Abstract
The bacterium Pseudomonas aeruginosa is an opportunistic pathogen that can cause lung, skin, wound, joint, urinary tract, and eye infections. While P. aeruginosa is known to exhibit a robust competitive response towards other bacterial species, this bacterium is frequently identified in polymicrobial infections where multiple species survive. For example, in prosthetic joint infections (PJIs), P. aeruginosa can be identified along with other pathogenic bacteria including Staphylococcus aureus, Enterococcus faecalis, and Corynebacterium striatum. Here we have explored the survival and behavior of such microbes and find that E. faecalis readily survives culturing with P. aeruginosa while other tested species do not. In each of the tested conditions, E. faecalis growth remained unchanged by the presence of P. aeruginosa, indicating a unique mutualistic interaction between the two species. We find that E. faecalis proximity leads P. aeruginosa to attenuate competitive behaviors as exemplified by reduced production of Pseudomonas quinolone signal (PQS) and pyocyanin. Reduced alkyl quinolones is important to E. faecalis as it will grow in supernatant from a quinolone mutant but not P. aeruginosa wildtype in planktonic culture. The reduced pyocyanin production of P. aeruginosa is attributable to production of ornithine by E. faecalis, which we recapitulate by adding exogenous ornithine to P. aeruginosa monocultures. Similarly, co-culture with an ornithine-deficient strain of E. faecalis leads P. aeruginosa to yield near mono-culture amounts of pyocyanin. Here, we directly demonstrate how notorious pathogens such as P. aeruginosa might persist in polymicrobial infections under the influence of metabolites produced by other bacterial species.
Introduction
Many infections are polymicrobial in nature, including those commonly associated with skin wounds, female or male urinary tract, prosthetic joints, and the lung of individuals with cystic fibrosis lung (1–3). Understanding the interspecies interaction between these polymicrobial communities is likely critical to understanding the survival of pathogens in such environments to include mechanisms of antibiotic resistance and evasion of host immune responses. The bacterium Pseudomonas aeruginosa is commonly identified in a variety of infections that are polymicrobial (3–8). P. aeruginosa virulence factors and toxic attributes are well documented in the literature (9, 10). While P. aeruginosa is well-known to exhibit competitive behavior towards other bacterial species (11–15), P. aeruginosa does not always dominate. For example, many studies have characterized competitive interactions between P. aeruginosa and Staphylococcus aureus (16, 17), as both are frequently co-isolated in infections of the lungs and skin and the ratio of P. aeruginosa to S. aureus does not necessarily change over time (18). Representative interactions between P. aeruginosa and other microbes have yet to receive the same research attention. For example, P. aeruginosa co-occurrence with Enterococcus faecalis (4–6, 19–23) is common as well, but little research has investigated the interaction between E. faecalis and P. aeruginosa. There are not clear hallmark outcomes associated with E. faecalis and P. aeruginosa co-infection. E. faecalis and P. aeruginosa have been specifically identified together in wound infections (4, 20, 24–27), catheter-associated urinary tract infection (CAUTI)(28), periodontal disease (29), and prosthetic joint infection (3, 30). With the rising concern about antibiotic resistance for the Enterococci and Pseudomonads, and high incidence of these pathogens among nosocomial infections, understanding the co-occurrence of these two specific bacterial species is especially important.
Here we examined P. aeruginosa in pairwise combination with a select handful of other bacteria that can be present in prosthetic joint infection (PJI). We find that E. faecalis survives with P. aeruginosa under conditions that outcompete all other tested species. We show that these two species can be co-cultured in planktonic culture and biofilms without compromising the growth of either species. Additionally, we demonstrate an effect of E. faecalis on P. aeruginosa Pseudomonas quinolone signal production, as well as the synthesis of pyocyanin, a P. aeruginosa virulence factor. These results highlight the ability of E. faecalis to dampen the competitive response of P. aeruginosa. We specifically identify and confirm a role of the amino acid ornithine in mediating this unique interaction. In addition to potential clinical applications, investigating the ability of E. faecalis to interfere with established P. aeruginosa alkyl quinolone (AQ) mediated pathways can further our understanding of the complex milieu of factors elicited and sensed by P. aeruginosa as part of polymicrobial infections.
Results
P. aeruginosa does not exhibit killing towards E. faecalis in co-culture
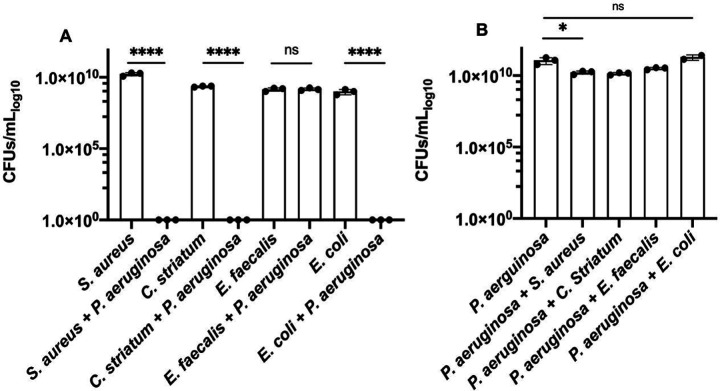
We were interested to better understand interactions between different bacterial species that have been identified in infection environments like prosthetic joint infection (PJI) (3, 31). We took an approach to characterize responses from a series of pairwise culture experiments that tested P. aeruginosa with other PJI microbes. We selected S. aureus, C. striatum, and E. faecalis, as representative PJI co-isolates. Each species was grown in rich Mueller Hinton broth, alone and in co-culture with P. aeruginosa. We found that only E. faecalis exhibited measurable CFUs when co-cultured with P. aeruginosa (Figure 1A) while S. aureus and C. striatum had no viable cell count. We were surprised that E. faecalis exhibited no significant difference in CFUs from its mono-culture control (Figure 1A). We also tested E. coli as a canonical laboratory control and found, as expected, that E. coli did not survive in co-culture with P. aeruginosa. In contrast, P. aeruginosa growth was largely unaffected by the inclusion of any competing species in these experiments as differences in P. aeruginosa CFUs from these different co-cultures were minor or insignificant in comparison to monoculture conditions (Figure 1B). While our results are in general agreement with several prior studies reporting that P. aeruginosa is generally antagonistic against competing microbes (11, 13, 16, 32, 33), we were struck by the magnitude of difference between the survival of E. faecalis in comparison to the killing of S. aureus, C. striatum, and E. coli measured in our experiments. We were interested to better understand the cooperative relationship between P. aeruginosa and E. faecalis.
Figure 1.
Colony forming units (CFUs) for bacterial species in monoculture and coculture with P. aeruginosa, show differences in survival after 24 hours in Mueller Hinton broth. A. S. aureus, C. striatum, and E. coli each have no viable CFUs after being cultured with P. aeruginosa. E. faecalis CFUs in coculture with P. aeruginosa remain unchanged from monoculture (limit of detection (LOD) = 1). B. P. aeruginosa CFUs have a slight decrease when cultured with S. aureus and C. striatum and are unchanged when cocultured with E. faecalis and E. coli. CFUs were determined from 3 replicates dilutions from the countable plate assay for the range of 100 – 10−9 dilution. Pairwise comparisons by Welch’s t-test are indicated on the plot: ns= P > 0.05. *=P ≤ 0.05, ****= P ≤ 0.0001.
Alkyl quinolone production by P. aeruginosa in colony biofilms is diminished near E. faecalis
One response elicited by other microbes upon P. aeruginosa can be measured in the production of heterocyclic aromatic 2-alkyl-4(1H)-quinolones (AQs)(13, 34–36). AQs such as 2-heptyl-3-hydroxy-4-quinolone (Pseudomonas Quinolone Signal; PQS) and 2-heptyl-4-hydroxyquinoline-N-oxide (HQNO) are known to be produced by P. aeruginosa in response to competition with other bacterial species. We have previously shown that P. aeruginosa exhibits earlier production of AQs when grown in proximity to other species such as E. coli or S. aureus (13). We hypothesized that E. faecalis would elicit a reduced AQ response, to enable survival in coculture with P. aeruginosa.
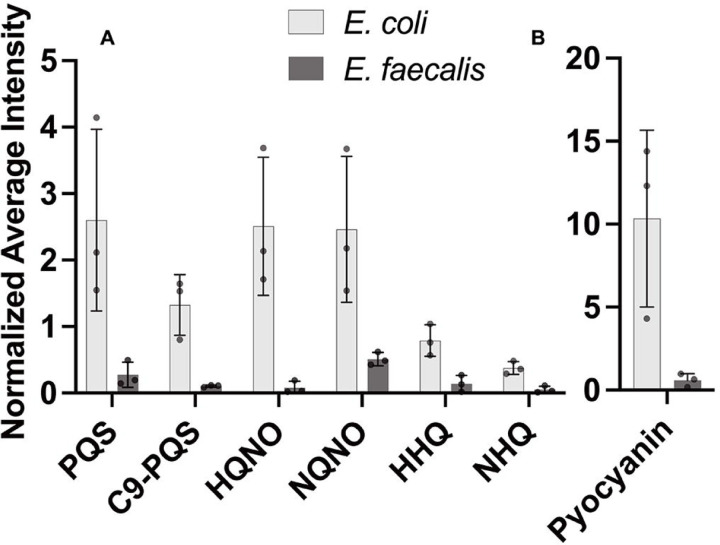
We examined a series of colony biofilm assay experiments inoculated with P. aeruginosa alone or side-by-side with E. faecalis. Separate assays included P. aeruginosa side-by-side with E. coli as an AQ-inducing control (13). To spatially assess relative abundance of P. aeruginosa AQ production in response to E. faecalis we used matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI)(37, 38). A representative spatial heatmap rendering of our results shows greater abundance of PQS near an E. coli colony, but not near E. faecalis, as compared to P. aeruginosa alone (Figure S1). Overall, we assessed PQS as well related quinolones HQNO, HHQ, and their nine carbon side-chain variants C9-PQS, NQNO and NHQ. Intensity profiles for each molecule produced by P. aeruginosa near to E. coli or E. faecalis were assessed relative to amounts produced by P. aeruginosa alone (Figure 2). For each of these AQs, we find 2–8× reduced signature is present when P. aeruginosa is proximal to E. faecalis. As expected, E. coli elicited an opposite response as 1.5–2.5× increased signal of PQS, C9-PQS, HQNO, and NQNO was detected above mono-culture levels. We perceived the reduced AQ response near E. faecalis as a lack of P. aeruginosa antagonism and hypothesized that E. faecalis surviving with P. aeruginosa is a result of directly altering the AQ response. We directly confirm the importance of reduced AQ production to E. faecalis survival as E. faecalis will grow when incubated with spent supernatant of a P. aeruginosa ΔpqsA AQ deficient strain but not with supernatant of P. aeruginosa wildtype (Figure S2).
Figure 2.
Alkyl quinolone production in P. aeruginosa biofilms is reduced by the presence of E. faecalis. The amount of alkyl quinolones and pyocyanin detected by MALDI-MSI relative to P. aeruginosa alone (i.e. 1.0) indicates E. coli and E. faecalis have opposite effects on alkyl quinolone production. Error bars show ± one standard deviation. A. Presence of E. coli elicits an increased PQS, C9-PQS, HQNO and NQNO response while E. faecalis reduces production for each. B. Additionally, pyocyanin production increased in the presence of E. coli (10.3× P. aeruginosa alone) but decreased near E. faecalis (0.59× P. aeruginosa alone).
E. faecalis reduces expression of the P. aeruginosa alkyl quinolone biosynthetic gene pqsH
We speculated that differences in P. aeruginosa AQ abundance observed with E. faecalis should result from a substantive shift in P. aeruginosa gene expression. The AQ biosynthetic pathway is complex and distinct branch points are known for several well-studied P. aeruginosa quinolones (34, 39). We focused our attention on the gene pqsH, the final gene required to synthesize the quorum sensing signals PQS and C9-PQS.
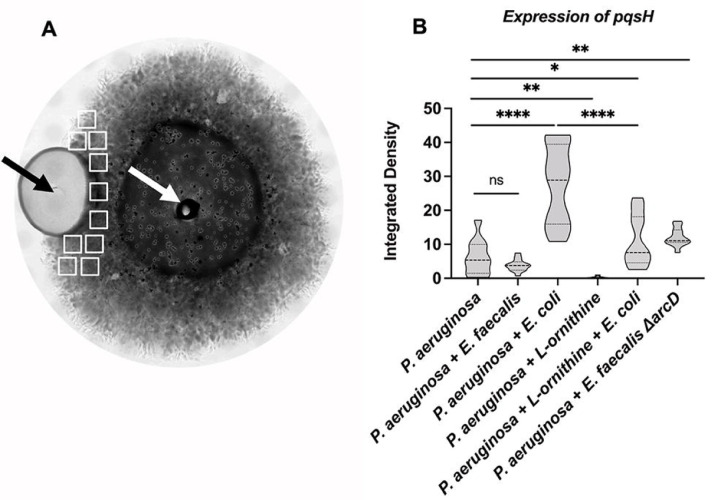
We imaged a fluorescent protein transcriptional reporter for pqsH and found patterns consistent with our MALDI-MSI results. In 48h biofilm colonies, a basal level of P. aeruginosa PpqsH-sfgfp fluorescence is observed (Figure 3B). Inclusion of E. faecalis near P. aeruginosa leads to a reduction of PpqsH-sfgfp expression that is not statistically significant but the spatial variability is reduced; while proximity to E. coli increased expression of PpqsH-sfgfp more than 4.0× (Figure 3B). In these side-by-side colony biofilm assays, any influence of co-culture was spatially dependent as expression of PpqsH-sfgfp in areas of the P. aeruginosa biofilm opposite from E. faecalis or E. coli were not significantly different (Figure S3). We find equivalent trends in planktonic culture where co-culture with E. faecalis leads to reduced PpqsH-sfgfp expression (67% of mono-culture), while co-culture with E. coli increased expression of PpqsH-sfgfp to 150% of mono-culture (Figure 4A).
Figure 3.
Alkyl quinolone gene expression is reduced in proximity to E. faecalis. Fluorescence intensity of PpqsH-sfgfp was determined after 48 hours incubation. A. A representative brightfield image shows the colonies of E. faecalis and P. aeruinogsa where their sites of inoculation are noted by the black and white arrows, respectively. B. No significant difference of pqsH-sfgfp was observed between P. aeruginosa and E. faecalis while E. coli significant increased expression. E. faecalis ΔarcD increased expression of pqsH-sfgfp. The addition of 12 mM L-ornithine decreased pqsH-sfgfp in monoculture and in coculture with E. coli. Integrated densities were acquired from 10 sample areas (white rectangles) on each plate for three biological replicates each for which results were statistically different by one-way ANOVA (P ≤ 0.0001). Pairwise comparisons by Welch’s t-test are indicated on the plot: ns= P > 0.05, *=P ≤ 0.05, **= P ≤ 0.01, ****= P ≤ 0.0001.
Figure 4.
Alkyl quinolone gene expression of pqsH is reduced in planktonic co-culture with E. faecalis or with exogenous ornithine. A. Fluorescence expression of PpqsH-sfgfp is reduced in planktonic co-culture with E. faecalis in comparison to P. aeruginosa monoculture or with E. coli (where PpqsH-sfgfp expression is elevated). (B) Fluorescence expression of PpqsH-sfgfp is reduced in planktonic co-culture with increasing amounts of L-ornithine. Values represent averages of ≥3 wells each for which reporter fluorescence was normalized to culture density.
Aside from the PQS regulon, several other competitive effectors and virulence factors produced by P. aeruginosa are regulated by the acyl homoserine lactone (AHL) quorum sensing regulons, Las and Rhl (40). Thus, we also tested if the presence of E. faecalis directly influenced P. aeruginosa las and rhl regulon responses by imaging reporters of the three robustly expressed QS genes hcnA and rsaL (Las) and rhlA (Rhl)(41, 42) respectively. The addition of E. faecalis near P. aeruginosa had minimal effect on the expression of genes regulated by either Las or Rhl as judged by the relative fluorescence of PhcnA-gfp, PrsaL-gfp, or PrhlA-gfp reporters (Figure S4). While a minimal effect on PhcnA-gfp was observed in our spatial plate assays, we judge this due to heterogeneity of cell density as testing of these same PhcnA-gfp, PrsaL-gfp, or PrhlA-gfp reporters in planktonic culture assays showed no difference in expression with inclusion of E. faecalis (Figure S5). Collectively, these results indicate that the influence of E. faecalis upon P. aeruginosa is specific to PQS and the AQs and not other community-level responses as E. faecalis does not affect a change in P. aeruginosa Las and Rhl QS gene expression.
Pyocyanin production is attenuated by the presence of E. faecalis
We noted that in some P. aeruginosa-E. faecalis co-culture experiments, P. aeruginosa qualitatively displayed less of its characteristic blue-green pigmentation (Figure S6A). The predominant blue P. aeruginosa pigment is pyocyanin, for which production is driven by the PQS quorum sensing regulon or interaction of pqsE with rhlR (9, 34, 43–50). Our MALDI-MSI intensity profile data showed 0.59× amount of pyocyanin near E. faecalis while proximity to E. coli showed a 10.3× increase of pyocyanin over mono-culture (Figure 2). We probed for a direct effect of E. faecalis on pyocyanin production.
To assess boundaries of the expected response, we first tested two separate planktonic culture conditions that yield different relative abundance of P. aeruginosa pyocyanin. When P. aeruginosa monocultures are grown on glutamate as the sole carbon source, pyocyanin production was maximal, while with glucose, pyocyanin production was minimal. Subsequently, with glutamate as the sole carbon source, E. faecalis reduced pyocyanin produced by 42% compared to P. aeruginosa monoculture and addition of E. coli increased pyocyanin production over monoculture by 115% (Figure 5A). While the amounts of pyocyanin produced with glucose as the sole carbon source are much lower for P. aeruginosa monoculture, the addition of E. coli increases production to 135% of monoculture. With glucose, pyocyanin production by P. aeruginosa-E. faecalis co-culture is equivalent to monoculture (Figure 5B), indicating P. aeruginosa typical PQS response associated with competitive interactions was attenuated. Thus, with both culture conditions, growing P. aeruginosa with E. faecalis led to low production, which was opposite of the response quantified with E. coli.
Figure 5.
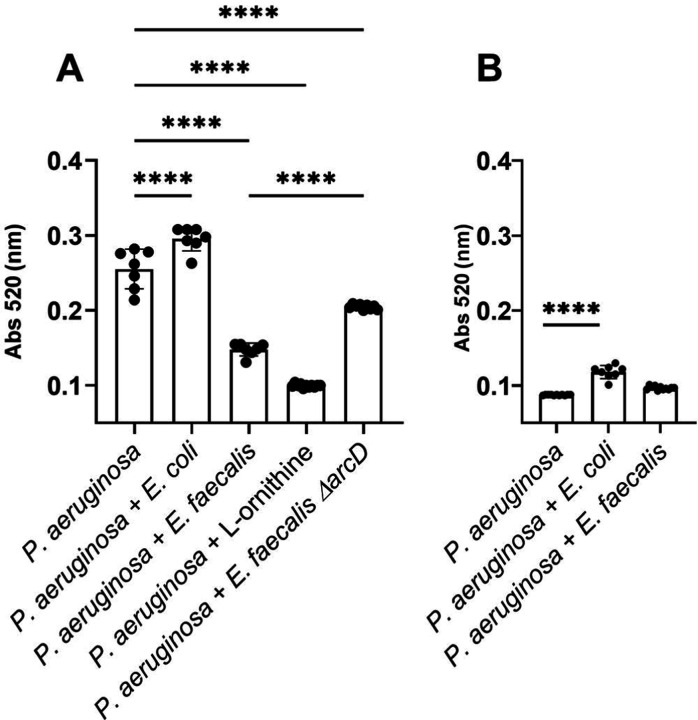
P. aeruginosa pyocyanin production is altered by L-ornithine production from E. faecalis. Quantification of pyocyanin extracted from P. aeruginosa monocultures and cocultures with E. coli, E. faecalis, ΔarcD, and L-ornithine grown in minimal media containing (A) glutamate and (B) glucose for which results were statistically different by one-way ANOVA (P ≤ 0.0001). Pairwise comparisons by Welch’s t-test are indicated on the plot: ****= P ≤ 0.0001.
Ornithine of E. faecalis promotes reduction in PQS response by P. aeruginosa
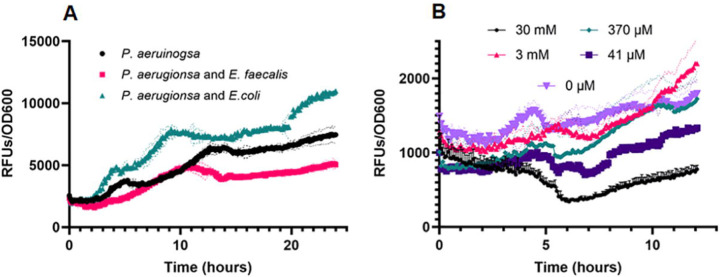
We were intrigued by a report of Keogh, et al (51) showing that L-ornithine produced by E. faecalis altered growth and behavior of E. coli in co-culture. We tested if ornithine might be important to the E. faecalis and P. aeruginosa interactions we observed. First, when equivalent PpqsH-sfgfp reporter colony biofilm assays to those described above were used, we found that supplementation of growth medium with L-ornithine reduced expression of PpqsH-sfgfp in P. aeruginosa alone to near zero (Figure 3B). Additionally, the addition of L-ornithine led to a reduction in PpqsH-sfgfp fluorescence even in the presence of E.coli (Figure 3B). In planktonic culture, the addition of increasing concentrations of L-ornithine led to greater reduction in PpqsH-sfgfp fluorescence (Figure 4B).
We further quantified the impact of L-ornithine upon P. aeruginosa by confirming a reduction in pyocyanin production. In planktonic culture, we found that increasing amounts of exogenous ornithine (0 – 30 mM) correlated with decreasing levels of pyocyanin (Figure S6B). Using 12 mM of L-ornithine decreased pyocyanin production more than presence of E. faecalis (Figure 4B), but concentrations as low as 50 μM also reduced pyocyanin ≥50% (Figure S6B). This effect was specific to L-ornithine, as addition of D-ornithine, or metabolically comparable amino acids L-arginine and L-lysine, did not influence pyocyanin production (Figure S7).
The ability to produce L-ornithine is key to E. faecalis mediated changes in P. aeruginosa alkyl quinolone pathways
We confirmed that L-ornithine produced by E. faecalis is the relevant factor needed to alter P. aeruginosa pqsH expression and pyocyanin production testing the E. faecalis arcD mutant. E. faecalis ΔarcD lacks the L-arginine/L-ornithine antiporter and is known to be deficient for ornithine production (51). Unlike wild-type E. faecalis, ΔarcD elicited increased PpqsH-sfgfp expression in our colony biofilm model experiments (Figure 3B), thus specifically indicating the importance of ornithine in mediating the interactions between E. faecalis and P. aeruginosa.
The ΔarcD mutant also elicited 1.5x pyocyanin production over wild-type E. faecalis (Figure 5A), which was equivalent to 80% of pyocyanin produced by P. aeruginosa monoculture. We postulate that L-ornithine may be the dominant factor of multiple made by E. faecalis to influence P. aeruginosa pyocyanin production.
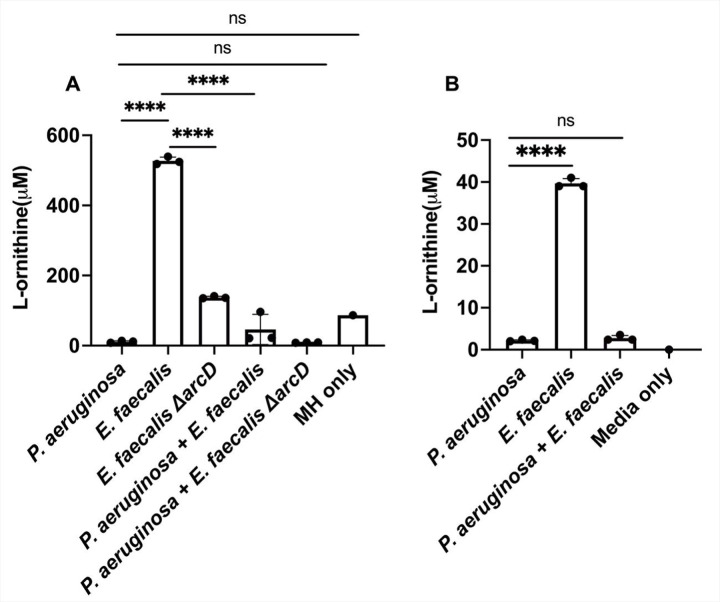
Lastly, we confirm and quantify production of L-ornithine in planktonic culture using ultra-high performance liquid chromatography (UHPLC) coupled with high-resolution mass spectrometry (HRMS). We find that E. faecalis produced L-ornithine in concentrations ranging from 518–539 μM when grown in Mueller Hinton broth (Figure 6A). In coculture with P. aeruginosa, L-ornithine was only detected at concentrations ranging from 21 μM - 96 μM (Figure 6A). In P. aeruginosa monoculture, L-ornithine was detected in the media at concentrations between 8–13.5 μM. This change in detectable L-ornithine between monocultures and co-culture suggests that P. aeruginosa is not just sensing, but also consuming the ornithine produced by E. faecalis. While overall ornithine production was substantially lower in minimal medium supplemented with glutamate, we observed similar trends as only E. faecalis monocultures show appreciable L-ornithine levels. (Figure 6B).
Figure 6.
The presence of L-ornithine differs between monocultures of P. aeruginosa and E. faecalis and co-culture. A. In Mueller Hinton broth detectable L-ornithine was decreased in co-culture compared to E. faecalis monoculture. P. aeruginosa and ΔarcD co-culture also had a reduction in L-ornithine compared to ΔarcD alone, the amount detected was comparable to L-ornithine already present in the media. B. Ornithine was detected in higher abundance from E. faecalis monoculture in minimal media supplemented with glutamate compared to P. aeruginosa alone and co-culture. Each condition represents three biological replicates for which results were statistically different by one-way ANOVA (P ≤ 0.0001). Pairwise comparisons by Welch’s t-test are indicated on the plot: ns= P > 0.05, ****= P ≤ 0.0001.
Discussion
Our research sheds light on a unique relationship between E. faecalis and P. aeruginosa. Specifically, we show a rare example of survival in the presence of P. aeruginosa under culture conditions that generally enable P. aeruginosa dominance and antagonism. In direct competition, E. faecalis dampens P. aeruginosa alkyl quinolone responses, which is the opposite of other known examples. Results from this work and prior reports generally show that P. aeruginosa alkyl quinolone responses are generally upregulated in the presence of other bacterial species (13, 17). It is not yet clear if the ability of E. faecalis to alter P. aeruginosa behaviors correlates to competitive or killing responses in infections where both species are present. We do find that absence of P. aeruginosa AQs allows for E. faecalis growth in planktonic culture. The proximity and range of interaction between E. faecalis and P. aeruginosa is clearly important as the effect of E. faecalis upon alkyl quinolone or pyocyanin production was localized to the area nearest to E. faecalis. This finer point was not apparent whatsoever from our planktonic culture results. Nonetheless, this interaction we have characterized between E. faecalis and P. aeruginosa may provide a useful framework for better understanding specific cross-talk and potential cooperative interactions within polymicrobial communities.
In addition to better understanding bacterial interactions between E. faecalis and P. aeruginosa, this work also highlights the role of shared metabolites in modulating stress responses and virulence factor production by P. aeruginosa, specifically related to the P. aeruginosa PQS system. This work demonstrates that an outside signal from E. faecalis, L-ornithine, is sufficient to alter the PQS response and reduce the production of the virulence factor, pyocyanin. This is confirmed by showing that an E. faecalis ΔarcD mutant, which is unable to export L-ornithine, lacks the capacity to limit pyocyanin production by P. aeruginosa. Showing this ability of E. faecalis to alter the aggressive behavior of P. aeruginosa by a specific amino acid represents an important step in understanding P. aeruginosa virulence and the potential to develop new treatments for polymicrobial infections.
Materials and Methods
Bacterial strains and culture conditions
All strains used in experiments are reported in Table S1. All bacterial strains were routinely grown planktonically for 18 hours in Luria-Bertani (LB) broth at 37°C with shaking at 240 rpm, except as noted for additional specific assays.
Plate assays were prepared using FAB medium supplemented with 12 mM glucose and 1.5% Noble agar. For monoculture plates, P aeruginosa was inoculated by pipetting 1 uL of planktonic culture onto the center of the plate. For co-culture biofilms, 1 uL of E. faecalis and E. coli K-12 were spotted at 12 mm apart from 1 uL P. aeruginosa. Plates were inverted and incubated for 24 or 48 hours respectively.
Viability counts
Colony forming units (CFUs) were determined by using monocultures of each strain inoculated into 1 mL of Mueller-Hinton broth (Sigma-Aldrich) to an OD600 of 0.01. Co-culture experiments were inoculated with each individual strain at an OD600 of 0.01, for an overall OD600 of 0.02 (i.e. at a 1:1 ratio).
Cultures were grown for 24 hours at 37°C shaking at 240 rpm. Triplicate serial dilutions from 100 – 10−9 of each monoculture and coculture were done in 1xPBS and 5 uL of each dilution spotted on Brain Heart Infusion agar (Dot Scientific, Burton, MI, USA) agar plates for all species as well as Sabouraud agar (Dot Scientific) for P. aeruginosa selection, Mueller-Hinton broth with 2μg/mL Ciproflaxin for E. faecalis, Blood agar for E. coli, and Mannitol Salt Agar for S. aureus and C. striatum. Colony forming units (CFUs) were determined for each species in monoculture and coculture using selective media.
Mass Spectrometry Imaging Analysis
Mass spectrometry images of alkyl quinolones and pyocyanin present on colony biofilm assays were obtained using a FT-ICR mass spectrometer (solariX 7T, Bruker, USA) equipped with matrix-assisted desorption/ionization (MALDI)(52). Additional details are included in the Supplemental Methods.
Reporter Strain Construction
Standard genetic techniques were used to construct a chromosomal (Tn7) transcriptional reporter strain PpqsH-sfgfp that fused chromosomal DNA upstream of the pqsH gene to sfgfp. Strains, plasmids, and DNA sequences are included in Tables S1, S2 and S3, respectively of the SI Appendix and additional details of construction are included in the Supplemental Methods.
Fluorescence microscopy of Reporter Strains.
Images of P. aeruginosa containing PpqsH-gfp, PrhlA-gfp, PhcnA-gfp and PrsaL-gfp were obtained using a Leica DM6B upright microscope equipped with a 10× Fluotar objective with simultaneous excitation at 475 nm with emission capture using settings of 525 ± 50 nm. Captured images were 1028 × 916 pixels with a DPI of 144 pixels/in. Grey scale images were obtained using brightfield. Fluorescence intensity was measured using ImageJ (53) where ten equal sized sections of 48×50 pixels each were analyzed individually from three sample replicates for each test condition to determine the resultant integrated density intensity for each condition.
Pyocyanin Quantification
Pyocyanin quantification extraction was adapted from Frank and Demoss (45). Additional details included in the Supplemental Methods.
Ornithine quantification
Ornithine was quantified from planktonic cultures using a hybrid Hydrophilic Interaction Liquid Chromatography (HILIC) coupled with a high-resolution mass spectrometer (HRMS). Additional details included in the Supplemental Methods.
Data analysis
Plots of data and statistical significance comparison between all tested conditions using one-way ANOVA and pairwise comparisons by Welch’s t-test were generated using GraphPad Prism 10 software.
Supplementary Material
Importance.
While we now appreciate that many infections are polymicrobial, we understand little of the specific actions between a given set of microbes to enable combinatorial survival and pathogenesis. The bacteria Pseudomonas aeruginosa and Enterococcus faecalis are both prevalent pathogens in wound, urinary tract, and bacteremic infections. While P. aeruginosa often kills other species in standard laboratory culture conditions, we present here that E. faecalis can be reliably co-cultured with P. aeruginosa. We specifically detail that ornithine produced by E. faecalis reduces the Pseudomonas Quinolone Signal response of P. aeruginosa. This reduction of the Pseudomonas Quinolone Signal response aids E. faecalis growth.
Acknowledgments
M.M.F. was funded by NSF Graduate Research Fellowship1841556. J.E.P was funded by National Institute of General Medical Sciences grant R01GM14436101, and J.D.S. and J.V.S. were supported by the National Institute of Allergy and Infectious Diseases grant R01AI113219.
Footnotes
Competing interests
The authors declare no competing interest.
References
- 1.Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappinscott HM. 1995. Microbial Biofilms. Annu Rev Microbiol 49:711–745. [DOI] [PubMed] [Google Scholar]
- 2.Hall-Stoodley L, Costerton JW, Stoodley P. 2004. Bacterial biofilms: From the natural environment to infectious diseases. Nat Rev Microbiol 2:95–108. [DOI] [PubMed] [Google Scholar]
- 3.Weaver AA, Hasan NA, Klaassen M, Karathia H, Colwell RR, Shrout JD. 2019. Prosthetic joint infections present diverse and unique microbial communities using combined whole-genome shotgun sequencing and culturing methods. J Med Microbiol 68:1507–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gjodsbol K, Christensen JJ, Karlsmark T, Jorgensen B, Klein BM, Krogfelt KA. 2006. Multiple bacterial species reside in chronic wounds: a longitudinal study. Int Wound J 3:225–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colombo AV, Barbosa GM, Higashi D, di Micheli G, Rodrigues PH, Simionato MRL. 2013. Quantitative detection of Staphylococcus aureus, Enterococcus faecalis and Pseudomonas aeruginosa in human oral epithelial cells from subjects with periodontitis and periodontal health. J Med Microbiol 62:1592–1600. [DOI] [PubMed] [Google Scholar]
- 6.Citron DM, Goldstein EJ, Merriam CV, Lipsky BA, Abramson MA. 2007. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J Clin Microbiol 45:2819–2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zmistowski B, Fedorka CJ, Sheehan E, Deirmengian G, Austin MS, Parvizi J. 2011. Prosthetic joint infection caused by gram-negative organisms. J Arthroplasty 26:104–108. [DOI] [PubMed] [Google Scholar]
- 8.Flurin L, Greenwood-Quaintance KE, Patel R. 2019. Microbiology of polymicrobial prosthetic joint infection. Diagn Microbiol Infect Dis 94:255–259. [DOI] [PubMed] [Google Scholar]
- 9.Passador L, Cook JM, Gambello MJ, Rust L, Iglewski BH. 1993. EXPRESSION OF PSEUDOMONAS-AERUGINOSA VIRULENCE GENES REQUIRES CELL-TO-CELL COMMUNICATION. Science 260:1127–1130. [DOI] [PubMed] [Google Scholar]
- 10.Bomberger JM, Maceachran DP, Coutermarsh BA, Ye S, O’Toole GA, Stanton BA. 2009. Long-distance delivery of bacterial virulence factors by Pseudomonas aeruginosa outer membrane vesicles. PLoS Pathog 5:e1000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaume M, Kohler T, Fontana T, Tognon M, Renzoni A, van Delden C. 2015. Metabolic pathways of Pseudomonas aeruginosa involved in competition with respiratory bacterial pathogens. Front Microbiol 6:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welp AL, Bomberger JM. 2020. Bacterial Community Interactions During Chronic Respiratory Disease. Front Cell Infect Microbiol 10:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao T, Sweedler JV, Bohn PW, Shrout JD. 2020. Spatiotemporal Distribution of Pseudomonas aeruginosa Alkyl Quinolones under Metabolic and Competitive Stress. mSphere 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott JE, Li K, Filkins LM, Zhu B, Kuchma SL, Schwartzman JD, O’Toole GA. 2019. Pseudomonas aeruginosa Can Inhibit Growth of Streptococcal Species via Siderophore Production. J Bacteriol 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niggli S, Kummerli R. 2020. Strain Background, Species Frequency, and Environmental Conditions Are Important in Determining Pseudomonas aeruginosa and Staphylococcus aureus Population Dynamics and Species Coexistence. Appl Environ Microbiol 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filkins LM, Graber JA, Olson DG, Dolben EL, Lynd LR, Bhuju S, O’Toole GA. 2015. Coculture of Staphylococcus aureus with Pseudomonas aeruginosa Drives S. aureus towards Fermentative Metabolism and Reduced Viability in a Cystic Fibrosis Model. J Bacteriol 197:2252–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nguyen AT, Oglesby-Sherrouse AG. 2016. Interactions between Pseudomonas aeruginosa and Staphylococcus aureus during co-cultivations and polymicrobial infections. Appl Microbiol Biotechnol 100:6141–6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer AJ, Singh SB, LaMarche MM, Maakestad LJ, Kienenberger ZE, Pena TA, Stoltz DA, Limoli DH. 2021. Sustained Coinfections with Staphylococcus aureus and Pseudomonas aeruginosa in Cystic Fibrosis. Am J Respir Crit Care Med 203:328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bowler PG, Duerden BI, Armstrong DG. 2001. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 14:244–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giacometti A, Cirioni O, Schimizzi AM, Del Prete MS, Barchiesi F, D’Errico MM, Petrelli E, Scalise G. 2000. Epidemiology and microbiology of surgical wound infections. J Clin Microbiol 38:918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dalton T, Dowd SE, Wolcott RD, Sun Y, Watters C, Griswold JA, Rumbaugh KP. 2011. An in vivo polymicrobial biofilm wound infection model to study interspecies interactions. PLoS One 6:e27317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuchimori N, Hayashi R, Shino A, Yamazaki T, Okonogi K. 1994. Enterococcus faecalis aggravates pyelonephritis caused by Pseudomonas aeruginosa in experimental ascending mixed urinary tract infection in mice. Infect Immun 62:4534–4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan CAZ, Lam LN, Biukovic G, Soh EY, Toh XW, Lemos JA, Kline KA. 2022. Enterococcus faecalis Antagonizes Pseudomonas aeruginosa Growth in Mixed-Species Interactions. J Bacteriol 204:e0061521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolcott RD, Hanson JD, Rees EJ, Koenig LD, Phillips CD, Wolcott RA, Cox SB, White JS. 2016. Analysis of the chronic wound microbiota of 2,963 patients by 16S rDNA pyrosequencing. Wound Repair and Regeneration 24:163–174. [DOI] [PubMed] [Google Scholar]
- 25.Wu M, Ruan H, Huang Y, Liu C, Ni P, Ye J, Lu S, Xie T. 2015. Bacteriological Investigation of Chronic Wounds in a Specialized Wound Healing Department: A Retrospective Analysis of 107 Cases. The International Journal of Lower Extremity Wounds 14:178–182. [DOI] [PubMed] [Google Scholar]
- 26.Dana AN, Bauman WA. 2015. Bacteriology of pressure ulcers in individuals with spinal cord injury: What we know and what we should know. J Spinal Cord Med 38:147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowd SE, Sun Y, Secor PR, Rhoads DD, Wolcott BM, James GA, Wolcott RD. 2008. Survey of bacterial diversity in chronic wounds using pyrosequencing, DGGE, and full ribosome shotgun sequencing. BMC Microbiol 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flores-Mireles AL, Walker JN, Caparon M, Hultgren SJ. 2015. Urinary tract infections: epidemiology, mechanisms of infection and treatment options. Nat Rev Microbiol 13:269–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colombo AV, Barbosa GM, Higashi D, di Micheli G, Rodrigues PH, Simionato MRL. 2013. Quantitative detection of Staphylococcus aureus, Enterococcus faecalis and Pseudomonas aeruginosa in human oral epithelial cells from subjects with periodontitis and periodontal health. J Med Microbiol 62:1592–1600. [DOI] [PubMed] [Google Scholar]
- 30.Thoendel MJ, Jeraldo PR, Greenwood-Quaintance KE, Yao JZ, Chia N, Hanssen AD, Abdel MP, Patel R. 2018. Identification of Prosthetic Joint Infection Pathogens Using a Shotgun Metagenomics Approach. Clin Infect Dis 67:1333–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmerli W, Trampuz A, Ochsner PE. 2004. Prosthetic-joint infections. N Engl J Med 351:1645–1654. [DOI] [PubMed] [Google Scholar]
- 32.Abisado RG, Benomar S, Klaus JR, Dandekar AA, Chandler JR. 2018. Bacterial Quorum Sensing and Microbial Community Interactions. mBio 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bisht K, Baishya J, Wakeman CA. 2020. Pseudomonas aeruginosa polymicrobial interactions during lung infection. Current opinion in microbiology 53:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubern J-F, Diggle SP. 2008. Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species. Mol Biosyst 4:882–888. [DOI] [PubMed] [Google Scholar]
- 35.Morales-Soto N, Dunham SJB, Baig NF, Ellis JF, Madukoma CS, Bohn PW, Sweedler JV, Shrout JD. 2018. Spatially dependent alkyl quinolone signaling responses to antibiotics in Pseudomonas aeruginosa swarms. J Biol Chem 293:9544–9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saalim M, Villegas-Moreno J, Clark BR. 2020. Bacterial Alkyl-4-quinolones: Discovery, Structural Diversity and Biological Properties. Molecules 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanni EJ, Masyuko RN, Driscoll CM, Aerts JT, Shrout JD, Bohn PW, Sweedler JV. 2014. MALDI-guided SIMS: multiscale imaging of metabolites in bacterial biofilms. Anal Chem 86:9139–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li B, Comi TJ, Si T, Dunham SJ, Sweedler JV. 2016. A one-step matrix application method for MALDI mass spectrometry imaging of bacterial colony biofilms. J Mass Spectrom 51:1030–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wade DS, Calfee MW, Rocha ER, Ling EA, Engstrom E, Coleman JP, Pesci EC. 2005. Regulation of Pseudomonas quinolone signal synthesis in Pseudomonas aeruginosa. J Bacteriol 187:4372–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pearson JP, Pesci EC, Iglewski BH. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol 179:5756–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schuster M, Lostroh CP, Ogi T, Greenberg EP. 2003. Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185:2066–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH. 2003. Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: Effects of growth phase and environment. J Bacteriol 185:2080–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polisetti S, Baig NF, Morales-Soto N, Shrout JD, Bohn PW. 2017. Spatial Mapping of Pyocyanin in Pseudomonas aeruginosa Bacterial Communities Using Surface Enhanced Raman Scattering. Appl Spectrosc 71:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brint JM, Ohman DE. 1995. Synthesis Of Multiple Exoproducts In Pseudomonas-Aeruginosa Is Under The Control Of RhlR-RhlI, Another Set Of Regulators In Strain PAO1 With Homology To The Autoinducer-Responsive LuxR-LuxI Family. J Bacteriol 177:7155–7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frank LH, Demoss RD. 1959. On the biosynthesis of pyocyanine. J Bacteriol 77:776–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grossowicz N, Hayat P, Halpern YS. 1957. Pyocyanine biosynthesis by Pseudomonas aeruginosa. J Gen Microbiol 16:576–583. [DOI] [PubMed] [Google Scholar]
- 47.Taylor IR, Paczkowski JE, Jeffrey PD, Henke BR, Smith CD, Bassler BL. 2021. Inhibitor Mimetic Mutations in the Pseudomonas aeruginosa PqsE Enzyme Reveal a Protein-Protein Interaction with the Quorum-Sensing Receptor RhlR That Is Vital for Virulence Factor Production. ACS Chem Biol 16:740–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Farrow JM 3rd, Sund ZM, Ellison ML, Wade DS, Coleman JP, Pesci EC. 2008. PqsE functions independently of PqsR-Pseudomonas quinolone signal and enhances the rhl quorum-sensing system. J Bacteriol 190:7043–7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drees SL, Fetzner S. 2015. PqsE of Pseudomonas aeruginosa Acts as Pathway-Specific Thioesterase in the Biosynthesis of Alkylquinolone Signaling Molecules. Chem Biol 22:611–618. [DOI] [PubMed] [Google Scholar]
- 50.Simanek KA, Taylor IR, Richael EK, Lasek-Nesselquist E, Bassler BL, Paczkowski JE. 2022. The PqsE-RhlR Interaction Regulates RhlR DNA Binding to Control Virulence Factor Production in Pseudomonas aeruginosa. Microbiol Spectr 10:e0210821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keogh D, Tay WH, Ho YY, Dale JL, Chen S, Umashankar S, Williams RBH, Chen SL, Dunny GM, Kline KA. 2016. Enterococcal Metabolite Cues Facilitate Interspecies Niche Modulation and Polymicrobial Infection. Cell Host Microbe 20:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weaver AA, Parmar D, Junker EA, Sweedler JV, Shrout JD. 2023. Differential Spreading of Rhamnolipid Congeners from Pseudomonas aeruginosa. ACS Appl Bio Mater 6:4914–4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.