Abstract
The properties and enzymic activity of endoglucanases (EC 3.2.1.4) of the fungus Trichoderma reesei were studied by means of immunological methods and by using polyglycosidic substrates. Endoglucanases exist in the culture liquid as a series of immunologically related components. The most active endoglucanase component has an Mr of 43 000 and pI value of 4.0. The most abundant components have a value of pI about 5.0, an Mr of 56 000-67 000 and specific activity only one-fifth of that of the pI-4.0 component. During purification and storage the endoglucanases are spontaneously modified; the relative proportion of components having greater Mr values, more alkaline pI values and lower specific activities is increased. The hexose content of the endoglucanase components is 2-7%. Endoglucanases hydrolyse soluble beta-1,4 glycans. The enzymes described here differ from endoglucanase preparations described previously in not showing activity towards insoluble substrates. The role of endoglucanases in wood hydrolysis is consequently limited to the stage where wood constituents are already in soluble form.
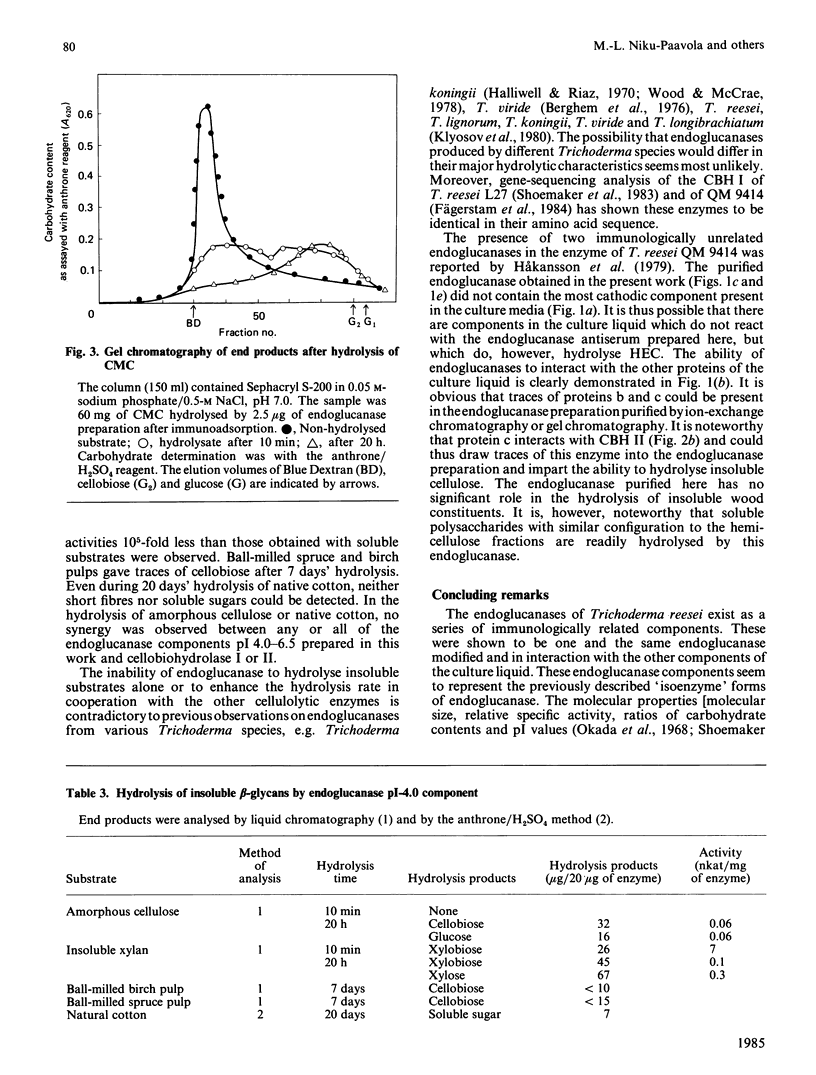
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almin K. E., Eriksson K. E., Jansson C. Enzymic degradation of polymers. II. Viscometric determination of cellulase activity in absolute terms. Biochim Biophys Acta. 1967 Jul 11;139(2):248–253. doi: 10.1016/0005-2744(67)90029-0. [DOI] [PubMed] [Google Scholar]
- Berghem L. E., Pettersson L. G., Axiö-Fredriksson U. B. The mechanism of enzymatic cellulose degradation. Purification and some properties of two different 1,4beta-glucan glucanohydrolases from Trichoderma viride. Eur J Biochem. 1976 Jan 15;61(2):621–630. doi: 10.1111/j.1432-1033.1976.tb10058.x. [DOI] [PubMed] [Google Scholar]
- Bertolini M., Pigman W. Action of alkali on bovine and ovine submaxillary mucins. J Biol Chem. 1967 Sep 10;242(17):3776–3781. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S., Schneider W. J., Hobgood K. K., Tolleshaug H., Brown M. S., Goldstein J. L. Biosynthesis of N- and O-linked oligosaccharides of the low density lipoprotein receptor. J Biol Chem. 1983 Dec 25;258(24):15261–15273. [PubMed] [Google Scholar]
- Diezel W., Kopperschläger G., Hofmann E. An improved procedure for protein staining in polyacrylamide gels with a new type of Coomassie Brilliant Blue. Anal Biochem. 1972 Aug;48(2):617–620. doi: 10.1016/0003-2697(72)90117-0. [DOI] [PubMed] [Google Scholar]
- Gong C. S., Chen L. F., Tsao G. T. Affinity chromatography of endoglucanase of Trichoderma viride by concanavalin A-agarose. Biotechnol Bioeng. 1979 Feb;21(2):167–171. doi: 10.1002/bit.260210203. [DOI] [PubMed] [Google Scholar]
- Halliwell G., Riaz M. The formation of short fibres from native cellulose by components of Trichoderma koningii cellulase. Biochem J. 1970 Jan;116(1):35–42. doi: 10.1042/bj1160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C., Cohen R. E., Zhang W. J., Ballou C. E. Carbohydrate chains on yeast carboxypeptidase Y are phosphorylated. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2244–2248. doi: 10.1073/pnas.78.4.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson U., Fägerstam L. G., Pettersson L. G., Andersson L. A 1,4-beta-glucan glucanohydrolase from the cellulolytic fungus Trichoderma viride QM 9414. Purification, characterization and preparation of an immunoadsorbent for the enzyme. Biochem J. 1979 Apr 1;179(1):141–149. doi: 10.1042/bj1790141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Nummi M., Niku-Paavola M. L., Enari T. M., Raunio V. Isolation of cellulases by means of biospecific sorption on amorphous cellulose. Anal Biochem. 1981 Sep 1;116(1):137–141. doi: 10.1016/0003-2697(81)90335-3. [DOI] [PubMed] [Google Scholar]
- Nummi M., Niku-Paavola M. L., Lappalainen A., Enari T. M., Raunio V. Cellobiohydrolase from Trichoderma reesei. Biochem J. 1983 Dec 1;215(3):677–683. doi: 10.1042/bj2150677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nummi M., Perrin J. M., Niku-Paavola M. L., Enari T. M. Measurement of xylanase activity with insoluble xylan substrate. Biochem J. 1985 Mar 1;226(2):617–620. doi: 10.1042/bj2260617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada G., Nisizawa K., Suzuki H. Cellulase components from Trichoderma viride. J Biochem. 1968 May;63(5):591–607. doi: 10.1093/oxfordjournals.jbchem.a128818. [DOI] [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Characterization of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):147–161. doi: 10.1016/0005-2744(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Wood T. M., McCrae S. I. The cellulase of Trichoderma koningii. Purification and properties of some endoglucanase components with special reference to their action on cellulose when acting alone and in synergism with the cellobiohydrolase. Biochem J. 1978 Apr 1;171(1):61–72. doi: 10.1042/bj1710061. [DOI] [PMC free article] [PubMed] [Google Scholar]