Abstract
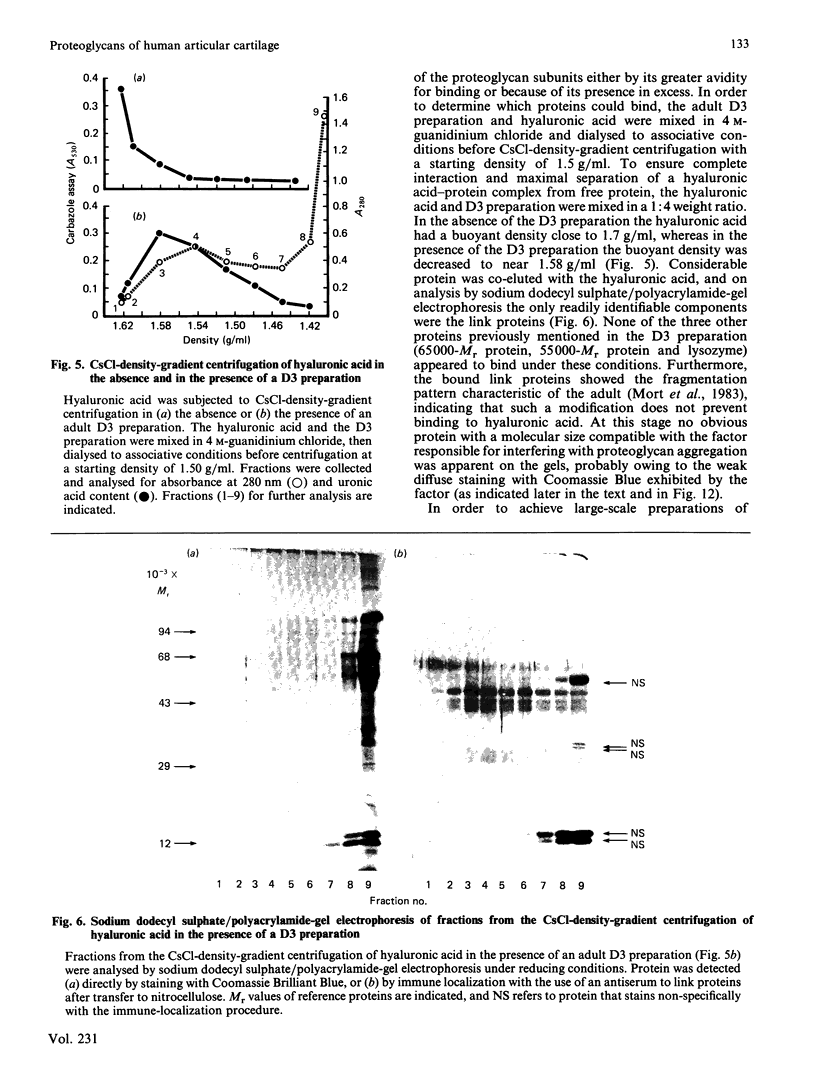
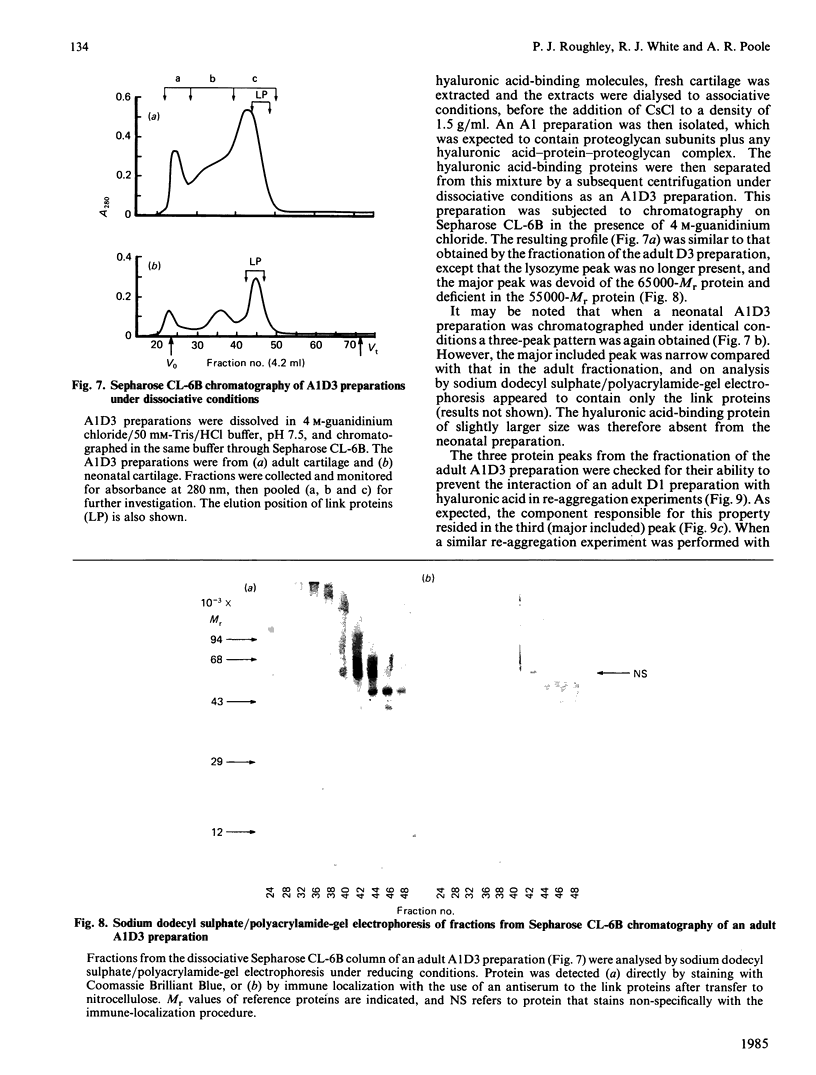
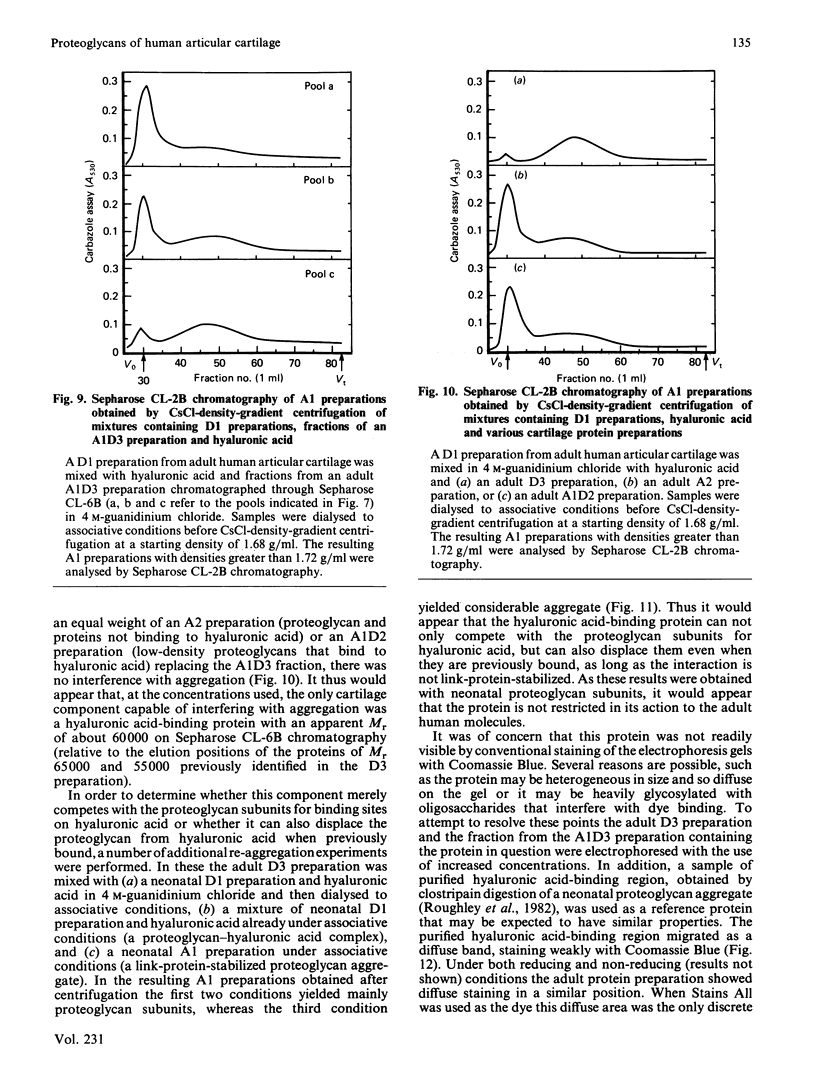
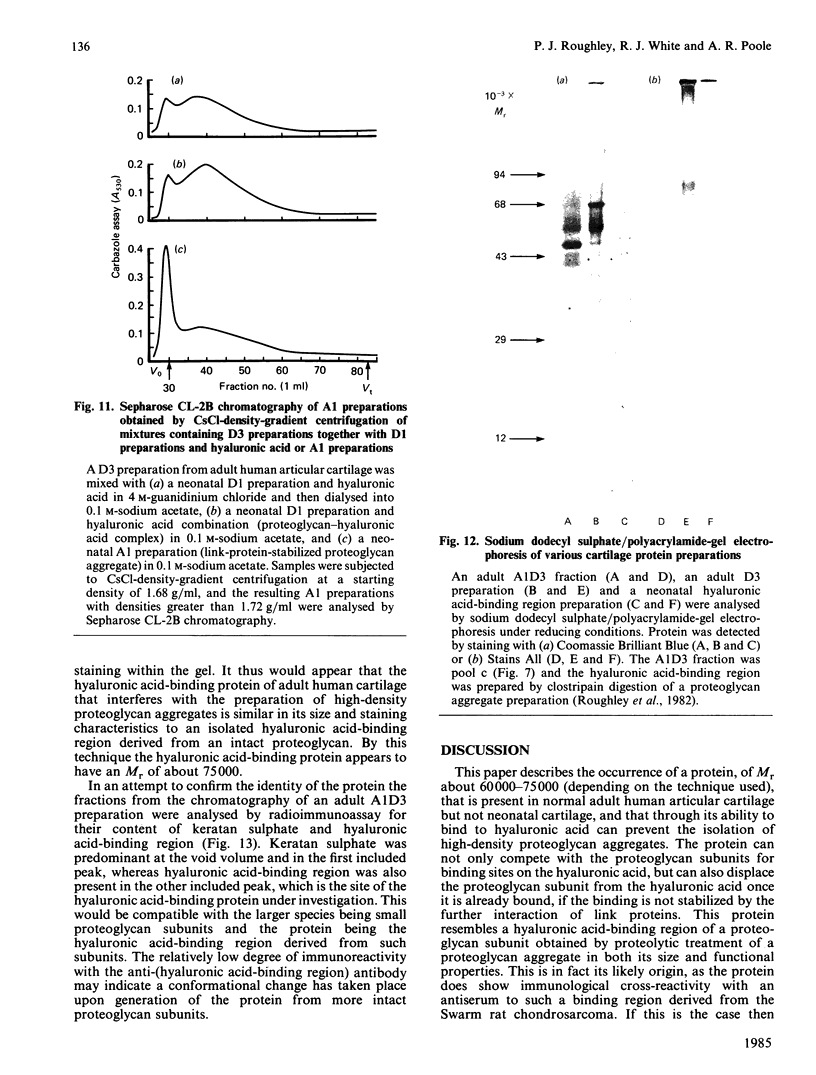
Adult human articular cartilage contains a hyaluronic acid-binding protein of Mr 60 000-75 000, which contains disulphide bonds essential for this interaction. The molecule can compete with proteoglycan subunits for binding sites on hyaluronic acid, and can also displace proteoglycan subunits from hyaluronic acid if their interaction is not stabilized by the presence of link proteins. The abundance of this protein in the adult accounts for the reported inability to prepare high-buoyant-density proteoglycan aggregates from extracts of adult human cartilage [Roughley, White, Poole & Mort (1984) Biochem. J. 221, 637-644], whereas the deficiency of the protein in newborn human cartilage allows the normal recovery of proteoglycan aggregates from this tissue. The protein shares many common features with a hyaluronic acid-binding region derived by proteolytic treatment of a proteoglycan aggregate preparation, and this may also represent its origin in the cartilage, with its production increasing during tissue maturation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983 Jul 25;258(14):8848–8854. [PubMed] [Google Scholar]
- Champion B. R., Poole A. R. Immunity to homologous cartilage proteoglycans in rabbits with chronic inflammatory arthritis. Coll Relat Res. 1981 Sep;1(5):453–473. doi: 10.1016/s0174-173x(81)80029-5. [DOI] [PubMed] [Google Scholar]
- Christner J. E., Caterson B., Baker J. R. Immunological determinants of proteoglycans. Antibodies against the unsaturated oligosaccharide products of chondroitinase ABC-digested cartilage proteoglycans. J Biol Chem. 1980 Aug 10;255(15):7102–7105. [PubMed] [Google Scholar]
- Cleland R. L., Sherblom A. P. Isolation and physical characterization of hyaluronic acid prepared from bovine nasal septum by cetylpyridinium chloride precipitation. J Biol Chem. 1977 Jan 25;252(2):420–426. [PubMed] [Google Scholar]
- Elliott R. J., Gardner D. L. Changes with age in the glycosaminoglycans of human articular cartilage. Ann Rheum Dis. 1979 Aug;38(4):371–377. doi: 10.1136/ard.38.4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Faltz L. L., Caputo C. B., Kimura J. H., Schrode J., Hascall V. C. Structure of the complex between hyaluronic acid, the hyaluronic acid-binding region, and the link protein of proteoglycan aggregates from the swarm rat chondrosarcoma. J Biol Chem. 1979 Feb 25;254(4):1381–1387. [PubMed] [Google Scholar]
- Franzén A., Björnsson S., Heinegård D. Cartilage proteoglycan aggregate formation. Role of link protein. Biochem J. 1981 Sep 1;197(3):669–674. doi: 10.1042/bj1970669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Pastewka J. V., Peacock A. C. Differential staining of phosphoproteins on polyacrylamide gels with a cationic carbocyanine dye. Anal Biochem. 1973 Nov;56(1):43–51. doi: 10.1016/0003-2697(73)90167-x. [DOI] [PubMed] [Google Scholar]
- Greenwald R. A. Lack of effect of lysozymes on cartilage proteoglycans. Arch Biochem Biophys. 1976 Aug;175(2):520–523. doi: 10.1016/0003-9861(76)90540-3. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Muir H. The specific interaction of hyaluronic acid with cartillage proteoglycans. Biochim Biophys Acta. 1972 Sep 15;279(2):401–405. doi: 10.1016/0304-4165(72)90160-2. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E. The role of link-protein in the structure of cartilage proteoglycan aggregates. Biochem J. 1979 Jan 1;177(1):237–247. doi: 10.1042/bj1770237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hascall V. C. Interaction of cartilage proteoglycans with hyaluronic acid. J Supramol Struct. 1977;7(1):101–120. doi: 10.1002/jss.400070110. [DOI] [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heinegård D., Hascall V. C. Aggregation of cartilage proteoglycans. 3. Characteristics of the proteins isolated from trypsin digests of aggregates. J Biol Chem. 1974 Jul 10;249(13):4250–4256. [PubMed] [Google Scholar]
- Heinegård D. Polydispersity of cartilage proteoglycans. Structural variations with size and buoyant density of the molecules. J Biol Chem. 1977 Mar 25;252(6):1980–1989. [PubMed] [Google Scholar]
- Kimura J. H., Thonar E. J., Hascall V. C., Reiner A., Poole A. R. Identification of core protein, an intermediate in proteoglycan biosynthesis in cultured chondrocytes from the Swarm rat chondrosarcoma. J Biol Chem. 1981 Aug 10;256(15):7890–7897. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., De Luca S., Nilsson B., Hascall V. C., Caputo C. B., Kimura J. H., Heinegard D. Oligosaccharides on proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1980 Jul 10;255(13):6084–6091. [PubMed] [Google Scholar]
- Mort J. S., Poole A. R., Roughley P. J. Age-related changes in the structure of proteoglycan link proteins present in normal human articular cartilage. Biochem J. 1983 Jul 15;214(1):269–272. doi: 10.1042/bj2140269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir H., Bullough P., Maroudas A. The distribution of collagen in human articular cartilage with some of its physiological implications. J Bone Joint Surg Br. 1970 Aug;52(3):554–563. [PubMed] [Google Scholar]
- Oegema T. R., Jr, Brown M., Dziewiatkowski D. D. The link protein in proteoglycan aggregates from the Swarm rat chondrosarcoma. J Biol Chem. 1977 Sep 25;252(18):6470–6477. [PubMed] [Google Scholar]
- Osserman E. F., Lawlor D. P. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966 Nov 1;124(5):921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. P., Mason R. M. Proteoglycan aggregates in adult human costal cartilage. Biochim Biophys Acta. 1979 Apr 3;583(4):512–526. doi: 10.1016/0304-4165(79)90068-0. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Rosenberg L. An immunoelectron microscope study of the organization of proteoglycan monomer, link protein, and collagen in the matrix of articular cartilage. J Cell Biol. 1982 Jun;93(3):921–937. doi: 10.1083/jcb.93.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole A. R., Pidoux I., Reiner A., Tang L. H., Choi H., Rosenberg L. Localization of proteoglycan monomer and link protein in the matrix of bovine articular cartilage: An immunohistochemical study. J Histochem Cytochem. 1980 Jul;28(7):621–635. doi: 10.1177/28.7.6156200. [DOI] [PubMed] [Google Scholar]
- Poole A. R., Reiner A., Tang L. H., Rosenberg L. Proteoglycans from bovine nasal cartilage. Immunochemical studies of link protein. J Biol Chem. 1980 Oct 10;255(19):9295–9305. [PubMed] [Google Scholar]
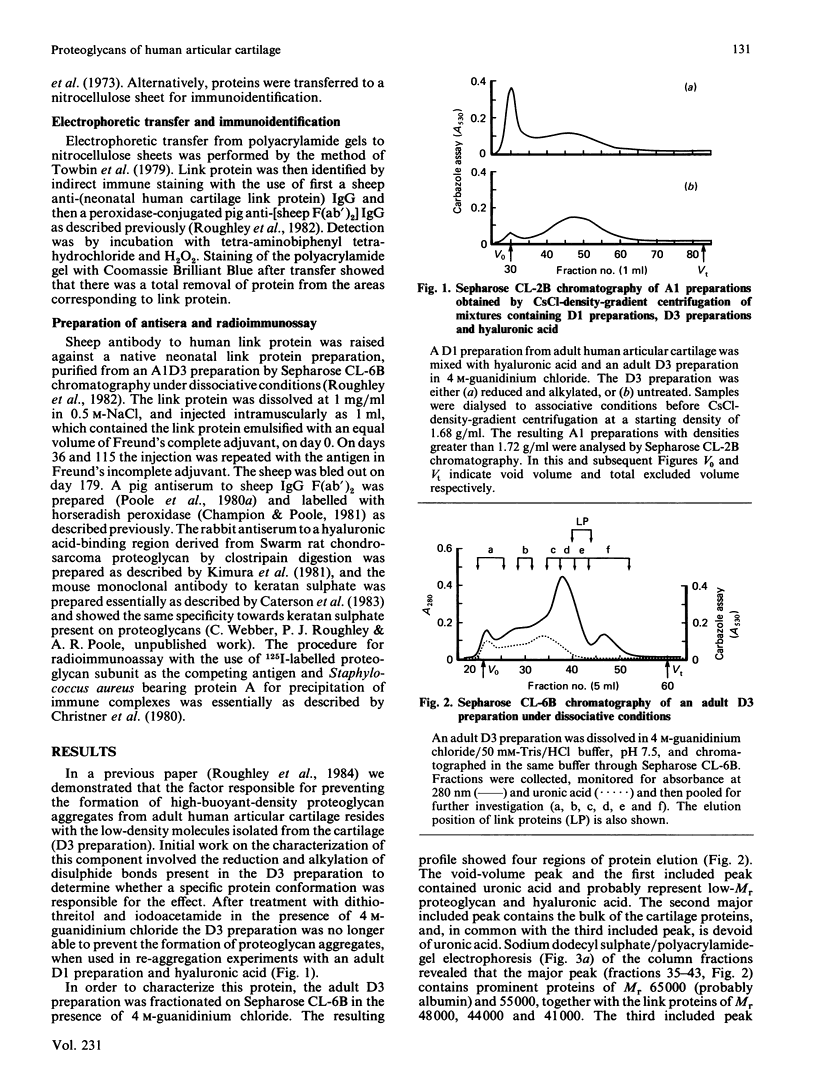
- Roughley P. J., Poole A. R., Mort J. S. The heterogeneity of link proteins isolated from human articular cartilage proteoglycan aggregates. J Biol Chem. 1982 Oct 25;257(20):11908–11914. [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Age-related changes in the structure of the proteoglycan subunits from human articular cartilage. J Biol Chem. 1980 Jan 10;255(1):217–224. [PubMed] [Google Scholar]
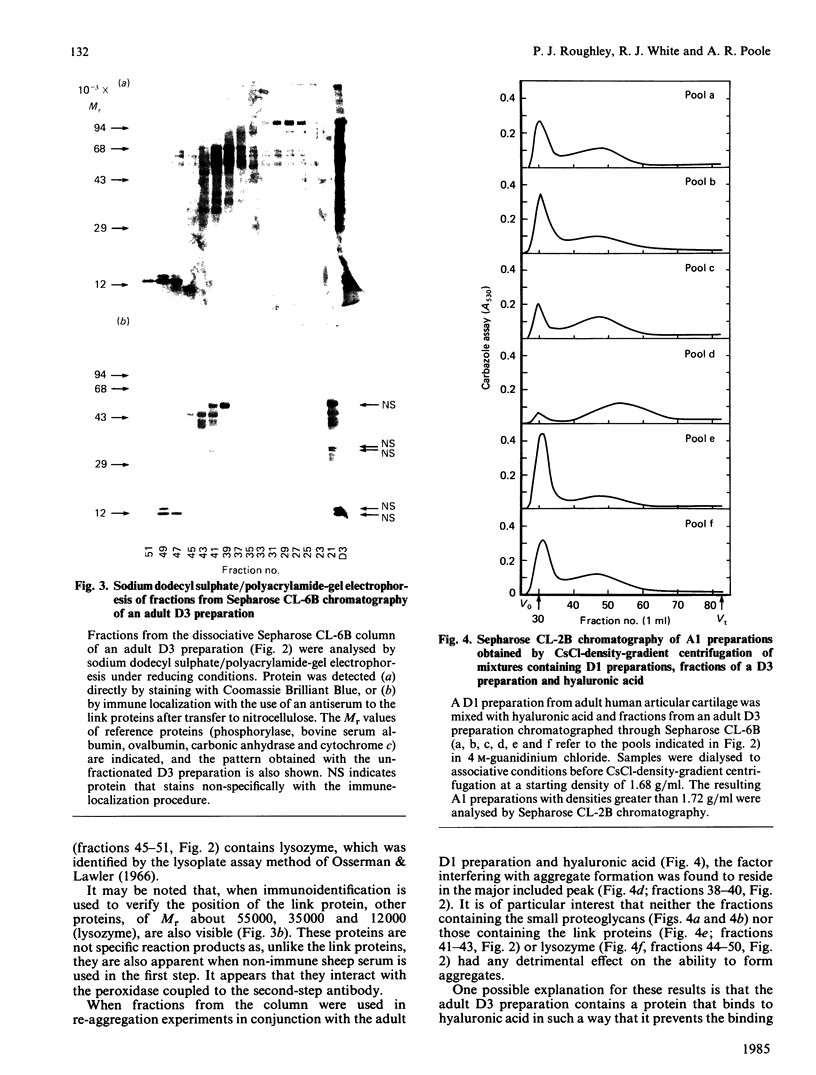
- Roughley P. J., White R. J., Poole A. R., Mort J. S. The inability to prepare high-buoyant-density proteoglycan aggregates from extracts of normal adult human articular cartilage. Biochem J. 1984 Aug 1;221(3):637–644. doi: 10.1042/bj2210637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang L. H., Rosenberg L., Reiner A., Poole A. R. Proteoglycans from bovine nasal cartilage. Properties of a soluble form of link protein. J Biol Chem. 1979 Oct 25;254(20):10523–10531. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venn M. F. Variation of chemical composition with age in human femoral head cartilage. Ann Rheum Dis. 1978 Apr;37(2):168–174. doi: 10.1136/ard.37.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]