Abstract
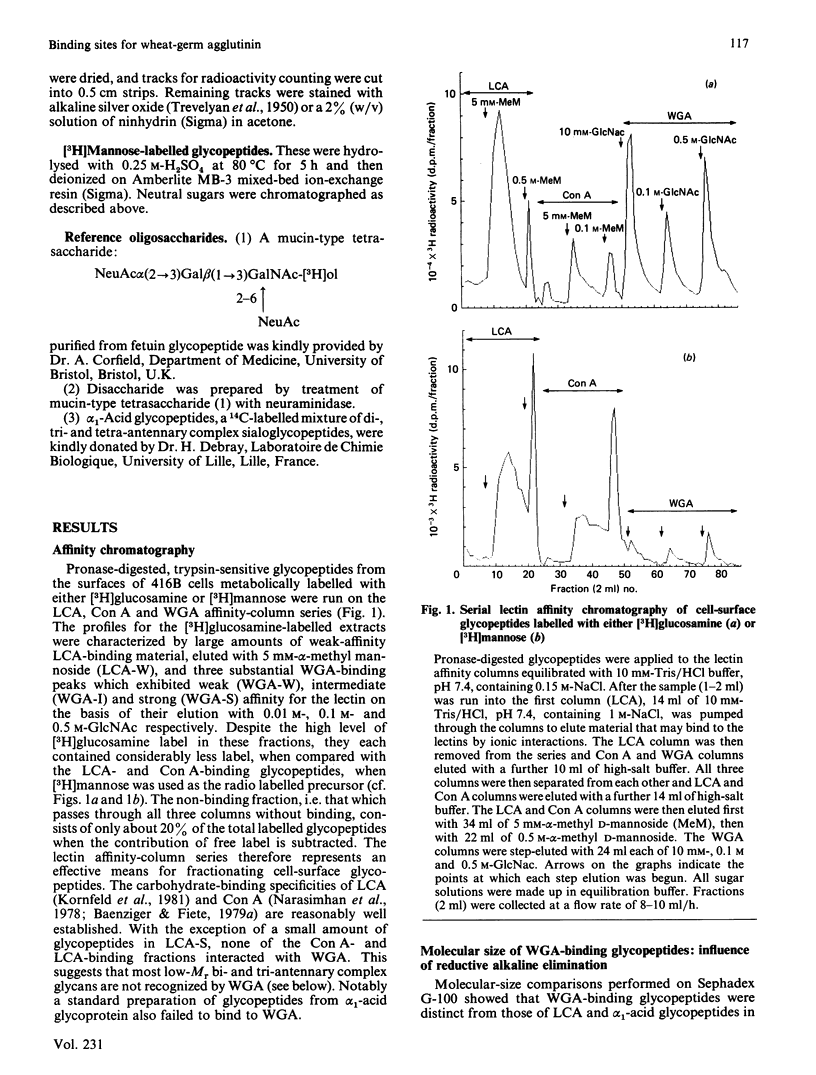
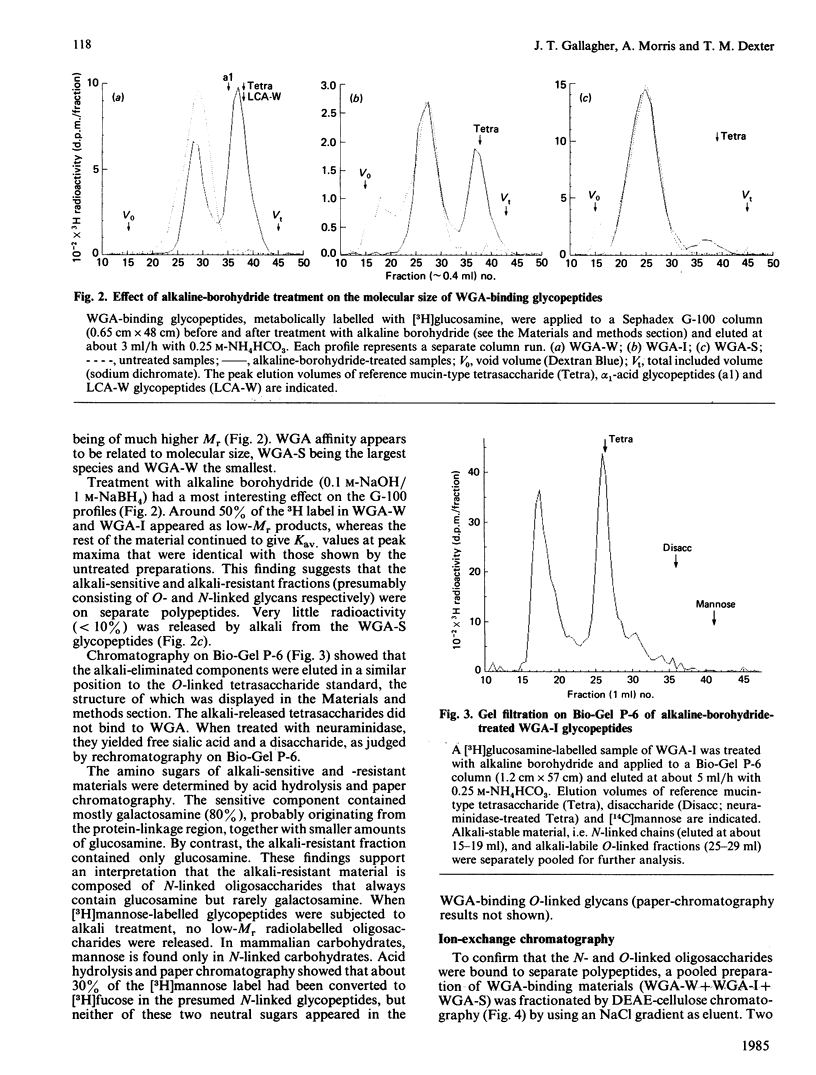
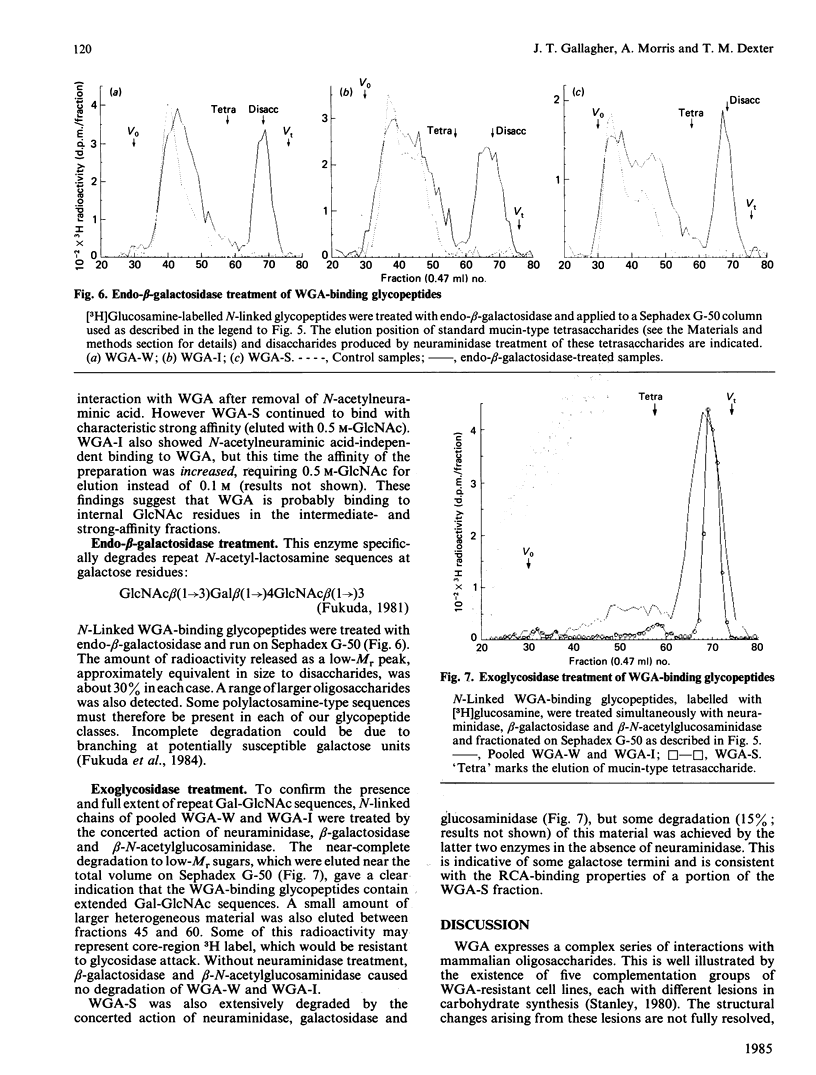
The carbohydrate-binding properties of wheat-germ agglutinin (WGA) have been studied by using glycopeptides isolated from the cell surfaces of a cultured murine myeloid cell line (416B). The glycopeptides were passed through affinity columns of lentil lectin (LCA), concanavalin A (Con A) and WGA arranged in series so that material reaching the WGA column had failed to bind to LCA or Con A. WGA-binding glycopeptides were step-eluted with 0.01 M, 0.1 M and 0.5 M-N-acetylglucosamine (GlcNAc), to yield weak (WGA-W), intermediate (WGA-I) and strong (WGA-S) affinity fractions. WGA-W and WGA-I contained 'N'- and 'O'-linked oligosaccharides bound to separate polypeptides. WGA-S consisted almost entirely of N-linked components. Our analytical work was concentrated mainly on the N-linked fractions. In these carbohydrates WGA affinity was directly proportional to molecular size but inversely related to N-acetylneuraminic acid content. The binding of the weak-affinity fraction was dependent on N-acetylneuraminic acid, but the intermediate- and strong-binding species interacted with the lectin by N-acetylneuraminic acid-independent mechanisms. N-linked glycopeptides in each WGA-binding class were almost totally degraded to monosaccharides by the concerted action of the exoglycosidases neuraminidase, beta-galactosidase and beta-N-acetylglucosaminidase. Treatment with endo-beta-galactosidase caused partial depolymerization, yielding some disaccharides but also a heterogeneous population of partially degraded components. These findings suggest that WGA binds with high affinity to internal GlcNAc residues in large oligosaccharides containing repeat sequences of Gal beta(1----4)GlcNAc beta(1----3) (i.e. polylactosamine-type glycans). N-Acetylneuraminic acid is involved only in low-affinity interactions with WGA. WGA therefore displays an intricate pattern of saccharide specificities that can be profitably utilized for structural analysis of complex carbohydrates.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W., Banks J., Kemper J. G., Davidson E. A. Partial characterization of sialoglycopeptides produced by cultured human melanoma cells and melanocytes. Biochemistry. 1981 Sep 15;20(19):5586–5594. doi: 10.1021/bi00522a036. [DOI] [PubMed] [Google Scholar]
- Bhavanandan V. P., Katlic A. W. The interaction of wheat germ agglutinin with sialoglycoproteins. The role of sialic acid. J Biol Chem. 1979 May 25;254(10):4000–4008. [PubMed] [Google Scholar]
- Carlsson H. E., Lönngren J., Goldstein I. J., Christner J. E., Jourdian G. W. The interaction of wheat germ agglutinin with keratan from cornea and nasal cartilage. FEBS Lett. 1976 Feb 1;62(1):38–40. doi: 10.1016/0014-5793(76)80011-7. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran E. V., Davidson E. A. Sialoglycoproteins of human mammary cells: partial characterization of sialoglycopeptides. Biochemistry. 1979 Dec 11;18(25):5615–5620. doi: 10.1021/bi00592a015. [DOI] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Debray H., Decout D., Strecker G., Spik G., Montreuil J. Specificity of twelve lectins towards oligosaccharides and glycopeptides related to N-glycosylproteins. Eur J Biochem. 1981 Jun;117(1):41–55. doi: 10.1111/j.1432-1033.1981.tb06300.x. [DOI] [PubMed] [Google Scholar]
- Dennis J. W., Waller C. A., Schirrmacher V. Identification of asparagine-linked oligosaccharides involved in tumor cell adhesion to laminin and type IV collagen. J Cell Biol. 1984 Oct;99(4 Pt 1):1416–1423. doi: 10.1083/jcb.99.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Scott D., Teich N. M. Isolation and characterisation of a bipotential haematopoietic cell line. Nature. 1979 Feb 8;277(5696):471–474. doi: 10.1038/277471a0. [DOI] [PubMed] [Google Scholar]
- Eckhardt A. E., Goldstein I. J. Isolation and characterization of a family of alpha-D-galactosyl-containing glycopeptides from Ehrlich ascites tumor cells. Biochemistry. 1983 Nov 8;22(23):5290–5297. doi: 10.1021/bi00292a007. [DOI] [PubMed] [Google Scholar]
- Feizi T. Monoclonal antibodies reveal saccharide structures of glycoproteins and glycolipids as differentiation and tumour-associated antigens. Biochem Soc Trans. 1984 Jun;12(3):545–549. doi: 10.1042/bst0120545. [DOI] [PubMed] [Google Scholar]
- Finne J., Tao T. W., Burger M. M. Carbohydrate changes in glycoproteins of a poorly metastasizing wheat germ agglutinin-resistant melanoma clone. Cancer Res. 1980 Jul;40(7):2580–2587. [PubMed] [Google Scholar]
- Fukuda M. N. Purification and characterization of endo-beta-galactosidase from Escherichia freundii induced by hog gastric mucin. J Biol Chem. 1981 Apr 25;256(8):3900–3905. [PubMed] [Google Scholar]
- Fukuda M., Dell A., Oates J. E., Fukuda M. N. Structure of branched lactosaminoglycan, the carbohydrate moiety of band 3 isolated from adult human erythrocytes. J Biol Chem. 1984 Jul 10;259(13):8260–8273. [PubMed] [Google Scholar]
- Gallagher J. T. Carbohydrate-binding properties of lectins: a possible approach to lectin nomenclature and classification. Review. Biosci Rep. 1984 Aug;4(8):621–632. doi: 10.1007/BF01121015. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Hammarström S., Sundblad G. Precipitation and carbohydrate-binding specificity studies on wheat germ agglutinin. Biochim Biophys Acta. 1975 Sep 9;405(1):53–61. doi: 10.1016/0005-2795(75)90313-x. [DOI] [PubMed] [Google Scholar]
- Irimura T., Nicolson G. L. Carbohydrate chain analysis by lectin binding to electrophoretically separated glycoproteins from murine B16 melanoma sublines of various metastatic properties. Cancer Res. 1984 Feb;44(2):791–798. [PubMed] [Google Scholar]
- Kornfeld K., Reitman M. L., Kornfeld R. The carbohydrate-binding specificity of pea and lentil lectins. Fucose is an important determinant. J Biol Chem. 1981 Jul 10;256(13):6633–6640. [PubMed] [Google Scholar]
- Krusius T., Finne J., Rauvala H. The poly(glycosyl) chains of glycoproteins. Characterisation of a novel type of glycoprotein saccharides from human erythrocyte membrane. Eur J Biochem. 1978 Dec 1;92(1):289–300. doi: 10.1111/j.1432-1033.1978.tb12747.x. [DOI] [PubMed] [Google Scholar]
- Lotan R., Nicolson G. L. Purification of cell membrane glycoproteins by lectin affinity chromatography. Biochim Biophys Acta. 1979 Dec 20;559(4):329–376. doi: 10.1016/0304-4157(79)90010-8. [DOI] [PubMed] [Google Scholar]
- Monsigny M., Roche A. C., Sene C., Maget-Dana R., Delmotte F. Sugar-lectin interactions: how does wheat-germ agglutinin bind sialoglycoconjugates? Eur J Biochem. 1980 Feb;104(1):147–153. doi: 10.1111/j.1432-1033.1980.tb04410.x. [DOI] [PubMed] [Google Scholar]
- Narasimhan S., Wilson J. R., Martin E., Schachter H. A structural basis for four distinct elution profiles on concanavalin A--Sepharose affinity chromatography of glycopeptides. Can J Biochem. 1979 Jan;57(1):83–96. doi: 10.1139/o79-011. [DOI] [PubMed] [Google Scholar]
- Scudder P., Hanfland P., Uemura K., Feizi T. Endo-beta-D-galactosidases of Bacteroides fragilis and Escherichia freundii hydrolyze linear but not branched oligosaccharide domains of glycolipids of the neolacto series. J Biol Chem. 1984 May 25;259(10):6586–6592. [PubMed] [Google Scholar]
- Stanley P., Carver J. P. Selective loss of wheat germ agglutinin binding to agglutinin-resistant mutants of Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5056–5059. doi: 10.1073/pnas.74.11.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Tsuji T., Matsumoto I., Osawa T. Structural requirements for the binding of oligosaccharides and glycopeptides to immobilized wheat germ agglutinin. Biochemistry. 1981 Sep 29;20(20):5894–5899. doi: 10.1021/bi00523a037. [DOI] [PubMed] [Google Scholar]


