Abstract
The binding of mono- and oligo-vanadates to sarcoplasmic reticulum was analysed by 51V-n.m.r. spectroscopy. The observations indicate that, in addition to monovanadate, the di-, tetra- and deca-vanadates are also bound to sarcoplasmic-reticulum membranes with high affinity. The binding of the vanadate oligoanions may explain some of the effects of vanadates on the conformation and crystallization of Ca2+-transport ATPase.
Full text
PDF

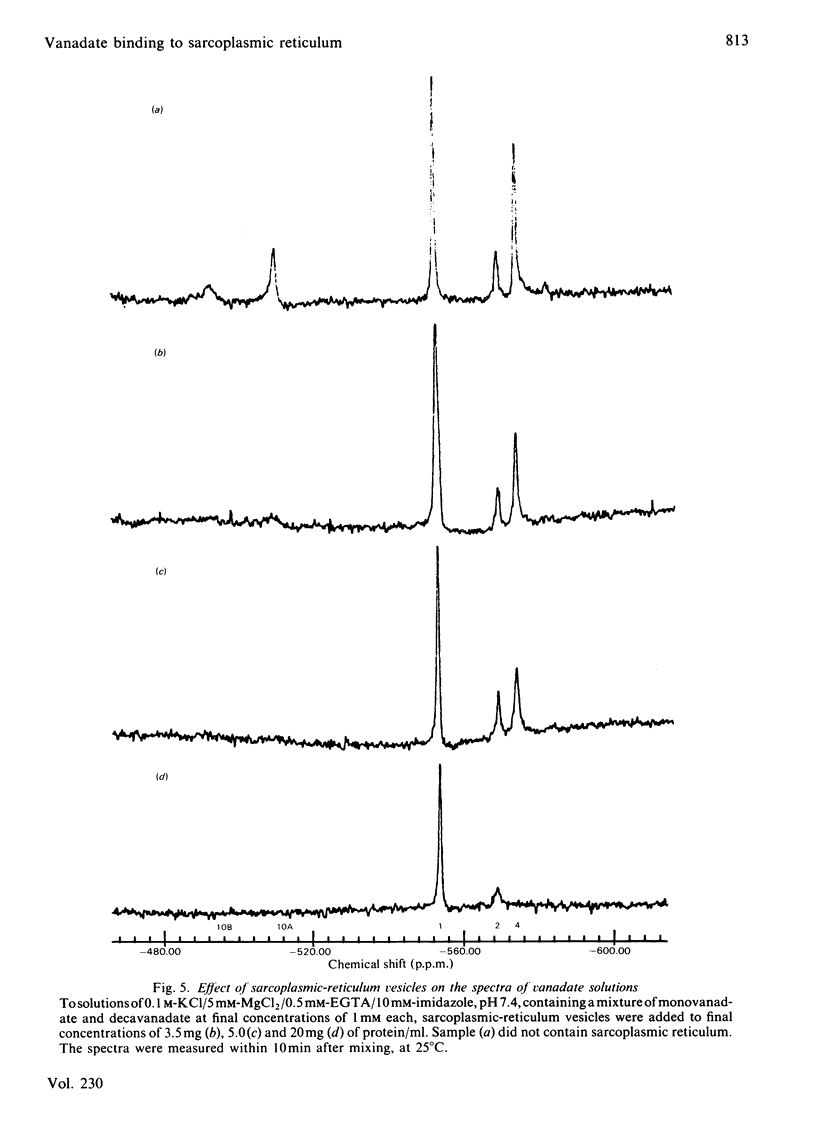


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd D. W., Kustin K. Vanadium: a versatile biochemical effector with an elusive biological function. Adv Inorg Biochem. 1984;6:311–365. [PubMed] [Google Scholar]
- Choate G., Mansour T. E. Studies on heart phosphofructokinase. Decavanadate as a potent allosteric inhibitor at alkaline and acidic pH. J Biol Chem. 1979 Nov 25;254(22):11457–11462. [PubMed] [Google Scholar]
- Climent F., Bartrons R., Pons G., Carreras J. Effect of vanadate on phosphoryl transfer enzymes involved in glucose metabolism. Biochem Biophys Res Commun. 1981 Jul 30;101(2):570–576. doi: 10.1016/0006-291x(81)91297-3. [DOI] [PubMed] [Google Scholar]
- DeMaster E. G., Mitchell A. A comparison of arsenate and vanadate as inhibitors or uncouplers of mitochondrial and glycolytic energy metabolism. Biochemistry. 1973 Sep 11;12(19):3616–3621. doi: 10.1021/bi00743a007. [DOI] [PubMed] [Google Scholar]
- Dux L., Martonosi A. Ca2+-ATPase membrane crystals in sarcoplasmic reticulum. The effect of trypsin digestion. J Biol Chem. 1983 Aug 25;258(16):10111–10115. [PubMed] [Google Scholar]
- Dux L., Martonosi A. The regulation of ATPase-ATPase interactions in sarcoplasmic reticulum membrane. I. The effects of Ca2+, ATP, and inorganic phosphate. J Biol Chem. 1983 Oct 10;258(19):11896–11902. [PubMed] [Google Scholar]
- Dux L., Martonosi A. Two-dimensional arrays of proteins in sarcoplasmic reticulum and purified Ca2+-ATPase vesicles treated with vanadate. J Biol Chem. 1983 Feb 25;258(4):2599–2603. [PubMed] [Google Scholar]
- Goodno C. C. Inhibition of myosin ATPase by vanadate ion. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2620–2624. doi: 10.1073/pnas.76.6.2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindquist R. N., Lynn J. L., Jr, Lienhard G. E. Possible transition-state analogs for ribonuclease. The complexes of uridine with oxovanadium(IV) ion and vanadium(V) ion. J Am Chem Soc. 1973 Dec 26;95(26):8762–8768. doi: 10.1021/ja00807a043. [DOI] [PubMed] [Google Scholar]
- Medda P., Hasselbach W. The vanadate complex of the calcium-transport ATPase of the sarcoplasmic reticulum, its formation and dissociation. Eur J Biochem. 1983 Dec 1;137(1-2):7–14. doi: 10.1111/j.1432-1033.1983.tb07788.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H., Jilka R. L., Boland R., Martonosi A. N. Mechanism of ATP hydrolysis by sarcoplasmic reticulum and the role of phospholipids. J Biol Chem. 1976 Sep 10;251(17):5414–5423. [PubMed] [Google Scholar]
- Pai E. F., Sachsenheimer W., Schirmer R. H., Schulz G. E. Substrate positions and induced-fit in crystalline adenylate kinase. J Mol Biol. 1977 Jul;114(1):37–45. doi: 10.1016/0022-2836(77)90281-9. [DOI] [PubMed] [Google Scholar]
- Pick U., Bassilian S. Modification of the ATP binding site of the Ca2+ -ATPase from sarcoplasmic reticulum by fluorescein isothiocyanate. FEBS Lett. 1981 Jan 12;123(1):127–130. doi: 10.1016/0014-5793(81)80035-x. [DOI] [PubMed] [Google Scholar]
- Pick U., Karlish S. J. Indications for an oligomeric structure and for conformational changes in sarcoplasmic reticulum Ca2+-ATPase labelled selectively with fluorescein. Biochim Biophys Acta. 1980 Nov 20;626(1):255–261. doi: 10.1016/0005-2795(80)90216-0. [DOI] [PubMed] [Google Scholar]
- Pick U., Karlish S. J. Regulation of the conformation transition in the Ca-ATPase from sarcoplasmic reticulum by pH, temperature, and calcium ions. J Biol Chem. 1982 Jun 10;257(11):6120–6126. [PubMed] [Google Scholar]
- Pick U. The interaction of vanadate ions with the Ca-ATPase from sarcoplasmic reticulum. J Biol Chem. 1982 Jun 10;257(11):6111–6119. [PubMed] [Google Scholar]
- Soman G., Chang Y. C., Graves D. J. Effect of oxyanions of the early transition metals on rabbit skeletal muscle phosphorylase. Biochemistry. 1983 Oct 11;22(21):4994–5000. doi: 10.1021/bi00290a018. [DOI] [PubMed] [Google Scholar]
- Varga S., Csermely P., Martonosi A. The binding of vanadium (V) oligoanions to sarcoplasmic reticulum. Eur J Biochem. 1985 Apr 1;148(1):119–126. doi: 10.1111/j.1432-1033.1985.tb08815.x. [DOI] [PubMed] [Google Scholar]


