Abstract
Background
The CDC recommends the more immunogenic adjuvanted and high-dose flu vaccines over standard-dose, non-adjuvanted vaccines for individuals above 65 years old. The current study compares adjuvanted trivalent inactivated flu vaccine (aTIV, FLUAD) versus high-dose flu vaccine (HD-IIV3, FLUZONE HD) to determine if they met non-inferiority standards for older long-term care facility (LTCF) residents.
Methods
We collected blood from long-term care facility residents participating in a randomized 1:1 active control trial comparing MF59C.1 adjuvanted trivalent inactivated flu vaccine, aTIV versus HD-IIV3 over the course of two flu seasons, 2018–2019 and 2019–2020 (Trial, NCT03694808). We assessed humoral immunity at set time points via hemagglutinin inhibition assays (HAI) and anti-neuraminidase (enzyme-linked lectin assays (ELLA)). The recombinant influenza vaccine (RIV, Flublok) was assessed similarly in year two for a small number of participants who were carried over from year 1 (n=32).
Results
We enrolled 387 volunteers and administered either aTIV (n=194), HD-IIV3 (n=193) over the course of the 2018–2019 and 2019–2020 flu seasons. Among those enrolled and randomized in year one, a subset were administered RIV and studied in year two (n = 32). At 28 days post-vaccination, aTIV exhibited non-inferiority to HD-IIV3 for HAI for both H1N1 and H3N2 strains (GMT ratios (95% CI) for HD-IIV3/aTIV of 1.03(0.76, 1.4) and 1.04(0.73, 1.48), respectively; both 95% CI upper bounds < 1.5 to meet non-inferiority criteria) but not for Influenza B (GMT ratio (95% CI) = 1.21 (0.91, 1.61)). Non-inferiority criteria for HAI seroconversion were not met for any of the three strains. Applying the same non-inferiority criteria to neuraminidase inhibition (NI), both day 28 titer and seroconversion in aTIV were non-inferior to HD-IIV3 for H1N1 and H3N2 strains.
Conclusions
Both aTIV and HD-IIV3 elicited similar immune responses with robust antibody responses. For the primary outcome, aTIV is non-inferior to HD-IIV3 for HAI titer of H1N1 and H3N2 but failed to meet non-inferiority criteria for Influenza B and seroconversion for all assessed strains. For the secondary outcome, aTIV was non-inferior to HD-IIV3 for both titer and seroconversion of anti-neuraminidase for both H1N1 and H3N2.
Keywords: Adjuvanted vaccine, High-Dose vaccine, Influenza vaccine, Long-term care, Aged, Immunogenicity
Background
Influenza continues to cause significant morbidity and mortality globally, particularly affecting adults over the age of 65 and those residing in long-term care facilities (LTCFs) Increased frailty, comorbidities, and communal living arrangements make this population especially vulnerable1. Standard-dose influenza vaccines have reduced effectiveness in older adults, prompting the development of formulations that elicit stronger immune responses. The Centers for Disease Control and Prevention (CDC) currently recommend the preferential use of adjuvanted (aTIV), high-dose (HD-IIV3) and recombinant (RIV) influenza vaccines for individuals over 65 years of age 2.
The CDC approved enhanced vaccines, such as Fluzone High-Dose (HD-IIV3; Sanofi Pasteur Inc.) in 2009, which contains four times the hemagglutinin (HA) antigen content of standard-dose vaccines. HD-IIV3 vaccine has demonstrated a greater effectiveness at preventing influenza in aging populations including LTCF residents compared to standard-dose vaccines 3, 4. The CDC also approved an influenza vaccine enhanced with the MF59 adjuvant, Fluad (aTIV; Seqirus USA Inc.), for older adults in 2016. Various studies have demonstrated the efficacy of adjuvants in enhancing immune responses, and greater effectiveness even in elderly populations 5, 6.
This study aims to compare the immunogenicity of the adjuvanted aTIV vaccine with the high-dose HD-IIV3 vaccine to determine whether aTIV is non-inferior in older adults residing in LTCFs. We conducted a randomized, controlled trial over two influenza seasons (2018–2019 and 2019–2020) to assess the humoral immune responses elicited by these vaccines using hemagglutination inhibition (HAI) and enzyme-linked lectin assays (ELLA assay). Our primary outcome measures included geometric mean titer (GMT) ratios for H1N1, H3N2, and influenza B strains at 28 days post-vaccination. Secondary outcomes included seroconversion rates and neuraminidase (NA) inhibition (NI) titers. Given the variability in vaccine performance across different influenza seasons, this study provides valuable insights into the comparative effectiveness of these two enhanced vaccines in a highly susceptible population.
With both seasons combined we found that aTIV met HAI GMT non-inferiority standards for H1N1 and H3N2 but not B, however, aTIV failed to meet non-inferiority standards for HAI seroconversion. For neuraminidase, aTIV is non-inferior to HD-IIV3 for both GMT and seroconversion. Similar to other studies 7, results differed between flu seasons suggesting that the ability of these two vaccines to elicit immune responses may not greatly differ.
Methods
Trial design and oversight
We conducted a phase 4 randomized active-control trial comparing the aTIV vaccine to the HD-IIV3 vaccine. The trial included participants aged 65 years and older residing in long-term care facilities across Ohio. The study spanned two influenza seasons, from September 23, 2018, to December 30, 2020. The University of Hospitals of Cleveland and the Cleveland VA Institutional Review Boards (IRB) approved the study which complied with international standards and was registered on ClinicalTrials.gov on 13 October 2018 (Identifier: NCT03694808). We obtained written informed consent from all participants or their legally authorized representatives.
Participants and group assignments
Participants were long-term care residents aged 65 and older who did not have recent acute illnesses, were not using immunomodulatory agents, and had no recent history of cancer requiring treatment, certain circulatory system issues, allergies or reactions to any component of the influenza vaccine, or a history of Guillain-Barré syndrome following influenza vaccination. We randomized participants in a 1:1 ratio to receive either the aTIV or HD-IIV3 vaccine. Blood samples were collected at baseline (D0, -7–0 days), day 28 (D28, 24–29 days), and for the 2018–2019 season, day 180 (D180, 192–215 days). Day 180 samples were not obtained for 2019–2020 due to the start of the SARS-CoV-2 pandemic. Samples were processed using standard methods and stored at −80°C until analysis.
Vaccines
HD-IIV3 is derived from inactivated influenza virus amplified in chicken eggs and concentrated to present fourfold more HA per strain (60 mcg HA per strain per dose) than the standard dose vaccine. aTIV is also generated from chicken egg grown influenza virus with the addition of MF59C.1 adjuvant (MF59®), a squalene-based oil-in-water emulsion at 15 mcg HA per strain per dose (Seqirus USA Inc., NJ USA). RIV comes from a baculovirus vector driven recombinant process generating the HA subunit, and includes the purified HA at a 45mcg/ml amount per strain per dose. The 2019–2020 RIV formulation was quadrivalent and contained 2 B strains while aTIV and HDIIV3 are both trivalent formulations.
Assays
Hemagglutinin Inhibition Assays (HAI)
HAI detects the presence of HA-specific antibodies in donor sera following previously established protocols (Klimov, 2012) using turkey red blood cells (Lampire Biological Laboratories). We acquired strain-matched virus for A/H1N1 (A/Michigan/45/2015, FR1483), A/H3N2 (A/Singapore/02/2018, FR-1590), and influenza B (B/Colorado/01/2018, FR-1588) from International Reagent Resource (IRR) for the 2018–2019 flu season. For the 2019–2020 flu season, A/H1N1 (A/Brisbane/02/2018, FR1665) and A/H3N2 (A/Kansas//14/2017, FR1666) from Scott Hensley, University of Pennsylvania, and influenza B (B/Colorado/06/Victoria, FR1667) were utilized. Immunogenicity was measured by geometric mean titers (GMT), seroprotection, and seroconversion rates.
Neuraminidase Inhibition (NI) measuring anti-neuraminidase titers
We used standard methods of the anti-NA enzyme-linked lectin assays (ELLA) to determine neuraminidase inhibition activity 8. Briefly, Immulon 4 HBX plates (ThermoFisher Scientific, Waltham, MA) were coated with fetuin (Sigma F3385 at 25 ug/ml) and incubated overnight at 4°C. Sera was heat treated at 56°C and serially diluted across the plate prior to the addition of strain-matched enzymatically active recombinant NA (SinoBiological, Chesterbrook, PA) for each season. Plates were incubated overnight at 37°C, washed, and peanut agglutinin horseradish peroxidase conjugate (SIgma L7759) was added for 2 hours at room temperature (RT). Plates were visualized with a citrate buffer and an o-phenylenediamine dihydrochloride tablet (Sigma; P8287). After a 10-minute incubation at RT, the reaction was stopped with 0.5M sulfuric acid and read at 490nm.
Analysis
All analyses were performed in R Version 4.2.2. Seroconversion was defined as a fourfold or greater increase in HAI titer at day 28, and seroprotection was defined as an HAI titer ≥40. The GMT at day 28 was used to assess non-inferiority, considering the ratio and 95% confidence interval of the HD-IIV3 GMT to that of aTIV. For aTIV to be considered non-inferior to HD-IIV3 by FDA guidelines, the upper bound of this two-sided 95% CI could not exceed 1.5. For seroconversion rates, non-inferiority of aTIV to HD-IIV3 was achieved if the upper bound of the two-sided 95% CI for the difference in rates (HD-IIV3 - aTIV) did not exceed 10 percentage points. We compared baseline levels of HAI and NI titers between aTIV and HD-IIV3 vaccines using standard mean differences (SMD) to assess the balance achieved by the randomization, with absolute values <0.1 considered well-balanced and >0.25 considered strongly unbalanced 9. Additionally, we summarized titers observed at day 180 where available in the year one cohort; and the day 0 and day 28 RIV, aTIV, and HD-IIV3 titers in the year two cohort in which the third vaccine RIV was administered to a subset as well.
Results
Study participants
We screened 478 participants for inclusion in the study and enrolled and randomized 387 to receive either aTIV or HD-IIV3. An additional 32 participants from the first year of the study also participated in the second year, receiving RIV as their administered vaccine. Common reasons for exclusion from the study were withdrawn consent or unsuccessful/missed blood draws at the designated time points. The participants ranged in age from 65 to 100 years at the time of enrollment, with an approximately equal distribution of male and female participants and 20% of participants identifying as non-White. Similar distributions of age, gender, and race were observed between the aTIV and HD-IIV3 groups (Table 1). Baseline levels of both HAI and NI titers were also found to be balanced between the vaccine groups (Supplemental Table 1), with absolute standardized mean differences not exceeding 0.1 for the two years combined nor 0.25 for either study year.
Table 1.
Demographics of subjects by vaccine
| HD-IIV3 | aTIV | RIV | |
|---|---|---|---|
| Number of Subjects | 193 | 194 | 32 |
| Age in Years: Mean +/− SD | 80.6+/−9.3 | 79.5+/−9.8 | 78.9+/−10.1 |
| Sex: Female | 93 (48.2%) | 102 (52.6%) | 6 (18.8%) |
| Sex: Male | 100 (51.8%) | 90 (46.4%) | 26 (81.2%) |
| Sex: Unknown/Not reported | 0 (0%) | 2 (1%) | 0 (0%) |
| Race: White | 161 (83.4%) | 156 (80.4%) | 25 (78.1%) |
| Race: Black | 21 (10.9%) | 27 (13.9%) | 6 (18.8%) |
| Race: Other/Unknown/Not Reported | 11 (5.7%) | 11 (5.7%) | 1 (3.1%) |
| Ethnicity: Hispanic or Latino | 5 (2.6%) | 3 (1.5%) | 1 (3.1%) |
| Ethnicity: Not Hispanic or Latino | 181 (93.8%) | 183 (94.3%) | 31 (96.9%) |
| Ethnicity: Unknown/Not Reported | 7 (3.6%) | 8 (4.1%) | 0 (0%) |
| Vaccine year: 2018–2019 | 86 (44.6%) | 91 (46.9%) | 0 (0%) |
| Vaccine year: 2019–2020 | 107 (55.4%) | 103 (53.1%) | 32 (100%) |
Primary Outcome
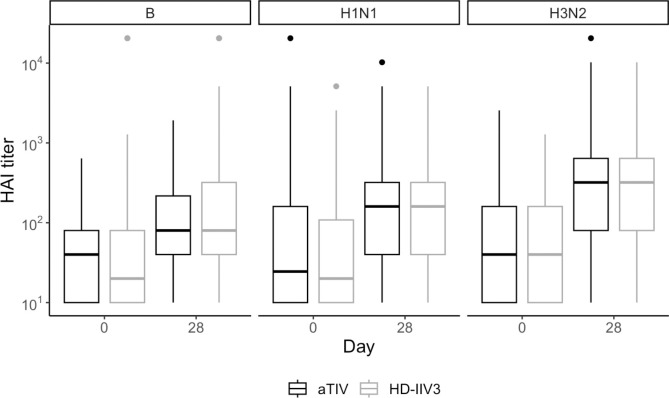
When combining data from both flu seasons (Figure 1), the HAI GMTs for H1N1, H3N2, and B strains for subjects administered aTIV were 117.8, 222.5, and 91.4, respectively. For subjects administered HD-IIV3, these GMTs were 121.7, 230.5, and 110.7. Based on the 95% confidence interval about the ratios of the HD-IIV3 GMT to the aTIV GMT, aTIV met non-inferiority criteria for HAI GMT ratios at 28 days post-vaccination for both H1N1 and H3N2 but not for the influenza B strain (Table 2).
Figure 1. Observed distributions of HAI titer for aTIV (black) and HD-IIV3 (gray), by day and strain.
For each box, the center line indicates the median and the bottom and top of the box indicate the first and third quartile, respectively. The lower and upper whiskers extend from the first and third quartile lines, respectively, to the smallest and largest values no more than 1.5 times the interquartile range (height of box) away from the first and third quartile values.
Table 2: Statistical summary of HAI GMT and seroconversion rate at Day 28 for aTIV and HD-IIV3.
HD-IIV3 shows GMT NI for H1N1 and H3N2 (bold & italicized) where upper bound of 95% confidence interval about the GMT ratio of HD-IIV3 to aTIV is < 1.5. This statistic exceeds 1.5 for B strain, and thus does not demonstrate non-inferiority. aTIV seroconversion fails to meet non-inferior criteria to HD-IIV3 for H1N1, H3N2 and B as all upper bounds of seroconversion differences 95% CI exceed 0.1.
| HD-IIV3 | aTIV | HD-IIV3 vs. aTIV comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Strain | N subjects | Day 28 GMT | N (%) Seroconversion | N subjects | Day 28 GMT | N (%) Seroconversion | GMT Ratio (95% CI) | Seroconversion difference (95% CI) |
| B | 193 | 110.7 | 83 (43%) | 194 | 91.4 | 69 (36%) | 1.21 (0.91,1.61) | 0.074 (−0.028, 0.177) |
| H1N1 | 190 | 121.7 | 83 (44%) | 192 | 117.8 | 76 (40%) | 1.03 (0.76,1.4) | 0.041 (−0.063, 0.145) |
| H3N2 | 193 | 230.5 | 124 (64%) | 194 | 222.5 | 105 (54%) | 1.04 (0.73,1.48) | 0.101 (−0.001, 0.204) |
Combining data from both flu seasons, seroconversion rates at day 28 for participants who received aTIV were 39.6% for H1N1, 54.1% for H3N2, and 35.6% for the B strain. In participants who received HD-IIV3, the seroconversion rates were 43.7% for H1N1, 64.2% for H3N2, and 43.0% for the B strain (Table 3), and the non-inferiority criteria was not met for any strain.
Table 3. Statistical summary of NI GMT and seroconversion for aTIV and HD-IIV3.
For both measures, aTIV shows non-inferiority to HD-IIV3 for both H1N1 and H3N2 (bold and italicized), with the upper limit of the HD-IIV3 to aTIV GMT ratio 95% confidence interval not exceeding 1.4 and the upper limit of the seroconversion difference 95% confidence interval not exceeding 0.1.
| HD-IIV3 | aTIV | HD-IIV3 vs. aTIV comparisons | ||||||
|---|---|---|---|---|---|---|---|---|
| Strain | N subjects | Day 28 GMT | N (%) Seroconversion | N subjects | Day 28 GMT | N (%) Seroconversion | GMT Ratio (95% CI) | Seroconversion difference (95% CI) |
| H1N1 | 189 | 159.4 | 54 (29%) | 192 | 351.5 | 119 (62%) | 0.45 (0.33,0.63) | −0.334 (−0.433, −0.235) |
| H3N2 | 192 | 34.4 | 31 (16%) | 194 | 36.3 | 52 (27%) | 0.95 (0.76,1.17) | −0.107 (−0.193, −0.02) |
When the HAI GMTs were summarized separately for the two study years, we observed higher values in the 2018–2019 season than 2019–2020 across all strains and for both aTIV and HD-IIV3 vaccines (Supplemental Table 2). The rates of HAI seroconversion were calculated for each individual season and generally show a similar pattern of responses, with the highest seroconversion rates observed for H3N2 strain in both seasons and both vaccines (Supplemental Table 3).
The proportion of participants who achieved seroprotection, defined as an HAI titer ≥40 at day 28, was similar between the aTIV and HD-IIV3 groups regardless of strain and observed at higher rates in 2018–2019 than in 2019–2020 across strains and vaccines (Supplemental Table 4). In both vaccines, over 80% seroprotection was observed for all strains when considering the seasons combined. In a subset of 2018–2019 season subjects at day 180 we measured HAI titers and observed a decrease from day 28 in both vaccine groups (Supplemental Figure 1).
Secondary Outcomes
When the non-inferiority criteria were applied to neuraminidase inhibition (NI) titers, aTIV was non-inferior to HD-IIV3 for both seroconversion and GMT at day 28 post-vaccination for both H1N1 and H3N2 strains. For H1N1, the NA seroconversion rate was 62% for aTIV compared to 29% for HD-IIV3, and for H3N2, the rates were 27% and 16%, respectively. NI GMT values for H1N1 were 351.5 for aTIV and 159.4 for HD-IIV3, while for H3N2, the NI GMTs were 36.3 and 34.4, respectively (Table 3).
In a subset of 2018–2019 season subjects at day 180 we measured NI titers and observed a decrease from day 28 in both vaccine groups for H1N1 with little to no decline observed for H3N2 (Supplemental Figure 1).
In the 2019–2020 season, we summarized HAI and NI for the participants who received RIV (n=32). Compared to aTIV and HD-IIV3 in the same season, RIV produced similar HAI titers. Unlike the aTIV and HD-IIV3 vaccines which contain some neuraminidase, RIV does not; subjects administered RIV had less change in NI from day 0 to Day 28 than those who received the other vaccines (Supplemental Figure 2).
Discussion
The US Advisory Committee on Immunization Practices (ACIP) recommends that individuals aged 65 and older receive an enhanced vaccine that includes two high-dose vaccines (HD-IIV3 and RIV) and adjuvanted influenza vaccine (aTIV) 2. Each of these enhanced vaccines has demonstrated better clinical efficacy or effectiveness than standard-dose vaccines 3, 4, 6, 10, and greater immunogenicity11. Three studies have directly compared the enhanced vaccines in older individuals and none in long-term care residents 12–14. This study provides the opportunity to contrast immunogenicity data with these 3 clinical effectiveness studies although our subjects are all long-term residents and the effectiveness studies are a general population of older individuals. Each of these effectiveness studies use metadata from large database systems with the strengths and weaknesses of such designs. Between the studies, they span 4 influenza seasons with two studies overlapping in one season. They all focus on the reduction of influenza associated clinical outcomes assessed primarily by inpatient or outpatient healthcare visits. The Imran et al and Boikes et al each overlap one year of our study.
Our immunogenicity studies observed a number of differences between the vaccines, strains and seasons. Our results demonstrate that aTIV failed to meet non-inferiority criteria for HAI seroconversion at day 28 post-vaccination for all three influenza strains when both flu seasons were combined. The small percentage differences, although statistically significant, may not represent a clinically important finding, considering the seroconversion rates for aTIV were 39.6% for H1N1, 54.1% for H3N2, and 35.6% for the B strain, compared to 43.7%, 64.2%, and 43.0%, respectively, for HD-IIV3. Despite the differences in HAI seroconversion rates, aTIV was non-inferior to HD-IIV3 in terms of NI titers. At day 28 post-vaccination, aTIV demonstrated higher seroconversion rates and NI GMTs for H1N1 and H3N2 compared to HD-IIV3.
The immunogenicity results favored HD-IIV3 in HAI titer seroconversion but favored aTIV in anti-NA titers. These distinct and opposing advantages may help explain the somewhat contradictory reported clinical effectiveness of which vaccine is better. Two studies found aTIV more effective relative to HD-IIV3 but in the modest range of a 7% reduction in influenza-related medical encounters (IRME) in those over age 65 and in a different season a 10–18% reduction in IRME specifically older persons CDC defined influenza risk factors and no difference in the older population without influenzas risk factors 13, 14. In contrast, Van Aalst et al found that HD-IIV3 was 12 % better than aTIV at reducing respiratory hospital admissions across two H3N2 predominant seasons 12.
The current study adds to the prior data by demonstrating potential relevance of anti-NA titers to protection. Several prior studies also indicate the importance and relevance of anti-NA titer to clinical effectiveness 15, 16. Yet, RIV does not have any NA in it but nonetheless has proven as a highly effective vaccine among non-institutionalized older adults 10. Thus we expect the role for anti-NA as a complementary one, and potentially independent from the protection provided by anti-HA antibody.
Our small group of nursing home residents who received RIV produced HAI titers similar to those of residents who received either of the other vaccines. Therefore, purely from an immunogenicity standpoint, this supports the inclusion of RIV in the enhanced category as assigned by the ACIP for preferential recommendation for older adults. Dunkle et al compared RIV to standard dose vaccine in those age 50 and older and found RIV to be superior to standard dose vaccine 10.
Limitations
While we utilized standard assays to assess humoral immunity, antibody responses in older long-term care residents have less consistent reliability as indicators of protection. Cellular immunity may play a more significant role in determining vaccine effectiveness in this population, and this was not assessed in our study. NI and HAI responses may also provide a proxy for cellular immunity, and evidence for protection that could supplant or eclipse the benefit provided by just increased antibody titers. While antibody titers can serve to prevent or reduce the impact of the initial infection, cellular immunity associates with clinical recovery, and therefore remains an unmeasured confounder of this analysis. Also, we did not measure antibody avidity, which may differ for these vaccines 11. Finally, participants with conditions that could impair immune response such as the use of immunomodulatory drugs or recent cancer requiring chemotherapy were excluded from the study, potentially limiting the generalizability of our findings to those individuals. Future studies should explore vaccine efficacy in this highly frail subset of the population.
Conclusion
Both aTIV and HD-IIV3 elicited strong humoral immune responses in older individuals residing in long-term care facilities. While aTIV did not meet non-inferiority standards for HAI seroconversion, it demonstrated non-inferiority for neuraminidase inhibition and outperformed HD-IIV3 in terms of NA seroconversion and GMTs. On balance, our data support the clinical and immunogenicity data from others and add nuance with respect to NI; our results support the ACIP recommendations for offering an enhanced to older individuals.
Supplementary Material
Figure 2. Observed distributions of NI titers for aTIV (black) and HD-IIV3 (gray), by day and strain.
For each box, the center line indicates the median and the bottom and top of the box indicate the first and third quartile, respectively. The lower and upper whiskers extend from the first and third quartile lines, respectively, to the smallest and largest values no more than 1.5 times the interquartile range (height of box) away from the first and third quartile values.
Acknowledgments.
We would like to thank the long-term care facility staff and residents who participated in this study, without whom this study would not have been feasible. The sponsor (NIH) plaed no roll in this study other than funding fuding it.
Financial support.
NIH Grant: 1R011AI129709–01A1 and Louis Stokes Cleveland VA Medical Center, Cleveland, OH
Footnotes
Conflicts of Interest. Potential conflicts of interest. S. G. and D. H. C. are recipients of investigator-initiated grants to their universities from Pfizer to study pneumococcal vaccines, Sanofi Pasteur and Seqirus to study influenza vaccines, and Moderna to study respiratory infection. S. G. is recipient of investigator-initiated grant to the university from Genentech on influenza antivirals; reports consulting for Seqirus, Sanofi, Merck, Vaxart, Novavax, Moderna, and Janssen; has served on the speaker bureaus for Seqirus and Sanofi; reports personal fees from Pfizer; and reports data and safety monitoring board fees from Longevoron and SciClone.
Disclaimer. The contents do not represent the views of the U.S. Department of Veterans Affairs or of the United States Government.
References
- 1.Moyo P, Zullo AR, McConeghy KW, et al. Risk factors for pneumonia and influenza hospitalizations in long-term care facility residents: a retrospective cohort study. BMC Geriatr 2020;20(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grohskopf LA, Ferdinands JM, Blanton LH, et al. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices - United States, 2024–25 Influenza Season. MMWR Recomm Rep 2024;73(5):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DiazGranados CA, Dunning AJ, Kimmel M, et al. Efficacy of high-dose versus standard-dose influenza vaccine in older adults. N Engl J Med 2014;371(7):635–645. [DOI] [PubMed] [Google Scholar]
- 4.Gravenstein S, Davidson HE, Taljaard M, et al. Comparative effectiveness of high-dose versus standard-dose influenza vaccination on numbers of US nursing home residents admitted to hospital: a cluster-randomised trial. Lancet Respir Med 2017;5(9):738–746. [DOI] [PubMed] [Google Scholar]
- 5.Gravenstein S, McConeghy KW, Saade E, et al. Adjuvanted influenza vaccine and influenza outbreaks in U.S. nursing homes: Results from a pragmatic cluster-randomized clinical trial. Clin Infect Dis 2021. [DOI] [PubMed] [Google Scholar]
- 6.McConeghy KW, Davidson HE, Canaday DH, et al. Cluster-randomized trial of adjuvanted vs. non-adjuvanted trivalent influenza vaccine in 823 U.S. nursing homes. Clin Infect Dis 2020. [DOI] [PubMed] [Google Scholar]
- 7.Schmader KE, Liu CK, Flannery B, et al. Immunogenicity of adjuvanted versus high-dose inactivated influenza vaccines in older adults: a randomized clinical trial. Immun Ageing 2023;20(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couzens L, Gao J, Westgeest K, et al. An optimized enzyme-linked lectin assay to measure influenza A virus neuraminidase inhibition antibody titers in human sera. Journal of virological methods 2014;210:7–14. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen TL, Xie L. Incomparability of treatment groups is often blindly ignored in randomised controlled trials - a post hoc analysis of baseline characteristic tables. J Clin Epidemiol 2021;130:161–168. [DOI] [PubMed] [Google Scholar]
- 10.Dunkle LM, Izikson R, Patriarca P, et al. Efficacy of Recombinant Influenza Vaccine in Adults 50 Years of Age or Older. N Engl J Med 2017;376(25):2427–2436. [DOI] [PubMed] [Google Scholar]
- 11.Li APY, Cohen CA, Leung NHL, et al. Immunogenicity of standard, high-dose, MF59-adjuvanted, and recombinant-HA seasonal influenza vaccination in older adults. NPJ Vaccines 2021;6(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Aalst R, Gravenstein S, Mor V, et al. Comparative effectiveness of high dose versus adjuvanted influenza vaccine: A retrospective cohort study. Vaccine 2020;38(2):372–379. [DOI] [PubMed] [Google Scholar]
- 13.Boikos C, Fischer L, O’Brien D, et al. Relative Effectiveness of Adjuvanted Trivalent Inactivated Influenza Vaccine Versus Egg-derived Quadrivalent Inactivated Influenza Vaccines and High-dose Trivalent Influenza Vaccine in Preventing Influenza-related Medical Encounters in US Adults >/= 65 Years During the 2017–2018 and 2018–2019 Influenza Seasons. Clin Infect Dis 2021;73(5):816–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imran M, Mills CW, McDermott KW, et al. Relative Effectiveness of the MF59-Adjuvanted Influenza Vaccine Versus High-Dose Influenza Vaccine in Older Adults With Influenza Risk Factors During the 2019–2020 US Influenza Season. Open forum infectious diseases 2024;11(8):ofae459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunning AJ, DiazGranados CA, Voloshen T, et al. Correlates of Protection against Influenza in the Elderly: Results from an Influenza Vaccine Efficacy Trial. Clin Vaccine Immunol 2016;23(3):228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Memoli MJ, Shaw PA, Han A, et al. Evaluation of Antihemagglutinin and Antineuraminidase Antibodies as Correlates of Protection in an Influenza A/H1N1 Virus Healthy Human Challenge Model. mBio 2016;7(2):e00417–00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.