Abstract
Background:
Parkinson’s disease (PD) is often characterized by altered rates and patterns of neuronal activity in the sensorimotor regions of the basal ganglia thalamocortical network. Little is known, however, regarding how neuronal activity in the executive control network of the brain changes in the parkinsonian condition.
Objective:
Investigate the impact of parkinsonism on neuronal activity in the dorsolateral prefrontal cortex (DLPFC), a key region in executive control, during a go/nogo reaching task.
Methods:
Using a within-subject design, single and multi-unit neuronal activity was recorded in the DLPFC of a nonhuman primate before and after the induction of mild parkinsonism using the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
Results:
Coincident with development of mild parkinsonian motor signs, there was a marked reduction in the percentage of DLPFC cells with significant task-related firing rate modulation during go and nogo conditions.
Conclusions:
These results suggest that DLPFC dysfunction may occur early in parkinsonism and contribute to cognitive impairments and disrupted executive function often observed in PD patients.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by disruptions in motor function, e.g., delayed movement initiation, decreased movement speed, rest tremor, and increased joint rigidity1. Non-motor symptoms, however, such as cognitive dysfunction, are also prevalent components of PD2,3. These cognitive impairments include changes in executive functions such as working memory, set shifting, and movement inhibition4–6. Functional imaging studies have shown that the DLPFC, a critical node in the BGTC network involved in executive function, is impaired in PD5,7–10. This impairment is likely secondary to the role of dopamine in mediating executive functions involving the DLPFC11,12. While some functional imaging studies suggest parkinsonism results in hypoactivation in the prefrontal cortex13,14, consistent with classic models of basal ganglia function in which excessive inhibitory activity from the internal segment of the globus pallidus is hypothesized to result in reduced excitatory thalamo-cortical drive15,16, other studies have found hyperactivation in DLPFC in PD patients compared to controls5,17,18. There are limited neuronal data at the single unit level characterizing changes in DLPFC activity in PD to support or refute either of these findings.
One task known to probe executive function in the context of DLPFC is the go/nogo task, which require subjects to discern between two target types that indicate either taking or avoiding an action19,20. Additionally, the go/nogo paradigm has been useful to show alterations in executive function in PD, such as movement preparation and response inhibition19,20. In this study, a go/nogo touch screen task was used to engage DLPFC, and to test the hypothesis that neuronal processing in the DLPFC is abnormal in early PD through the induction of a mild parkinsonian state. We compared task-related single and multi-unit neuronal firing characteristics in the DLPFC of a nonhuman primate before and after induction of mild parkinsonism using the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP).
Methods
Surgical procedures:
All procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and complied with US Public Health Service policy on the humane care and use of laboratory animals. One adult female rhesus macaque (Macaca mulatta, 20 years of age) was used in this study. Surgery was performed under isoflurane anesthesia using aseptic techniques. The animal was implanted in the right DLPFC with a 96-channel Utah microelectrode array (Pt-Ir, 1.5 mm depth, 400 um inter-electrode spacing, Blackrock Microsystems) using surgical methods described previously21–23. DLPFC was identified based on sulcal landmarks during the array implantation surgery (Fig. 2A).
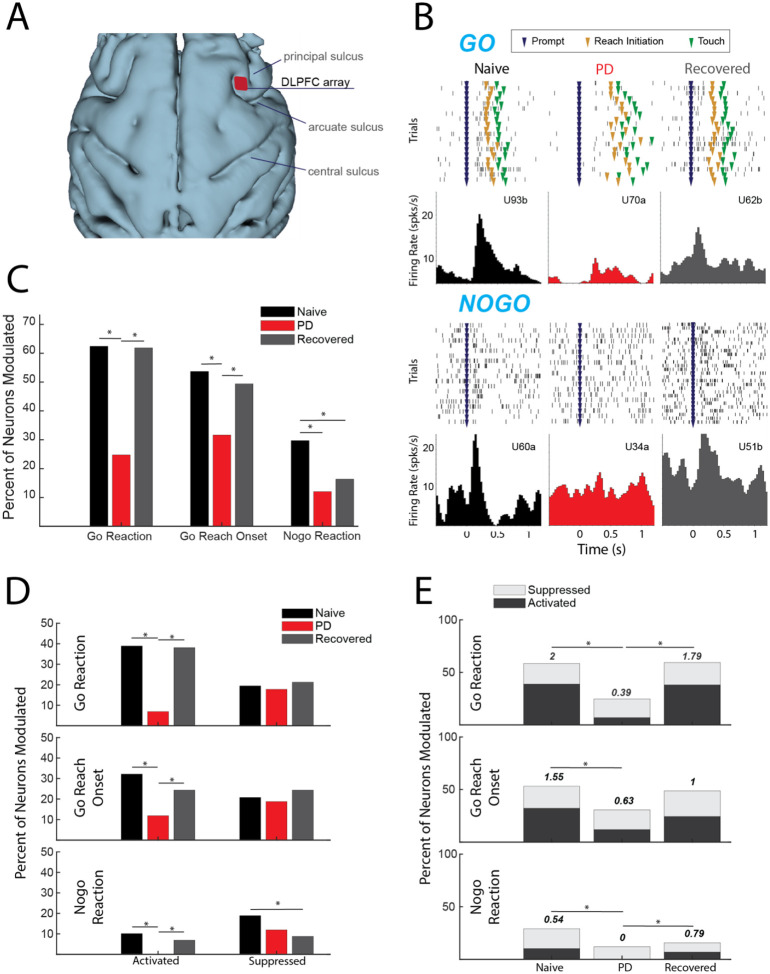
Fig 2.
(A) 3D reconstruction of cortex with DLPFC array location. (B) Example cells during go (upper) and nogo (lower) trials from each condition. (C) Percentage of cells modulating during comparison window (p < 0.05, X2). (D) Percent of total cells activated (left) and suppressed (right) (p < 0.05, X2). (E) Ratio of percent activated over percent suppressed, indicated by number above the bars (p < 0.05, X2).
Go/nogo task and data collection:
The animal was trained to perform a visually cued go/nogo reaching task (Fig 1A). Trials were initiated when the animal placed its left hand on a capacitive touchpad (“start-pad”) and, after a two second delay, a cue appeared in one of three randomly selected locations. Two seconds following this cue a “go” target appeared at the selected location in 80% of the trials, and a “nogo” target would appear in 20% of the trials. A successful go trial required the primate to leave the start-pad within 1.5 seconds and touch the target within another 1.5 seconds. A successful nogo trial required the animal to hold on the start-pad for 1.5 seconds following target presentation. Successful trials resulted in a juice reward. Reaction time was defined as the time between presentation of the go target and reach initiation (the time when the animal’s hand left the start-pad). Reach duration was defined as the time between reach initiation and contact with the target. The animal initiated the next trial by voluntarily returning to the start-pad. Go and nogo target appearance timepoints were extracted from the task software and reach initiation timepoints were recorded from the start-pad. Raw neurophysiological data were collected using a TDT workstation (Tucker Davis Technologies) operating at ~ 25 kHz sampling rate. Activity of DLPFC units was recorded while the animal was seated, head fixed, in a primate chair performing the reaching task.
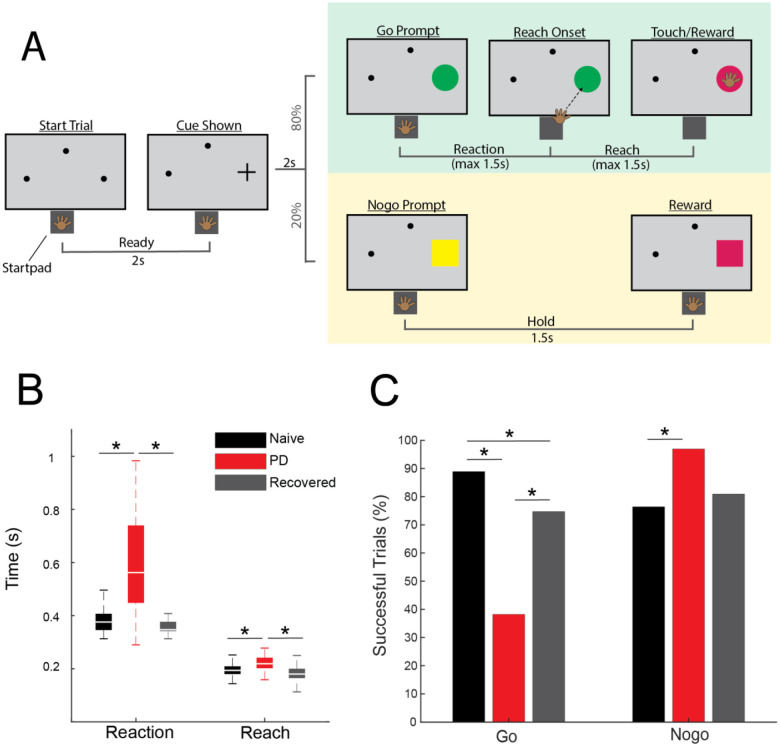
Fig 1.
(A) Go/nogo task paradigm. (B) Reach and reaction times during successful go trials in naive (black), PD (red), and recovered (gray) (WRS, p < 0.05). (C) Percentage of successful go and nogo trials (X2, p < 0.05).
Once data were collected in the normal state, the animal was rendered parkinsonian by one intracarotid injection of MPTP (0.4mg/kg). Overall parkinsonian severity was assessed using a modified Unified Parkinson’s Disease Rating Scale (mUPDRS), which rated appendicular motor symptoms (upper and lower limb rigidity, bradykinesia, akinesia, and tremor) on the hemi-body contralateral to neural recordings using a 0–3 scale (0 = normal, 3 = severe, maximum total score = 27)24. We observed mild parkinsonian signs (mUPDRS: 2.8±1.14, 5 ratings) after MPTP injection; however motor signs improved over subsequent weeks and returned to a baseline mUPDRS score of 0 after 11 days (defined here as the “recovered” state). Post-MPTP neural data were obtained in both the mild parkinsonian and recovered states.
Statistical analysis of neuronal data:
Neuronal recordings were analyzed offline using custom software developed in MATLAB (Mathworks) and Offline Sorter (Plexon). Raw data were bandpass filtered 300–5000 Hz, and single and multi-units were isolated and sorted using principal component and template-based methods in Offline Sorter (hereafter referred to collectively as “units”). Spike trains were aligned to go target appearance, nogo target appearance, or reach onset. A trial-averaged spike density function for each unit was generated by convolving each spike with a gaussian kernel (60 ms variance) and a time resolution of 10 ms. The baseline firing rate for each unit was defined as the mean of the spike density function during a 1.5 second period beginning 0.25 seconds after trial initiation, before cue presentation. Units with an extremely low firing rate were excluded from further analysis (less than 0.75 spikes per second). To investigate neuronal modulation during the reaction time period, spiking activity immediately following target appearance until the minimum reaction time across all trials (255 ms) was used. The same time frame was used for nogo trials. To investigate neuronal modulation during reach initiation, activity 50 ms prior to reach onset until the minimum reach duration across all trials (144 ms) was used. A one-sample 2-tailed t-test was used to compare the mean baseline firing rate of each unit to the firing rates during the reaction or reach periods. Units with a significant change in firing rate during the analysis window (p ≤ 0.002) compared to baseline were classified as modulated.25 With a time resolution of 10 ms and a maximum window of 255 ms, the maximum number of comparisons was 25, so 0.002 (0.05/25) was chosen as a conservative threshold for determining whether a cell was modulated. Units with a significant increase in firing rate were further classified as activated, and those with a significant decrease as suppressed. Reaction times were compared across states using the Wilcoxon rank sum test (WRS), as were the reach durations. Chi-squared tests [X2(DoF, N)] were used to compare the percentage changes in the number of modulated, activated and suppressed units, the ratio of activated over suppressed units, and changes in task success rates, between the naïve, PD, and recovered conditions.
Results
Effects of MPTP on task performance:
MPTP administration induced a mild parkinsonian state based on clinical assessments (2.8±1.14, 5 ratings). In the recovered state the mUPDRS score returned to zero. The NHP completed 288 go trials and 72 nogo trials in the naïve state and recovered state. In the parkinsonian condition the NHP completed 123 go trials and 31 nogo trials. As indicated in Fig. 1B, reaction times increased in the parkinsonian condition compared to the naïve (WRS; z = −8.2862, p < 0.001) and recovered states (WRS; z = 8.7851, p < 0.001). Reach times were also longer in the parkinsonian condition compared to naïve (WRS; z = −5.4332, p < 0.001) and recovered states (WRS; z = 6.1389, p < 0.001). There was a decrease in task success rate during go trials from 81% in naïve to 36% in the parkinsonian condition (Fig. 1C, left) [X2(1,411) = 85.1, p < 0.001]. Task success rate during go trials was higher in the recovered state compared to the parkinsonian condition [X2(1,411) = 38.0233, p < 0.001]. Nogo trial success rates were increased in the parkinsonian condition compared to the naïve state [X2(1,103) = 6.2442, p = 0.0125]. In the recovered state, nogo task performance returned to a level similar to that observed in the naïve state (Fig. 1C, right).
Parkinsonism alters neuronal modulation in DLPFC:
A total of 410 units in DLPFC were recorded in this study (n = 149 naïve, n = 101 parkinsonian, and n = 160 recovered. Representative neurons (Fig. 2B) illustrate our main finding that there is a significant reduction in task-related unit activity in DLPFC in the mild parkinsonian condition. While 62.4% of units had significant firing rate modulation during the go reaction time period in the naïve state, in the parkinsonian condition only 24.8% were modulated ([X2(1,250) = 34.2638, p < 0.001]). Similarly, there was a reduction in the percent of units with significant modulation in the go reach period (53.7% in naïve compared to 31.7% in the parkinsonian condition, [X2(1, 250) = 11.7901, p < .001]) (Fig. 2C). During the recovered state the percent of units with a significant firing rate modulation returned to levels similar to the naïve state for both go reaction (naive: 62.4%, PD: 24.8%, recovered: 61.9%) [X2(1,261) = 34.2148, p < 0.001] and go reach periods (naive: 53.7%, PD: 31.7%, recovered: 49.4%) [X2(1,261) = 7.9289, p = 0.005]. Modulation during the nogo reaction period decreased from 29.7% in the naïve state to 12.0% in the parkinsonian condition [X2(1, 250) = 10.7307, p = 0.001], but did not return to naïve levels in the recovered state [X2(1,261) = 0.9288, p = 0.3352].
Parkinsonism decreases neuronal activation:
The results presented in Figure 2C showed that fewer DLPFC cells were modulated in the mild PD condition, irrespective of whether that modulation was due to significant increases (activation) or decreases (suppression) in firing rate. We then examined how the parkinsonian state impacted the proportion of cells classified into these modulation subcategories (Fig. 2D) As described below, we found that the reduction in modulation in the PD condition was driven predominantly by a loss of cells that were significantly activated during the go/nogo task.
Activation:
The percentage of cells activated during the go reaction period decreased from 38.9% in the naïve state to 6.9% in the parkinsonian condition [X2(1,250) = 32.0287, p < 0.001)] and the percentage of cells activated during the reach period decreased from 32.2% to 11.9% [X2(1,250) = 10.0132, p < 0.001]. In addition, the percentage of cells activated during the nogo reaction period decreased to zero from naïve to the parkinsonian condition [X2(1,250) = 10.7876, p < 0.001]. In the recovered state the percentage of activated units increased back to naïve levels during go reaction (38.1%) [X2(1,261) = 31.2728, p < 0.001], reach (24.4%) [X2(1,261) = 6.1473, p = 0.0132], and nogo reaction (6.9) [X2(1,261) = 7.2251, p = 0.0072].
Suppression:
There was no significant difference in the percentage of suppressed units during the go reaction period [X2(1,250) = 0.1062, p = 0.7445], reach [X2(1,250) = 0.1495, p = 0.699], or the nogo reaction period [X2(1,250) = 2.1119, p = 0.1462] between naïve and the parkinsonian condition. Similarly, there was no significant difference in the percentage of suppressed units between the parkinsonian condition and recovered state during the go reaction period [X2(1,261) = 0.4561, p = 0.4994], reach period [X2(1,261) = 1.1087, p = 0.2924], or nogo reaction period [X2(1,261) = 0.6939, p = 0.4048]. During the nogo reaction period there was a significant decrease in suppression between the naïve and recovered states [X2(1,309) = 6.6395, p = 0.01] (Fig. 2D).
Ratio of activation to suppression:
The loss of activation without a significant change in suppression resulted in a decrease in the ratio of activation to suppression from naïve to the parkinsonian state during all three analysis periods (Fig. 2E). This ratio decreased from 2 to 0.39 during go reaction [X2(1,118) = 13.6973, p < 0.001], 1.55 to 0.63 during reach [X2(1,112) = 6.7274, p = 0.01], and 0.54 to 0 during nogo reaction [X2(1,56) = 6.1091, p = 0.0134]. In the recovered state, the ratio of activated to suppressed cells increased during go reaction [X2(1,124) = 11.6236, p < 0.001] and nogo reaction [X2(1,38) = 8.0947, p = 0.004], returning closer to that observed in the naïve state.
Discussion
The present study investigated the effects of MPTP-induced parkinsonism on neuronal activity in the DLPFC of a nonhuman primate. A unique advantage of this animal model not feasible in human studies is that it allows for a within-subject comparison of changes in activity of neuronal cell populations between healthy and parkinsonian conditions. Induction of the parkinsonian state was associated with a decrease in task-dependent neuronal modulation of firing rates. This reduced modulation was driven by a decrease in the number of activated neurons, leading to a decrease in the ratio of activated to suppressed neurons. The recovered state was associated with an increase in task-dependent modulation, driven by increased activation, compared to the parkinsonian condition. Importantly, these changes in neural activity occurred even in a mild state of parkinsonism, suggesting that the mechanisms involved in cognitive dysfunction may be initiated in the early stages of PD.
Comparison to previous studies of DLPFC in PD:
Our results suggest DLPFC is hypoactive during motor preparation and execution in mild parkinsonism. Consistent with this observation, studies utilizing PET and fMRI have found decreased activation during both self-initiated and externally cued timing tasks in the right DLPFC in PD patients compared to healthy control subjects13,14,26. These groups hypothesized that the decrease in DLPFC activation is a result of reduced thalamic output to the cortex as a result of decreased dopamine in the basal ganglia, consistent with the classical model of PD pathophysiology15. Nevertheless, there are multiple imaging studies that have identified increased rather than decreased activation in the DLPFC during motor tasks5,17,18. Martin et al. used fMRI to identify increased activation in the DLPFC during motor planning in early-stage PD patients performing a finger tapping task, but found no change in DLPFC activity during movement execution17. Similarly, Disbrow et al. found increased BOLD signal in DLPFC bilaterally prior to un-cued movement5. Some suggest that this relative hyperactivity in DLPFC could be a compensatory mechanism to accommodate for disrupted function of motor areas in PD17,18. Evidence of compensatory mechanisms in these studies were supported by a lack of change in task performance despite increased motor symptoms based on UPDRS-III motor signs, though this was not a phenomenon observed in the present study (i.e., motor signs were observed and quantified by clinical exam as well as during the task).
While our findings are consistent with the classical model of PD, it is also possible that though our task clearly engaged DLPFC, it may not have required the conceptualization of movement prior to target appearance that may involve compensatory mechanisms, as suggested by Martin et al.17. Furthermore, the high success rate in the parkinsonian condition suggests that the primate may not have been habituated to the go condition, and therefore may have been simply waiting for the go cue to appear before planning movement27. There is also the possibility that the parkinsonian condition obtained was too mild to have induced any compensatory effects. Some studies, however, finding hyperactivation in DLPFC suggested compensatory effects were present in mild PD patients17,18. While we did not find evidence of an overactive DLPFC in the PD state as might be hypothesized based on these imaging studies, future studies are necessary to fully probe the hypothesis of compensatory mechanisms triggered in the frontal cortex in PD. For example, while our data was collected in a mild PD state, more severe PD states should be investigated to determine whether hyperactivity develops when motor signs are more severe, and compensation is necessary to perform the task.
Recovery from MPTP injection:
Recovery from a mild state of parkinsonism following MPTP administration has been previously documented28. The mechanisms of this recovery, however, are not fully understood. Hypotheses include reactive synaptogenesis (temporary, quick onset synapse formation) and denervation hypersensitivity (increased sensitivity to a neurotransmitter after loss of synapses), and uptake of excess dopamine in the nigrostriatal circuit29–31. The most likely mechanism underlying this recovery however, is that MPTP administration causes cell injury, but some cells recover over time31. By including the recovered condition in this study we were able to show a possible correlation between DLPFC activity and go/nogo task behaviors such as success rate and reaction time. Although the changes in DLPFC activity in the parkinsonian condition that resolved following recovery provide compelling evidence is support of the role of DLPFC deficits in the observed motor dysfunction, whether the change in behavior was the cause or the result of the change in DLPFC activity needs to be determined with additional studies.
Limitations and future directions:
This study included only one NHP, but represents our early findings that are part of a larger study where multiple animals are being enrolled to validate these findings. Another potential limitation is that the task may not have probed the response inhibition aspects of the DLPFC that we had intended, which may be reflected in the increased success rate of nogo trials in the parkinsonian condition. In the future we will modify the task paradigm to induce response inhibition while allowing us to investigate the changes in the DLPFC in early PD where the animals are still able to perform the task. There are challenges, however, to designing tasks that are both cognitively complex and feasible for a parkinsonian animal given their impaired cognitive and motor functions. Regardless, this study provided data to support the finding that even in a mild disease state there are salient changes to neural activity in the DLPFC. In the future we may look to quantify cognitive performance of the primate while parkinsonian to characterize neurophysiological changes related specifically to cognitive disruption and identify how dopamine replacement therapy and DBS alter pre-frontal cortical activity. While deep brain stimulation is effective at modulating motor cortex activity in PD patients, its effect on frontal cortical regions is less well understood32. Understanding the neural mechanisms underlying frontal cortical dysfunction in PD will help motivate and inform neuromodulation techniques that would allow us to improve neural function for both parkinsonian motor and cognitive behaviors.
Acknowledgement
We would like to thank our colleagues in the Neuromodulation Research Center for helpful comments and critiques related to this study and especially thank our animal core team of Claudia Hendrix, Hannah Baker, Adele DeNicola, and Elizabeth McDuell as well as our veterinary and animal care colleagues at the University of Minnesota Research Animal Resources (RAR).
Funding Sources and Conflict of Interest
This work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS) R01-NS058945, R01-NS037019, R37-NS077657, R01NS117822, R01NS110613, P50-NS123109, MnDRIVE (Minnesota’s Discovery Research and Innovation Economy) Brain Conditions Program, and the Engdahl Family Foundation.
Jerrold L. Vitek serves as a consultant for Medtronic, Boston Scientific, and Abbott. He also serves on the Executive Advisory Board for Abbott and is a member of the scientific advisory board for Surgical Information Sciences. JLV has no non-financial conflicts to disclose. The remaining authors have no competing interests to disclose.
Funding Statement
This work was supported by the National Institutes of Health, National Institute of Neurological Disorders and Stroke (NINDS) R01-NS058945, R01-NS037019, R37-NS077657, R01NS117822, R01NS110613, P50-NS123109, MnDRIVE (Minnesota’s Discovery Research and Innovation Economy) Brain Conditions Program, and the Engdahl Family Foundation.
Jerrold L. Vitek serves as a consultant for Medtronic, Boston Scientific, and Abbott. He also serves on the Executive Advisory Board for Abbott and is a member of the scientific advisory board for Surgical Information Sciences. JLV has no non-financial conflicts to disclose. The remaining authors have no competing interests to disclose.
Footnotes
Ethical Compliance Statement
This study was approved by the Institutional Animal Care and Use Committee (IACUC). Informed patient consent was not necessary for this work. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
References
- 1.Jankovic J. Parkinson’s disease: clinical features and diagnosis. Journal of Neurology, Neurosurgery & Psychiatry 79, 368–376 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Michely J. et al. Differential effects of dopaminergic medication on basic motor performance and executive functions in Parkinson’s disease. Neuropsychologia 50, 2506–2514 (2012). [DOI] [PubMed] [Google Scholar]
- 3.Elgh E. et al. Cognitive function in early Parkinson’s disease: a population-based study. European Journal of Neurology 16, 1278–1284 (2009). [DOI] [PubMed] [Google Scholar]
- 4.Dujardin K. et al. The spectrum of cognitive disorders in Parkinson’s disease: A data-driven approach. Movement Disorders 28, 183–189 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Disbrow E. A. et al. Movement Activation and Inhibition in Parkinson’s Disease: a Functional Imaging Study. J Parkinsons Dis 3, 181–192 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zgaljardic D. J. et al. An Examination of Executive Dysfunction Associated with Frontostriatal Circuitry in Parkinson’s Disease. Journal of Clinical and Experimental Neuropsychology 28, 1127–1144 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cools R. & D’Esposito M. Inverted-U shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry 69, e113–e125 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caspers J. et al. Differential Functional Connectivity Alterations of Two Subdivisions within the Right dlPFC in Parkinson’s Disease. Frontiers in Human Neuroscience 11, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owen A. M. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist 10, 525–537 (2004). [DOI] [PubMed] [Google Scholar]
- 10.Leh S. E., Petrides M. & Strafella A. P. The Neural Circuitry of Executive Functions in Healthy Subjects and Parkinson’s Disease. Neuropsychopharmacol 35, 70–85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dauer W. & Przedborski S. Parkinson’s Disease: Mechanisms and Models. Neuron 39, 889–909 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Durstewitz D., Seamans J. K. & Sejnowski T. J. Dopamine-mediated stabilization of delay-period activity in a network model of prefrontal cortex. J Neurophysiol 83, 1733–1750 (2000). [DOI] [PubMed] [Google Scholar]
- 13.Yu H., Sternad D., Corcos D. M. & Vaillancourt D. E. Role of Hyperactive Cerebellum and Motor Cortex in Parkinson’s Disease. Neuroimage 35, 222–233 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis S. J. G., Dove A., Robbins T. W., Barker R. A. & Owen A. M. Cognitive Impairments in Early Parkinson’s Disease Are Accompanied by Reductions in Activity in Frontostriatal Neural Circuitry. J. Neurosci. 23, 6351–6356 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeLong M. R. Primate models of movement disorders of basal ganglia origin. Trends in Neurosciences 13, 281–285 (1990). [DOI] [PubMed] [Google Scholar]
- 16.Albin R. L., Young A. B. & Penney J. B. The functional anatomy of basal ganglia disorders. Trends in Neurosciences 12, 366–375 (1989). [DOI] [PubMed] [Google Scholar]
- 17.Martin J. A. et al. Disentangling motor planning and motor execution in unmedicated de novo Parkinson’s disease patients: An fMRI study. NeuroImage: Clinical 22, 101784 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ranchet M. et al. Changes in Prefrontal Cortical Activity During Walking and Cognitive Functions Among Patients With Parkinson’s Disease. Frontiers in Neurology 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Menon V., Adleman N. E., White C. d., Glover G. h. & Reiss A. l. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping 12, 131–143 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garavan H., Ross T. J. & Stein E. A. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A 96, 8301–8306 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rousche P. J. & Normann R. A. A method for pneumatically inserting an array of penetrating electrodes into cortical tissue. Ann Biomed Eng 20, 413–422 (1992). [DOI] [PubMed] [Google Scholar]
- 22.Maynard E. M., Nordhausen C. T. & Normann R. A. The Utah Intracortical Electrode Array: A recording structure for potential brain-computer interfaces. Electroencephalography and Clinical Neurophysiology 102, 228–239 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Escobar Sanabria D. et al. Parkinsonism and vigilance: alteration in neural oscillatory activity and phase-amplitude coupling in the basal ganglia and motor cortex. Journal of Neurophysiology 118, 2654–2669 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitek J. L., Zhang J., Hashimoto T., Russo G. S. & Baker K. B. External pallidal stimulation improves parkinsonian motor signs and modulates neuronal activity throughout the basal ganglia thalamic network. Experimental Neurology 233, 581–586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquereau B., DeLong M. R. & Turner R. S. Primary motor cortex of the parkinsonian monkey: altered encoding of active movement. Brain 139, 127–143 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jahanshahi M. et al. Self-initiated versus externally triggered movements: I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118, 913–933 (1995). [DOI] [PubMed] [Google Scholar]
- 27.Casey B. J. et al. A Developmental Functional MRI Study of Prefrontal Activation during Performance of a Go-No-Go Task. Journal of Cognitive Neuroscience 9, 835–847 (1997). [DOI] [PubMed] [Google Scholar]
- 28.Taylor J. R., Elsworth J. D., Roth R. H., Sladek J. R. & Redmond D. E. Severe long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism in the vervet monkey (Cercopithecus aethiops sabaeus). Neuroscience 81, 745–755 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Eidelberg E., Brooks B. A., Morgan W. W., Walden J. G. & Kokemoor R. H. Variability and functional recovery in the N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of parkinsonism in monkeys. Neuroscience 18, 817–822 (1986). [DOI] [PubMed] [Google Scholar]
- 30.Franke F., Quian Quiroga R., Hierlemann A. & Obermayer K. Bayes optimal template matching for spike sorting – combining fisher discriminant analysis with optimal filtering. J Comput Neurosci 38, 439–459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogerts B., Häntsch J. & Herzer M. A morphometric study of the dopamine-containing cell groups in the mesencephalon of normals, Parkinson patients, and schizophrenics. Biol Psychiatry 18, 951–969 (1983). [PubMed] [Google Scholar]