Abstract
Adenoid cystic carcinoma (ACC) is a rare and lethal malignancy that originates in secretory glands of the head and neck. A prominent molecular feature of ACC is the overexpression of the proto-oncogene MYB. ACC has a poor long-term survival due to its high propensity for recurrence and protracted metastasis. Currently, clinical technologies lack the efficiency to distinguish patient prognosis prior to its redevelopment. We hypothesize that metastatic ACC can be detected by monitoring tumor-specific MYB expression in patients’ blood. We developed a quantitative polymerase chain reaction (qPCR) assay for MYB transcripts and screened blood samples from four patient cohorts: no history or evidence of ACC (n=23), past history of ACC and no evidence of disease (NED) for greater than three years (n=15), local ACC (n=6), and metastatic ACC (n=5). Our assay detected significantly elevated levels of MYB transcripts in the metastatic ACC cohort (p < 0.01). Receiver operating characteristic (ROC) curves comparing metastatic to NED and metastatic to local disease were significant, with p values < 0.0001 and 0.0008, respectively. Single-cell RNA sequencing (scRNA-seq) of blood from metastatic ACC identified a cluster of circulating tumor cells (CTCs) expressing MYB. Here, we report a sensitive, cost-effective, and minimally invasive diagnostic test that leverages tumor-specific signatures to screen for metastatic ACC disease, potentially enhancing detection earlier than the current clinical standard.
Keywords: Adenoid cystic carcinoma, rare cancer, MYB, Liquid Biopsy, Diagnostic biomarkers
1. Background
Adenoid cystic carcinoma (ACC) is a rare but lethal epithelial cancer that predominantly arises from the secretory glands of the head and neck (1, 2). Despite surgical excision followed by radiation, the prognosis for patients with ACC remains poor due to high rates of recurrence and metastasis, with the 5, 10, and 20-year survival rates reported as 68–80%, 60%, and 28% respectively (3–5). Current surveillance methods involve periodic scans; however, technical complexities, limited access, and financial constraints contribute to patient attrition.
Here, we evaluate whether the known molecular signatures that give rise to ACC tumors could be harnessed for the development of a blood-based molecular assay to monitor patients with ACC. The most prominent molecular feature of ACC tumors is the overexpression of the MYB proto-oncogene, which is often translocated with other genomic loci to establish a feedforward mechanism that amplifies its expression in ACC (Figure 1A–B) (6–9). The reliance of ACC tumors on MYB expression to drive its survival and proliferation allows us to evaluate whether qPCR probes mapping to the 5’ regulatory elements of MYB can detect and discern metastatic disease from blood of patients with ACC from those with non-disseminated tumors and other, non-cancerous sources of MYB expression.
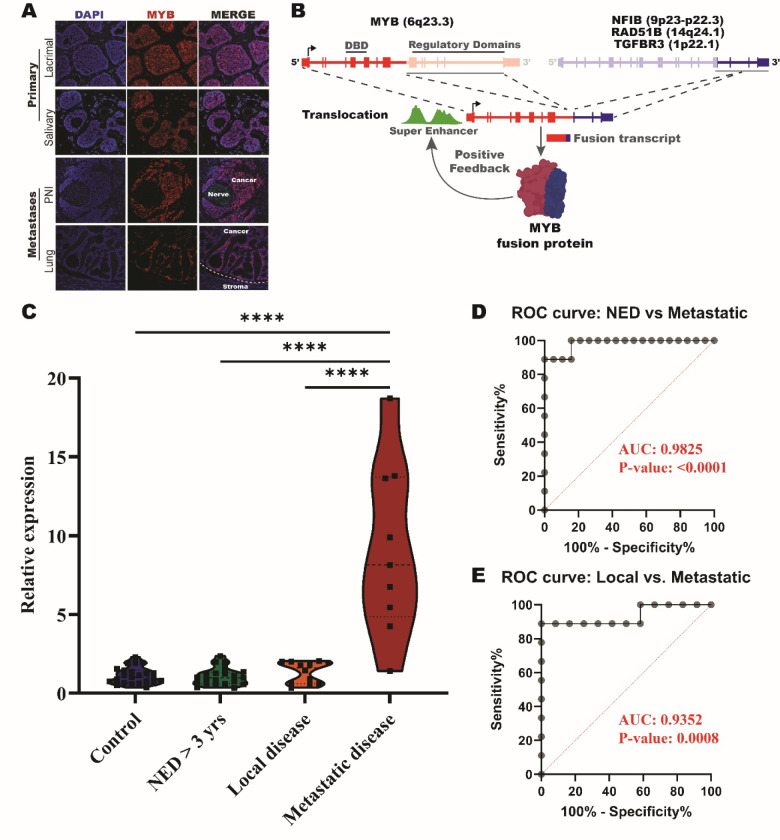
Figure 1: Detection of MYB in whole blood for patients with metastatic ACC.
(A) Immunofluorescent imaging of MYB expression in primary and metastatic tumor samples. (B) Schematic of MYB translocation events that occur with NFIB, RAD51B, and TGFBR3. (C) Violin plot of relative expression of MYB primer pair spanning exons 2–3 in control, NED, local, and metastatic patients. **** = p-value < 0.0001. (D) ROC curve of NED > 3 years verse metastatic results of whole blood MYB relative expression test. (E) ROC curve of local disease verse metastatic results of whole blood MYB relative expression test.
2. Methods
See supplemental information for detailed methods.
2.1. Peripheral blood sample collection and processing
The University of Miami Institutional Review Board approved this study (IRB 20090524 and 20210471). The study was conducted in a Health Insurance Portability and Accountability Act of 1996–compliant manner. Blood samples were collected from individuals with no history or evidence of ACC (n=23), past history of ACC and no evidence of disease (NED) (n=15), local ACC (n=6), and metastatic ACC (n=5) (Table 1) and frozen at −80°C until processed. Blood RNA was extracted and reverse-transcribed to cDNA. RT-qPCR was conducted and relative expression of MYB was quantified using the geometric mean of the Ct values for the two housekeeping genes and MYB amplification in the control samples (Supplementary Table 1) (10).
Table 1:
Clinical demographic table
| Sample (n = 25) | |
|---|---|
|
| |
| Patient demographics | |
|
| |
| Age at diagnosis | 46.4 (SD = 13.6, Range: 20 – 71) |
|
| |
| Gender | |
| Male | 16 |
| Female | 9 |
|
| |
| Affected Side | |
| Right | 14 |
| Left | 9 |
| Not sided | 2 |
|
| |
| Tumor characteristics | |
|
| |
| ACC location | |
| Salivary | 2 |
| Sinus | 4 |
| Oropharyngeal | 1 |
| Larynx | 1 |
| Lacrimal | 17 |
| ACC subtype | |
| Crib | 6 |
| Solid variant | 2 |
| Crib/Solid | 1 |
| Crib/Basa | 1 |
| Crib/Tub | 4 |
| Unavailable | 11 |
|
| |
| Bone involvement | |
|
| |
| T stage on presentation | |
| 1 | 5 |
| 2 | 5 |
| 3 | 3 |
| 4 | 10 |
| Unavailable | 3 |
|
| |
| N stage on presentation | |
| 0 | 21 |
| 3 | 1 |
| Unavailable | 3 |
|
| |
| M stage on presentation | |
| 0 | 22 |
| 1 | 0 |
| Unavailable | 3 |
|
| |
| Current clinical status | |
|
| |
| Post treatment local recurrence/metastasis | |
| Local | 6 |
| Met (total) | 5 |
| Deceased | 3 |
| NED | 15 |
2.2. Preparation of peripheral blood mononuclear cell (PBMC) fraction for single-cell RNA sequencing (scRNA-seq).
PBMC fractions were isolated by centrifugation. scRNA-seq library was prepared using the 10X Genomics Chromium Single Cell 5’ v2.0 chemistry (10x Genomics, Pleasanton, CA) according to manufacturer’s instructions. We used the CellRanger pipeline to process and align to the GRCh38 human genome reference. Further analysis was computed using various programs and references.
3. Results
3.1. Probes spanning the exon 2–3 boundary of MYB discern metastatic blood samples from other cohorts
We designed qPCR primer pairs spanning exon-exon boundaries throughout the 5’ end of the MYB gene body and validated all probes against a primary ACC tumor (Supplementary Figure 1). Subsequently, we tested six of these MYB probes in blood from patients with metastatic ACC. Only the primer pair spanning the exon 2 to 3 boundary detected an elevated MYB signal (Supplementary Figure 2). Utilizing this primer pair, we observed a significantly elevated level of MYB expression (p < 0.0001) in whole blood from our patients with metastatic ACC. In contrast, no significant level of MYB expression in blood was detected among the control, NED, or local disease cohorts (Figure 1C and Supplementary Figure 3). Using primer pairs that amplify products between exon 14 and 15 of MYB, we further validated that the elevated MYB signal detected in metastatic ACC blood originates from the enriched 5’ MYB transcription (Supplementary Figure 4).
ROC curves for metastatic versus NED and metastatic versus local disease were significant with p values of <0.0001 and 0.0008, respectively. The area under the curve (AUC) for both ROC curves were 0.9825 and 0.9352, respectively, indicating a highly significant and sensitive test (Figure 1C and D).
3.2. Circulating tumor cells (CTCs) are the source of elevated MYB in metastatic ACC blood
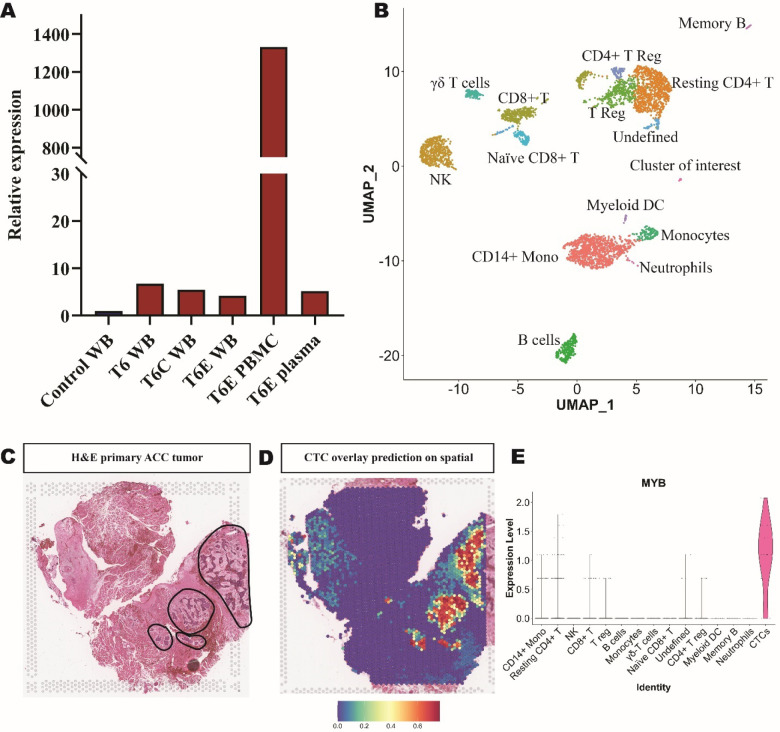
To elucidate the source of the elevated MYB signal detected in metastatic ACC blood samples, we isolated RNA from the plasma and mononuclear cell (PBMC) fractions to determine if the MYB signal arose from cell-free (plasma) or cell based (PBMCs) RNA sources. Our analysis revealed that the MYB level in the PBMC layer was 258.5-fold higher than that of the plasma (Figure 2A). Expanding on this, we performed scRNA-seq on the PBMC fraction of metastatic ACC blood (T6). The sequencing run captured 4,852 cells. Immune cell clusters were identified based on gene expression, whereas one cluster presented with the highest expression of MYB and did not have any immune cell gene expression signatures (indicated as cluster of interest in UMAP (n = 18 cells)) (Figure 2B). Upon integration of the scRNA-seq dataset with our previously reported spatial transcriptomic profiling of ACC (11), this cluster of interest was determined to have significant overlap in several ACC-specific markers, indicating that this cluster represents a circulating tumor cell (CTC) population (Figure 2C–E and Supplementary Figure 5).
Figure 2: Circulating tumor cell identification in PBMCs of metastatic ACC patient.
(A) Tracking patient T6 MYB relative expression over time in whole blood (WB), PMBC, and plasma samples. (B) UMAP of 15 clusters from sample T6 PBMC single-cell sequencing experiment with cell type identifications. (C) Hematoxylin and eosin (H&E) image of primary ACC tumor used for spatial transcriptomics with circled tumor foci indicated. (D) Probability prediction of single-cell cluster of interest cell type overlaid on spatial transcriptomic primary ACC tumor sample. (E) Violin plot of MYB expression in PBMC single-cell sequencing.
4. Discussion
We have developed a liquid biopsy that can detect a cancer specific signature from blood samples of patients with metastatic ACC. Our RT-qPCR assay detects tumor-specific MYB expression specifically and sensitively in a rapid and cost-effective manner. We show that the relative expression of MYB significantly differentiates metastatic ACC blood samples from samples collected from controls, NED, and local disease patients. Longitudinal follow-up revealed elevated MYB signal in one patient (T5) who was diagnosed with metastatic ACC by imaging 11-months after our first detection of elevated MYB in their blood sample. Subsequent blood draws from this patient (T5B, T5C) after their clinical metastatic diagnosis show progressively higher levels of MYB expression, commensurate with their disease burden (Supplementary Figure 6). About 10% ACCs do not exhibit MYB overexpression, which would require initial screening for MYB expression in primary tumor before employing out assay for surveillance. Yet, for the vast majority of patients with ACC, our assay may decrease unnecessary imaging, enable earlier interventions in the setting of protracted metastasis, and ultimately improve long-term prognosis.
In interrogating the source of the MYB signal, we discovered a CTC population in metastatic ACC blood (Figure 2C–D and Supplementary Figure 5). To our knowledge, only one previous report of CTCs was identified in ACC patients’ blood, which were isolated using the general marker pan-cytokeratin (12). In our analysis, keratin 8 had minor preferential expression in the ACC CTC cluster (Supplementary Figure 7). Transcriptomic analysis revealed high Sox4 expression in ACC CTCs, which may be a potential additional biomarker. However, qPCR probing of Sox4 expression lacked the diagnostic power provided by MYB (Supplementary Figure 8).
We acknowledge that a limitation of the current findings is the size of our sample cohorts, partly due to the rarity and lethality of ACC itself. A randomized prospective clinical study is warranted to ascertain the true diagnostic power and sensitivity of this assay to discern regional lymph node metastases in addition to distal metastases. We are currently working on establishing the protocols for such as study at our institution.
5. Conclusions
The findings of this study provide encouraging evidence that leveraging the key signature of ACC can facilitate the development of a simple, sensitive, and cost-effective metastatic screening tool for ACC management. Upon clinical validation, we envision a test that could be implemented for continuous monitoring of MYB levels in patients with a history of ACC, thereby enabling the stratification of individuals who may require confirmatory imaging or more frequent screenings.
Supplementary Material
Acknowledgements
We would like to thank all participants who donated blood to this study. We would also like to thank University of Miami Miller School of Medicine Onco-Genomics Sequencing Core.
Funding
Research reported in this publication was supported in part by the Dr. Nasser Al-Rashid Orbital Vision Research Endowment 700975 (D.T.T. and D.P.), a Department of Defense (DOD) Rare Cancers Research Program Grant W81XWH2211079 (D.P.), and the Adenoid Cystic Carcinoma Research Foundation (ACCRF) AWD006393 (D.P.). The Bascom Palmer Eye Institute is supported by NIH Center Core Grant P30EY014801 and a Research to Prevent Blindness Unrestricted Grant GRoo4596 (New York, NY, USA). The Sylvester Comprehensive Cancer Center shared resources are supported by the NCI Core Grant P30CA240139. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- ACC
Adenoid cystic carcinoma
- AUC
Area under the curve
- CTC
Circulating tumor cells
- NED
No evidence of disease
- PBMC
Peripheral blood mononuclear cells
- ROC curve
Receiver operating characteristic curve
Footnotes
Competing interests
The authors declare that there are no competing interests.
Ethics approval and consent to participate
The University of Miami Institutional Review Board approved this study (IRB 20090524 and 20210471). All subjects gave a written informed consent to participate in the study. The study was conducted in a Health Insurance Portability and Accountability Act of 1996–compliant manner.
Consent for publication
All authors give consent for the publication of the following content.
Availability for data and materials
Data presented is all available upon request to the corresponding author.
References
- (1).Bradley PJ. Adenoid cystic carcinoma evaluation and management: progress with optimism! Curr Opin Otolaryngol Head Neck Surg. 2017;25(2):147–53. [DOI] [PubMed] [Google Scholar]
- (2).Chummun S, McLean NR, Kelly CG, Dawes PJ, Meikle D, Fellows S, et al. Adenoid cystic carcinoma of the head and neck. Br J Plast Surg. 2001;54(6):476–80. [DOI] [PubMed] [Google Scholar]
- (3).Chae YK, Chung SY, Davis AA, Carneiro BA, Chandra S, Kaplan J, et al. Adenoid cystic carcinoma: current therapy and potential therapeutic advances based on genomic profiling. Oncotarget. 2015;6(35):37117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Van Weert S, Bloemena E, van der Waal I, de Bree R, Rietveld DH, Kuik JD, et al. Adenoid cystic carcinoma of the head and neck: a single-center analysis of 105 consecutive cases over a 30-year period. Oral Oncol. 2013;49(8):824–9. [DOI] [PubMed] [Google Scholar]
- (5).Jang S, Patel PN, Kimple RJ, McCulloch TM. Clinical Outcomes and Prognostic Factors of Adenoid Cystic Carcinoma of the Head and Neck. Anticancer Res. 2017;37(6):3045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Drier Y, Cotton MJ, Williamson KE, Gillespie SM, Ryan RJ, Kluk MJ, et al. An oncogenic MYB feedback loop drives alternate cell fates in adenoid cystic carcinoma. Nat Genet. 2016;48(3):265–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Persson M, Andren Y, Moskaluk CA, Frierson HF Jr., Cooke SL, Futreal PA, et al. Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes Chromosomes Cancer. 2012;51(8):805–17. [DOI] [PubMed] [Google Scholar]
- (8).Persson F, Fehr A, Sundelin K, Schulte B, Loning T, Stenman G. Studies of genomic imbalances and the MYB-NFIB gene fusion in polymorphous low-grade adenocarcinoma of the head and neck. Int J Oncol. 2012;40(1):80–4. [DOI] [PubMed] [Google Scholar]
- (9).Mitani Y, Li J, Rao PH, Zhao YJ, Bell D, Lippman SM, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res. 2010;16(19):4722–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Taylor SC, Nadeau K, Abbasi M, Lachance C, Nguyen M, Fenrich J. The Ultimate qPCR Experiment: Producing Publication Quality, Reproducible Data the First Time. Trends Biotechnol. 2019;37(7):761–74. [DOI] [PubMed] [Google Scholar]
- (11).Moeyersoms AHM, Gallo RA, Zhang MG, Stathias V, Maeng MM, Owens D, et al. Spatial Transcriptomics Identifies Expression Signatures Specific to Lacrimal Gland Adenoid Cystic Carcinoma Cells. Cancers (Basel). 2023;15(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Fisher BM, Tang KD, Warkiani ME, Punyadeera C, Batstone MD. A pilot study for presence of circulating tumour cells in adenoid cystic carcinoma. Int J Oral Maxillofac Surg. 2021;50(8):994–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.