-551, in general accord with previous reports assigning to cytochrome c-551 the higher affinity for the enzyme and to azurin a slightly higher catalytic rate. However, when the cytochrome c-551 concentration was extended well beyond the value of Km, the initial velocity increased, and eventually almost doubled at a substrate concentration greater than or equal to 100 microM. This result suggests a 'half-hearted' behaviour, since at relatively low cytochrome c-551 concentrations only one of the two identical binding sites of the dimeric enzyme seems to be catalytically active, possibly because of unfavourable interactions influencing the stability of the Michaelis-Menten complex at the second site. When reduced azurin and cytochrome c-551 are simultaneously exposed to Ps. aeruginosa cytochrome oxidase, the observed steady-state oxidation kinetics are complex, as expected in view of the rapid electron transfer between cytochrome c-551 and azurin in the free state. In spite of this complexity, it seems likely that a mechanism involving a simple competition between the two substrates for the same active site on the enzyme is operative. Addition of a chemically modified and redox inactive form of azurin (Hg-azurin) had no effect on the initial rate of oxidation of either azurin and cytochrome c-551, but clearly altered the time course of the overall process by removing, at least partially, the product inhibition. The results lead to the following conclusions: (i) reduced azurin and cytochrome c-551 bind at the same site on the enzyme, and thus compete; (ii) Hg-azurin binds at a regulatory site, competing with the product rather than the substrate; (iii) the two binding sites on the dimeric enzyme, though intrinsically equivalent, display unfavourable interactions. Since water is the product of the reduction of oxygen, point (iii) has important implications for the reaction mechanism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonini E., Finazzi-Agrò A., Avigliano A., Guerrieri P., Rotilio G., Mondovì B. Kinetics of electron transfer between azurin and cytochrome 551 from Pseudomonas. J Biol Chem. 1970 Sep 25;245(18):4847–4849. [PubMed] [Google Scholar]
- Azoulay E., Couchoud-Beaumont P. Etude de la cytochrome-oxidase de Pseudomonas aeruginosa. Biochim Biophys Acta. 1965 Nov 22;110(2):301–311. [PubMed] [Google Scholar]
- Barber D., Parr S. R., Greenwood C. Some spectral and steady-state kinetic properties of Pseudomonas cytochrome oxidase. Biochem J. 1976 Aug 1;157(2):431–438. doi: 10.1042/bj1570431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barisas B. G., Gill S. J. Thermodynamic analysis of carbon monoxide binding by hemoglobin trout I. Biophys Chem. 1979 Mar;9(3):235–244. doi: 10.1016/0301-4622(79)85006-1. [DOI] [PubMed] [Google Scholar]
- Blatt Y., Pecht I. Allosteric cooperative interactions among redox sites of Pseudomonas cytochrome oxidase. Biochemistry. 1979 Jun 26;18(13):2917–2922. doi: 10.1021/bi00580a037. [DOI] [PubMed] [Google Scholar]
- Brunori M., Greenwood C., Wilson T. A temperature-jump study of the reaction between azurin and cytochrome c-551 from Pseudomonas aeruginosa. Biochem J. 1974 Jan;137(1):113–116. doi: 10.1042/bj1370113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggleby R. G. A nonlinear regression program for small computers. Anal Biochem. 1981 Jan 1;110(1):9–18. doi: 10.1016/0003-2697(81)90104-4. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finazzi-Agrò A., Giovagnoli C., Avigliano L., Rotilio G., Mondovì B. Luminescence quenching in azurin. Eur J Biochem. 1973 Apr 2;34(1):20–24. doi: 10.1111/j.1432-1033.1973.tb02723.x. [DOI] [PubMed] [Google Scholar]
- Finazzi-Agrò A., Rotilio G., Avigliano L., Guerrieri P., Boffi V., Mondovì B. Environment of copper in Pseudomonas fluorescens azurin: fluorometric approach. Biochemistry. 1970 Apr 28;9(9):2009–2014. doi: 10.1021/bi00811a023. [DOI] [PubMed] [Google Scholar]
- Gudat J. C., Singh J., Wharton D. C. Cytochrome oxidase from Pseudomonas aeruginosa. I. Purification and some properties. Biochim Biophys Acta. 1973 Feb 22;292(2):376–390. doi: 10.1016/0005-2728(73)90044-3. [DOI] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., SASAGAWA M., KUSAI K., NAKAI M., OKUNUKI K. Preparation of crystalline Pseudomonas cvtochrome c-551 and its general properties. Biochem J. 1960 Oct;77:194–201. doi: 10.1042/bj0770194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORIO T., HIGASHI T., YAMANAKA T., MATSUBARA H., OKUNUKI K. Purification and properties of cytochrome oxidase from Pseudomonas aeruginosa. J Biol Chem. 1961 Mar;236:944–951. [PubMed] [Google Scholar]
- Hill H. A., Smith B. E. Characteristics of azurin from Pseudomonas aeruginosa via 270-MHz 1H nuclear magnetic resonance spectroscopy. J Inorg Biochem. 1979 Oct;11(2):79–93. doi: 10.1016/s0162-0134(00)80174-9. [DOI] [PubMed] [Google Scholar]
- Matsushita K., Shinagawa E., Adachi O., Ameyama M. Membrane-bound cytochromes c of Pseudomonas aeruginosa grown aerobically. Purification and characterization of cytochromes c-551 and c-555. J Biochem. 1982 Nov;92(5):1607–1613. doi: 10.1093/oxfordjournals.jbchem.a134086. [DOI] [PubMed] [Google Scholar]
- Mitra S., Bersohn R. Location of the heme groups in cytochrome cd1 oxidase from Pseudomonas aeruginosa. Biochemistry. 1980 Jul 8;19(14):3200–3203. doi: 10.1021/bi00555a015. [DOI] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C. A purification procedure for the soluble cytochrome oxidase and some other respiratory proteins from Pseudomonas aeruginosa. Biochem J. 1976 Aug 1;157(2):423–430. doi: 10.1042/bj1570423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr S. R., Barber D., Greenwood C., Brunori M. The electron-transfer reaction between azurin and the cytochrome c oxidase from Pseudomonas aeruginosa. Biochem J. 1977 Nov 1;167(2):447–455. doi: 10.1042/bj1670447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr S. R., Wilson M. T., Greenwood C. The reaction of Pseudomonas aeruginosa cytochrome c oxidase with carbon monoxide. Biochem J. 1975 Oct;151(1):51–59. doi: 10.1042/bj1510051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter W. R., Trager W. F. Improved non-parametric statistical methods for the estimation of Michaelis-Menten kinetic parameters by the direct linear plot. Biochem J. 1977 Feb 1;161(2):293–302. doi: 10.1042/bj1610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen P., Pecht I. Conformational equilibria accompanying the electron transfer between cytochrome c (P551) and azurin from Pseudomonas aeruginosa. Biochemistry. 1976 Feb 24;15(4):775–786. doi: 10.1021/bi00649a008. [DOI] [PubMed] [Google Scholar]
- Silvestrini M. C., Colosimo A., Brunori M., Walsh T. A., Barber D., Greenwood C. A re-evaluation of some basic structural and functional properties of Pseudomonas cytochrome oxidase. Biochem J. 1979 Dec 1;183(3):701–709. doi: 10.1042/bj1830701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini M. C., Tordi M. G., Colosimo A., Antonini E., Brunori M. The kinetics of electron transfer between pseudomonas aeruginosa cytochrome c-551 and its oxidase. Biochem J. 1982 May 1;203(2):445–451. doi: 10.1042/bj2030445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T., Dickerson R. E., Schichman S. A., Meyer T. E. Crystal data, molecular dimensions and molecular symmetry in cytochrome oxidase from Pseudomonas aeruginosa. J Mol Biol. 1979 Sep 5;133(1):185–188. doi: 10.1016/0022-2836(79)90257-2. [DOI] [PubMed] [Google Scholar]
- Timkovich R., Robinson M. K. Evidence for water as the product for oxygen reduction by cytochrome cd. Biochem Biophys Res Commun. 1979 May 28;88(2):649–655. doi: 10.1016/0006-291x(79)92097-7. [DOI] [PubMed] [Google Scholar]
- Wharton D. C., Gudat J. C., Gibson Q. H. Cytochrome oxidase from Pseudomonas aeruginosa. I. Reaction with copper protein. Biochim Biophys Acta. 1973 Apr 5;292(3):611–620. doi: 10.1016/0005-2728(73)90009-1. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., KIJIMOTO S., OKUNUKI K. Some properties of Pseudomonas blue protein and its apo-protein. J Biochem. 1963 Mar;53:256–259. doi: 10.1093/oxfordjournals.jbchem.a127689. [DOI] [PubMed] [Google Scholar]
- YAMANAKA T., OTA A., OKUNUKI K. A nitrite reducing system reconstructed with purified cytochrome components of Pseudomonas aeruginosa. Biochim Biophys Acta. 1961 Oct 28;53:294–308. doi: 10.1016/0006-3002(61)90442-5. [DOI] [PubMed] [Google Scholar]



