Abstract
Healthy aging is associated with structural and functional brain changes. However, cognitive abilities differ from one another in how they change with age: whereas executive functions, like working memory, show age-related decline, aspects of linguistic processing remain relatively preserved (Hartshorne et al., 2015). This heterogeneity of the cognitive-behavioral landscape in aging predicts differences among brain networks in whether and how they should change with age. To evaluate this prediction, we used individual-subject fMRI analyses (‘precision fMRI’) to examine the language-selective network (Fedorenko et al., 2024) and the Multiple Demand (MD) network, which supports executive functions (Duncan et al., 2020), in older adults (n=77) relative to young controls (n=470). In line with past claims, relative to young adults, the MD network of older adults shows weaker and less spatially extensive activations during an executive function task and reduced within-network functional synchronization. However, in stark contrast to the MD network, we find remarkable preservation of the language network in older adults. Their language network responds to language as strongly and selectively as in younger adults, and is similarly lateralized and internally synchronized. In other words, the language network of older adults looks indistinguishable from that of younger adults. Our findings align with behavioral preservation of language skills in aging and suggest that some networks remain young-like, at least on standard measures of function and connectivity.
Keywords: language network, aging, multiple demand network, functional localization, functional connectivity, lateralization
Introduction
Healthy aging is associated with slower information processing and a reduction in working memory and attentional resources (Balota et al., 2000; Hartshorne & Germine, 2015; Li et al., 2001; Mooij et al., 2018). These behavioral changes have been argued to result from changes in the structure and function of different large-scale brain networks. One common claim is that brain networks become generally less segregated with age (Ballard et al., 2023; Chan et al., 2014; Chong et al., 2019; Damoiseaux, 2017; Geerligs et al., 2015; Malagurski et al., 2020; Varangis, Habeck, et al., 2019; Zhang & Diaz, 2023b, 2023a), manifesting in reduced within-network synchronization (or functional connectivity) and increased between-network synchronization (Chan et al., 2014). Other claims concern particular networks, including those that support executive functions (Andrews-Hanna et al., 2007; Ballard et al., 2023; Chan et al., 2014; Geerligs et al., 2015; Grady et al., 2016; Malagurski et al., 2020; Varangis, Habeck, et al., 2019).
In contrast to the well-documented changes in executive abilities, language is often described as one of the better-preserved functions in older adults. In spite of occasional word-finding difficulties (e.g., Connor et al., 2004; for a review, see Mortensen et al., 2006), vocabulary keeps growing with age, the ability to extract meaning from texts continues to improve, and the ability to predict upcoming words remains relatively intact (Dave et al., 2018; Federmeier et al., 2010; Hartshorne & Germine, 2015; Hubbard & Federmeier, 2024; Ryskin et al., 2020; Samu et al., 2017; Verhaeghen, 2003; for reviews, see Craik & Bialystok, 2006; Park & Bischof, 2011; Park & Reuter-Lorenz, 2009; Schaie & Willis, 2017; Shafto & Tyler, 2014; Wingfield & Stine-Morrow, 2000). However, whether and how the neural infrastructure for language processing changes with age remains debated. Some studies have argued that the topography of the language network—a set of temporal and frontal brain areas that respond strongly and selectively during language processing (Fedorenko et al., 2024)—is unchanged in older adults (Shafto & Tyler, 2014), but others have argued for more bilateral language processing (Diaz et al., 2016; Hoyau et al., 2017; Tyler et al., 2010). The magnitude of response during a variety of language tasks has been argued to increase (Peelle et al., 2013; Tyler et al., 2010; Zhang et al., 2021), decrease (Johnson et al., 2001; Samu et al., 2017), or show no change with age (Campbell et al., 2016; Fitzhugh et al., 2019; Samu et al., 2017; Tyler et al., 2010) across various regions of the language network. For within-network synchronization, some have found no change (Campbell et al., 2016; Pistono et al., 2021), but others have reported a reduction with age (Zhang et al., 2021; Zhang & Diaz, 2023a).
These inconsistencies could be due to at least two factors. First, many past studies have used paradigms that conflate language processing and general task demands (Fedorenko & Thompson-Schill, 2014). Because such paradigms recruit both the language-selective network and domain-general networks that support executive functions (e.g., Diachek et al., 2020; Wolna, Szewczyk, et al., 2024), the results are difficult to attribute to a particular network and thus difficult to interpret. And second, the majority of past fMRI studies have relied on group analyses, where activation maps or connectivity maps (Ferré et al., 2020; Geerligs et al., 2018; Gonzalez-Burgos et al., 2021; Grossman et al., 2002; Schill et al., 2023; Tyler et al., 2010; Zhang et al., 2021; Zhang & Diaz, 2023b, 2023a) are averaged voxel-wise across participants. This approach leads to a lot of blurring of the boundaries between distinct networks and associated interpretive challenges (Fedorenko, 2021; Gratton & Braga, 2021; Nieto-Castañón & Fedorenko, 2012) because of a) substantial inter-individual variability in the precise locations of functional areas (Finn et al., 2015; Mueller et al., 2013; Wang et al., 2015)—including language areas (Fedorenko et al., 2010; Frost & Goebel, 2012; Lipkin et al., 2022; Ojemann et al., 1989)—and b) proximity of different functional networks to each other in the association cortex (Braga et al., 2020; Fedorenko & Blank, 2020).
In the present study, we use individual-subject fMRI analyses (‘precision fMRI’; Gratton & Braga, 2021) to examine age-related changes in the language-selective network (Fedorenko et al., 2024). This network supports computations related to accessing word meanings and combining them into phrases and sentences during both comprehension and production (Fedorenko et al., 2010; Fedorenko & Blank, 2020; Hu et al., 2023; Menenti et al., 2011)—functions that are largely preserved in aging (Hartshorne & Germine, 2015; Shafto & Tyler, 2014; Wingfield & Stine-Morrow, 2000), cf. (Federmeier et al., 2003, 2010; Hubbard & Federmeier, 2024)—and is strongly selective for language over diverse non-linguistic inputs and tasks (Braga et al., 2020; Fedorenko et al., 2011; Monti et al., 2012). For comparison, we also examine the domain-general Multiple Demand (MD) network (Duncan et al., 2020), which supports executive abilities, like working memory, cognitive control, and attention, and has been linked to fluid intelligence and problem solving (Duncan, 2010, 2013; Duncan et al., 2020; Woolgar et al., 2010)—functions that exhibit age-related decline (Bedard et al., 2002; Buckner, 2004; Cepeda et al., 2001; De Luca et al., 2003; Ferguson et al., 2021; Kray et al., 2002; Lustig & Jantz, 2015; Smith et al., 2001; Spieler et al., 1996). In line with increasing emphasis on robustness and replicability (Adali & Calhoun, 2022; Bossier et al., 2020; Poldrack et al., 2017), we examine two independent cohorts of older adults (n=38 and n=39) along with a large cohort of young adults (n=470). To foreshadow our results, we find that a) the language and the MD networks remain robustly segregated in older adults, b) for the MD network, the magnitude and extent of activation as well as the within-network synchronization are lower in older adults, but critically, c) for the language network, the magnitude and selectivity of response, extent of activation, degree of lateralization, and the within-network synchronization do not differ between older and younger adults, in line with the relative preservation of linguistic functions in aging.
Results
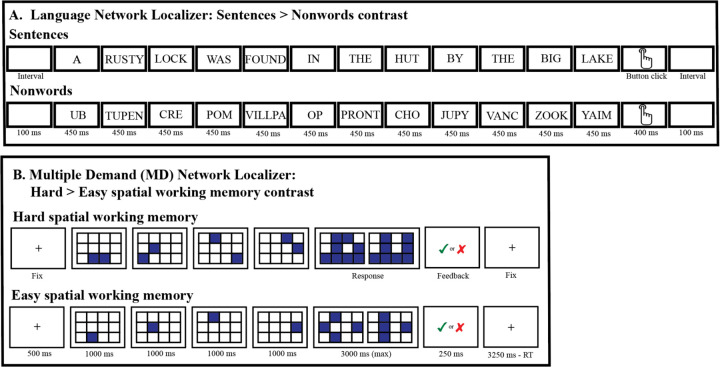
We use extensively validated ‘localizer’ paradigms (Figure 1), which reliably and selectively activate the target networks of interest—the language and the MD networks—in spite of their close proximity within the left frontal lobe (Blank et al., 2014; Braga et al., 2020; Fedorenko et al., 2012; Quillen et al., 2021; Wolna, Szewczyk, et al., 2024). For the analyses of response magnitude, responses to each condition of each localizer task were extracted using across-runs cross-validation to ensure independence between the data used to define the functional regions of interest (fROIs) vs. to characterize their responses (Kriegeskorte et al., 2009); see Methods for details.
Figure 1.
The paradigms that were used to localize the language network (A) and the Multiple Demand (MD) network (B). In the language localizer task (Fedorenko et al., 2010), participants were asked to attentively read sentences and lists of pronounceable nonwords in a blocked design, one word or nonword at a time, and press a button at the end of each sentence/nonword-list. In the MD localizer task (a spatial working memory task; (Assem et al., 2020; Fedorenko et al., 2013), participants were asked to keep track of eight (hard condition) or four (easy condition) spatial locations (presented two at a time, or one at a time, respectively) in a 3 × 4 grid. At the end of each trial (in both conditions), participants were asked to perform a two-alternative forced-choice task to indicate the set of locations they just saw. (See Methods for details of both paradigms.) Importantly, each of these paradigms has been shown to be robust at the individual-participant level and to generalize across many variants that use alternate materials and tasks (Blank et al., 2014, 2016; Fedorenko et al., 2011, 2013). (For a subset of participants, alternate versions of the localizer tasks were used; see Figure SI-1.)
1. The Multiple Demand network shows pronounced age-related changes in activation and functional synchronization.
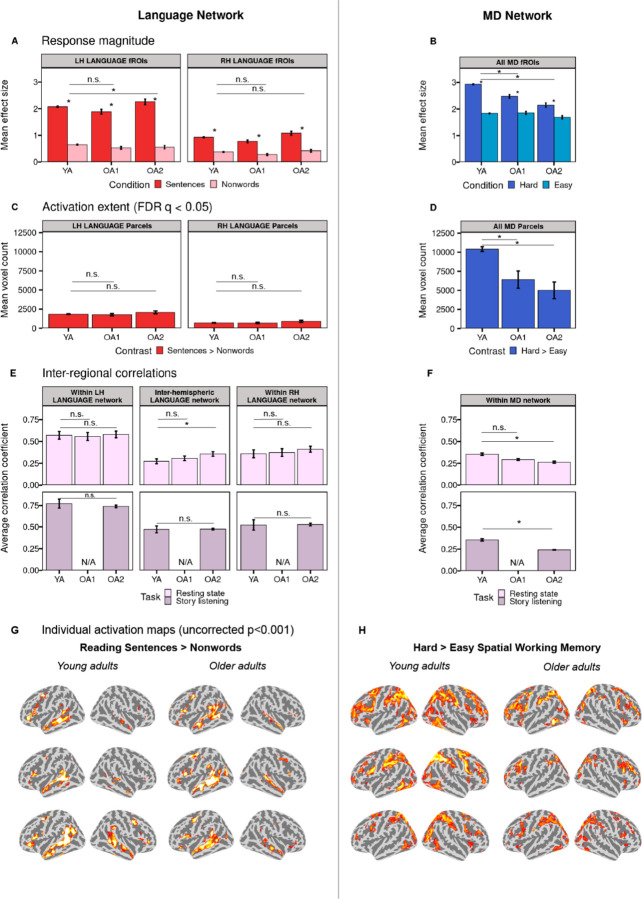
In line with past claims (Geerligs et al., 2015; Mitchell et al., 2023; Samu et al., 2017; Varangis, Razlighi, et al., 2019), the Multiple Demand (MD) network of older adults showed a reduction in the magnitude and spatial extent of activation during an executive function task (a spatial working memory task), and in the within-network synchronization of activity during naturalistic cognition, compared to younger adults. First, relative to younger adults (YA), both older adult cohorts (OA1 and OA2) showed significantly lower responses in the MD functional regions of interest (fROIs) to the spatial working memory task contrast (i.e., hard > easy). This effect held both at the network level (Figure 2B, False Discovery Rate (FDR) qs < 0.001) and in all MD fROIs individually (Figure SI-2B, FDR qs < 0.05) except in the right posterior parietal fROI, where the OA2 cohort did not significantly differ from the YA cohort (FDR q = 0.090). Post-hoc analyses revealed that this reduction in the size of the hard > easy effect was driven by a lower response during the hard condition in older adults compared to younger adults (ps < 0.04); in contrast, the groups did not differ in their response during the easy condition. Furthermore, both OA cohorts showed less extensive activation during the spatial working memory task compared to younger adults. This effect held both when considering activations across the brain (Figure 2D, FDR qs < 0.002) and when restricting the analysis to the masks that cover broad areas of typical MD network response to executive-function tasks (Figure SI-3B, FDR qs < 0.05). Finally, synchronization among the regions of the MD network, as measured by Pearson’s correlations between BOLD signal timeseries across pairs of MD fROIs was weaker in the OA2 cohort compared to younger adults during both a resting-state scan (Figure 2F, FDR q < 0.001) and a story listening paradigm (FDR q = 0.004) when considering all fROI pairs (within and across the two hemispheres) and when considering pairs within each hemisphere separately (FDR qs < 0.007). For OA1, within-network synchronization during a resting-state paradigm (the only naturalistic paradigm available for this cohort) was numerically, but not significantly, lower than in young adults (FDR qs > 0.20). These neural changes were accompanied by a decline in behavioral performance, with both OA cohorts showing reliably lower accuracies on the spatial working memory task for both the hard and easy conditions compared to the YA group (ps < 0.001; Figure SI-4).
Figure 2.
A comparison between older adults (two cohorts: OA1 and OA2) and younger adults (YA) in the activation and functional synchronization (or functional connectivity) measures of the language network (left) and the Multiple Demand (MD) network (right). A. Mean BOLD response magnitude during the critical (sentence reading) condition (red bars) and the control (nonword reading) condition (pink bars) in the left hemisphere (LH) and right hemisphere (RH) language fROIs (all fROIs are defined within individual participants, and responses are estimated using across-runs cross-validation; see Methods). Significant task contrast effects (FDR-corrected q < .05) are marked with an asterisk. Here and elsewhere, significant group differences (FDR-corrected q < .05) are marked with an asterisk above a bar. B. Mean BOLD response magnitude during the conditions of the spatial working memory task (hard condition: dark blue bars; easy condition: light blue bars) in bilateral MD fROIs. C-D. Extent of activation (number of significant voxels at the FDR-corrected q < 0.05 whole-brain threshold) for the language (C) and spatial working memory (D) tasks. E-F. Inter-regional timeseries correlations among language fROIs (E) and among MD fROIs (F) during a resting-state paradigm (pink bars, top row) and story listening (purple bars, bottom row). G-H. Sample activations maps of individual participants for the sentences > nonwords contrast (G) and hard > easy spatial working memory contrast (H) (see https://osf.io/2q65t/?view_only=ab1833db12c64eb0a7cc61c5795d35cd for the full set of individual activation maps). Threshold: uncorrected p<0.001 whole-brain (note that the maps are included solely for illustrative purposes; all the statistical analyses are performed on the neural measures extracted from these activation maps; see Methods).
2. The language network does not change with age in activation or functional synchronization.
In contrast to the MD network, the language network of older adults did not differ consistently (across the two OA cohorts) from that of younger adults in any neural measures examined. First, for the left-hemisphere (LH) network, the OA1 cohort did not differ from the YA group in the magnitude of response to the language task contrast (i.e., sentences > nonwords) at the network level or in individual fROIs. The OA2 cohort did show a small significant difference at the network (FDR q = 0.002) and left-hemisphere level due to a slightly higher response (Figure 2A, FDR q = 0.003) but at the fROI level, the difference was only significant in the left posterior temporal fROI (Figure SI-2A, FDR q < 0.001). No differences between OA cohorts and the YA group in the magnitude of response were found for the RH homotopic network. Similarly, neither of the OA cohorts differed from YA in the extent of activation during the language task at the network level in either hemisphere (Figure 2C, FDR qs > 0.10) or in any individual language region (Figure SI-3A, FDR qs > 0.06). In addition, no significant differences were observed between OA cohorts and the YA group in the lateralization of the language network (FDR qs > 0.81). Finally, activity synchronization within the LH language network, as measured by Pearson’s correlations between BOLD signal timeseries across pairs of language fROIs during naturalistic cognition paradigms, did not show any differences between the OA cohorts and the YA group (Figure 2E, FDR qs > 0.61). The same was true for the RH homotopic network (FDR qs > 0.10), and for the inter-hemispheric (left-to-right) correlations (FDR qs > 0.10), with the exception of OA2 showing a significant increase from the YA group during rest (Figure 2E, FDR q = 0.007).
3. The language and the MD networks remain robustly dissociated in older adults.
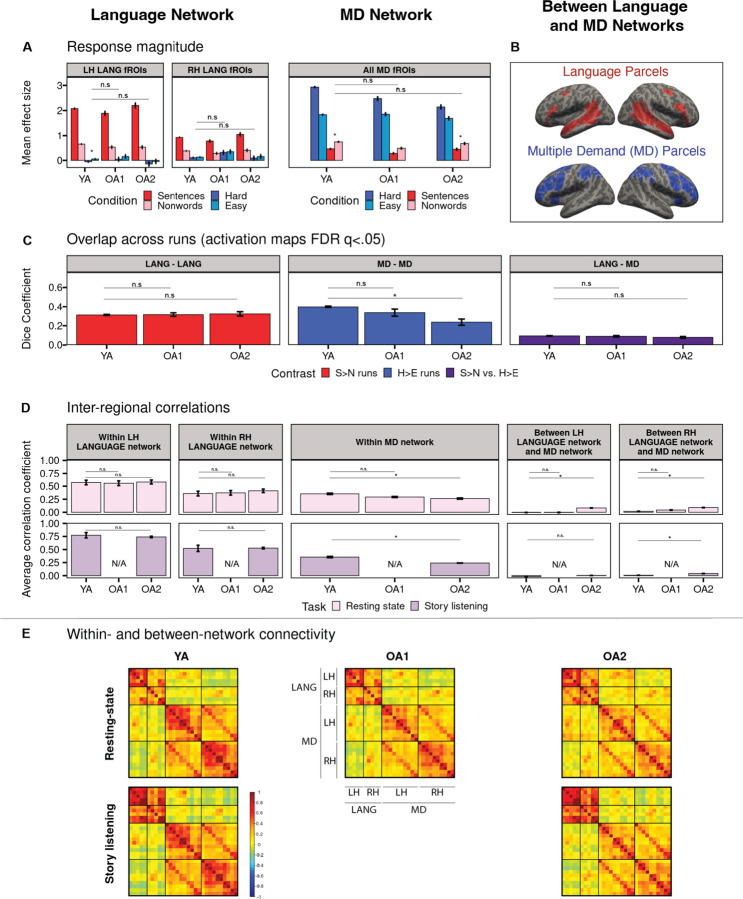
Similar to young adults, older adults show a clear dissociation between the language and the MD networks. First, in both younger and older adults, the language network shows an overall low response during the spatial working memory task and does not show a significant hard > easy effect either at the network level (Figure 3A), or in any individual language region (Figure SI-2A). The MD fROIs show an overall low response during language processing and show the opposite effect compared to the language network: a stronger response to nonword-lists than meaningful sentences. No significant differences were found between OA and YA in the responses to the spatial working memory task in the language network (Figure 3A left panel, FDR qs > 0.92), nor in the responses to the language task in the MD network (Figure 3A right panel, FDR qs > 0.07). Similarly, OA cohorts did not differ from the YA group in the degree of spatial overlap between the language and the MD networks, which was minimal for all groups, as measured by the Dice coefficient (Figure 3C right panel, FDR qs > 0.21). For all cohorts, the overlap between the language and the MD networks was significantly lower than the overlap within either network across runs (Figure 3C left and middle panels, all FDR qs < 0.001). Finally, similar to younger adults, OA cohorts showed a clear dissociation between the language and the MD networks in the functional correlation patterns, with higher within-network synchronization (Figure 3D left and middle panels) than between-network synchronization (Figure 3D right panel) (FDR qs < 0.001). However, a small increase in the degree of between-network synchronization was found in one cohort of older adults (OA2) compared to YA. This difference was found across both naturalistic paradigms between the RH language fROIs and the MD fROIs, and, during rest only, between the LH language fROIs and the MD fROIs (resting-state: OA2 mean r = 0.090 vs. YA mean r = 0.008, LH and RH language fROIs to MD fROIs FDR qs < 0.001; story listening: OA2 mean r = 0.011 vs. YA mean r = −0.006, LH language fROIs to MD fROIs q = 0.17 and RH language fROIs to MD fROIs FDR q < 0.001).
Figure 3.
A comparison between older adults (two cohorts: OA1 and OA2) and younger adults (YA) in the activation and functional synchronization (or functional connectivity) measures within and between the language (LANG) network and the Multiple Demand (MD) network. A. Mean BOLD response magnitude during the conditions of the language task (sentence reading: red bars; nonword reading: pink bars) and the conditions of the spatial working memory task (hard condition: dark blue bars; easy condition: light blue bars) in the left hemisphere (LH) and right hemisphere (RH) language fROIs and in bilateral MD fROIs (all fROIs are defined within individual participants, and responses are estimated using across-runs cross-validation; see Methods). The significance (FDR-corrected q < .05) of the task contrast effects for the non-preferred domain (i.e., the hard vs. easy spatial working memory effect in the language fROIs, and the sentences vs. nonwords effect in the MD fROIs; see Fig. 2 for response to the preferred domain) is marked with an asterisk. Here and elsewhere, significant group differences (FDR-corrected q < .05) are marked with an asterisk above a bar. B. Language and MD parcels (i.e., brain areas within which most individuals in prior studies showed activity for the localizer contrast) or “search spaces” used to defined fROIs (i.e., top 10% most responsive voxels within these parcels) within individuals. C. Spatial overlap of significantly activated voxels across the runs within a localizer task (language: left, MD: middle), and between the two tasks (right), as measured with the Dice coefficient. All activation maps were thresholded at FDR q <0.05. D. Inter-regional timeseries correlations among the language regions (left), among the MD regions (middle), and between the language and MD regions (right) during a resting-state paradigm (pink bars, top row) and story listening (purple bars, bottom row). E. Inter-regional functional correlation matrices for pairs of regions within each network and between the two networks during a resting-state paradigm (top row) and story listening (bottom row). The color scale represents Fisher-transformed Pearson correlation coefficients. LH: left-hemispheric fROIs. RH: right-hemispheric fROIs.
Discussion
We used individual-subject fMRI analyses (‘precision fMRI’; Gratton & Braga, 2021) and extensively validated ‘localizer’ paradigms to examine age-related changes in the language-selective network and, for comparison, in the domain-general Multiple Demand (MD) network. Our findings—replicated across two independent cohorts of older adults relative to a large group of younger adults—reveal a striking dissociation in the impact of aging on these two cognitive networks. In particular, both activity and functional synchronization in the MD network show a reduction in older adults, in line with age-related decay in executive functions (Buckner, 2004; Cepeda et al., 2001; De Luca et al., 2003; Ferguson et al., 2021; Hedden & Gabrieli, 2004; Kray et al., 2002; Lustig & Jantz, 2015; Smith et al., 2001; Spieler et al., 1996), which this network supports. In contrast, the language network shows remarkable preservation with age, reflected in both activity and functional synchronization measures, in line with relative preservation of linguistic abilities in aging (Craik & Bialystok, 2006; Hartshorne & Germine, 2015; Shafto & Tyler, 2014; Wingfield & Stine-Morrow, 2000). In the remainder of the Discussion, we contextualize these findings with respect to the prior literature on cognitive aging and discuss their implications.
The Multiple Demand network in older brains
One of the most replicated findings in the cognitive-behavioral aging literature is age-related decay in executive abilities: older adults exhibit slower processing speed, a reduced working memory capacity, and greater difficulty inhibiting distracting information (Craik & Bialystok, 2006; Park & Reuter-Lorenz, 2009). Lower behavioral performance of older adults on the spatial working memory task in the current study aligns with these findings. To better understand the neural basis of these age-related changes in executive abilities, we examined the Multiple Demand (MD) network, which has long been associated with executive functions (Duncan, 2010; Fedorenko et al., 2013). This network is engaged by diverse working memory and cognitive control tasks (Assem et al., 2020; Duncan & Owen, 2000; Fedorenko et al., 2013; Shashidhara et al., 2019); certain aspects of reasoning, such as mathematical reasoning or understanding computer code, also recruit this network (Amalric & Dehaene, 2019; Ivanova et al., 2020; Liu et al., 2020). Age-related functional changes in this network have been reported across numerous studies (Heckner et al., 2021; Turner & Spreng, 2012), but the effects have not been consistent. For example, some have reported overall increased activation in the frontal and parietal regions in older adults compared to younger adults (sometimes referred to as a ‘compensatory increase’) (Cabeza, 2002; Nielson et al., 2002). Others have instead found an increase only at lower cognitive loads (Reuter-Lorenz & Cappell, 2008; Schneider-Garces et al., 2010), and suggested that at higher cognitive loads, the older adults’ neural activity may reach a plateau, leading to reduced activation compared to the younger cohort (Reuter-Lorenz & Cappell, 2008). This proposal, where the direction of activation differences between older and younger adults depends on task difficulty is known as the Compensation-Related Utilization of Neural Circuits Hypothesis (CRUNCH; Reuter-Lorenz & Cappell, 2008). In our study, consistent with the CRUNCH hypothesis, the response magnitude during the more difficult condition of the spatial working memory task was reduced in OA relative to YA. However, in contrast to the CRUNCH hypothesis, the magnitude of response was similar across the age cohorts for the easier condition of the task, in spite of a clear behavioral difference in task performance.
An earlier investigation of the relationship between the MD network’s activity and behavioral performance for the spatial working memory task that was used here found that the size of the hard > easy effect in the MD network was positively associated with task performance in young adults (Assem et al., 2020). In particular, individuals with a larger hard > easy effect exhibited higher accuracies (and faster reaction times) for the working memory task and higher intelligence quotient (IQ) scores as measured with an independent test. Our findings of a smaller hard > easy effect in older adults (due to the lower magnitude of neural response in the hard condition; Fig. 2B) and worse performance on the spatial WM task are consistent with these earlier findings. This concordance suggests that the same underlying factors may explain a) inter-individual differences in executive abilities within an age group, and b) differences between age cohorts. It also aligns with studies that have shown that functional activity and synchronization patterns in the MD network are better predicted by performance on working memory tasks than by chronological age (Nagel et al., 2009, 2011; Samu et al., 2017).
Preservation of the language network with age
As outlined in the Introduction, language is one of the better-preserved cognitive functions in older age (Craik & Bialystok, 2006; Park & Bischof, 2011; Park & Reuter-Lorenz, 2009; Ryskin et al., 2020; Samu et al., 2017; Shafto & Tyler, 2014; Verhaeghen, 2003; Wingfield & Stine-Morrow, 2000). In line with this behavioral preservation, we found that the language network’s magnitude and selectivity of response, spatial extent, and activity synchronization strength remain stable with age. This neural preservation is in sharp contrast with the neural changes we observed for the MD network.
The language-selective network supports our ability to interpret and generate linguistic messages across spoken, written, signed, and other modalities (Blank et al., 2016; Fedorenko et al., 2011; Friederici, 2012; Hagoort & Indefrey, 2014; Hickok & Poeppel, 2007; Price, 2012) and across languages (Malik-Moraleda et al., 2022). The areas comprising this network likely store our linguistic knowledge representations—including knowledge of what words mean and how words go together to create complex meanings—and use these representations to encode thoughts into word sequences and decode others’ thoughts from their linguistic productions. The level of response in the language network has been shown to scale with how competent the individual is as a language user. For example, responses increase across the developmental trajectory, between age 4 and late adolescence, at which point they asymptote (Hiersche et al., 2024; Ozernov-Palchik et al., 2024; Weiss-Croft & Baldeweg, 2015). Similarly, responses are stronger in non-native speakers who are more proficient in the language compared to those who are less proficient (Malik-Moraleda et al., 2024). These effects are presumably due to the fact that children and adults with limited proficiency may not be able to engage linguistic computations to the full degree because they may be unfamiliar with certain words or constructions, leading to the inability to piece together the complete meaning. Older native speakers, like the participants in the current study, are of course highly proficient language users: the ability to generate and interpret linguistic messages does not appear to decay with age (in sharp contrast to executive abilities). This behavioral preservation presumably reflects the preservation of the underlying brain network. These findings are consistent with the brain maintenance hypothesis (Düzel et al., 2011; Nyberg et al., 2012), whereby healthy aging, as measured by the stability of cognitive performance in some domain over the adult life course, is due to the preservation of the structure and function of the relevant neural substrate (Cabeza et al., 2018; Düzel et al., 2011; Nyberg et al., 2012). Most of the prior evidence for this hypothesis has come from individual-differences investigations in the domains of long-term episodic memory or working memory: individuals with superior memory performance also show greater preservation of task-related neural responses (Cabeza et al., 2018; Nyberg et al., 2012). Here, we provide evidence from the language network, complementing prior evidence of the preservation of linguistic abilities and the language brain areas in aging (Diaz et al., 2016; Samu et al., 2017).
Why have some past studies found differences between younger and older adults in their brain response during language tasks? For example, some have reported reduced lateralization of the language network (Tyler et al., 2010)—and sometimes other networks—with age (Agcaoglu et al., 2015), giving rise to the Hemispheric Asymmetry Reduction in Old Adults (HAROLD) (Cabeza, 2002) hypothesis. However, in the current study, we find no difference in the degree of lateralization of the language network between younger and older adults. One possible explanation for this discrepancy is that past studies have not isolated the language network proper from the nearby MD network, which can get engaged when language processing is accompanied by external task demands (e.g., Diachek et al., 2020; Wright et al., 2011; for reviews see Campbell & Tyler, 2018; Fedorenko, 2014). Because the MD network is bilateral, a task that engages both the language-selective network and the MD network—as many language tasks do—may result in more bilateral activations in older adults due to the greater reliance on the MD network. A similar explanation may underlie the reports of reduced synchronization within the language network (Antonenko et al., 2013; Zhang et al., 2021; Zhang & Diaz, 2023b), and the findings of more bilateral language responses in young children (Olulade et al., 2020).
Another possibility is that previous studies showing age-related changes in activity levels in regions overlapping with the domain-general fronto-parietal MD network during language processing typically used production tasks (e.g., Hoyau et al., 2017; La et al., 2016; Meinzer et al., 2012). In contrast to comprehension, some aspects of language production (specifically, word retrieval) exhibit age-related decline (Samu et al., 2017; Shafto & Tyler, 2014; Zhang & Diaz, 2023a). Some have argued that this decline has to do with changes in parts of the language production pipeline (e.g., phonological encoding, Burke & Shafto, 2007), but others have attributed these effects to general executive function deficits (Baciu et al., 2016; Hoffman et al., 2018). Indeed, word production tasks, such as confrontation naming, appear to engage domain-general MD brain regions, in addition to the language areas (Hu et al., 2023), and as discussed above, the MD network deteriorates with age. Language comprehension tasks, on the other hand, do not engage Multiple Demand areas in the absence of extra-linguistic task demands (Diachek et al., 2020; Fedorenko & Shain, 2021). One previous study demonstrated a neural dissociation in the effect of aging on language comprehension versus production (Samu et al., 2017). They reported reduced activity levels in older adults during a picture naming task, but stable activity levels across age during a sentence comprehension task. Unfortunately, no independent functional localizers for the language versus domain-general cognitive control areas were included, making the attribution of these effects to a particular brain system challenging.
Persistent dissociation between the language and the MD networks with age
Another prominent hypothesis that has been put forward to explain age-related cognitive decline is the dedifferentiation hypothesis (Park et al., 2001, 2004) whereby areas and networks that are distinct at a younger age become less segregated in older brains (for a review, see Sala-Llonch et al., 2015). Much evidence for the dedifferentiation hypothesis comes from non-human animal studies in sensorimotor cortices (e.g., Kamal et al., 2013; Schmolesky et al., 2000; Yang et al., 2008). In humans, evidence for dedifferentiation comes from fMRI studies where some have reported a) reduced selectivity of brain regions for their preferred stimulus/task and/or increased responses to some stimulus/task in regions that previously did not respond to that stimulus/task (Park et al., 2004; Rajah & D’Esposito, 2005), as well as b) reduced within-network and/or increased between-network synchronization (Ballard et al., 2023; Chan et al., 2014; Chong et al., 2019; Damoiseaux, 2017; Malagurski et al., 2020; Varangis, Habeck, et al., 2019). We do not find support for the dedifferentiation hypothesis in response magnitude data: the language areas of older adults respond as strongly and selectively during language processing as those of younger adults. The selectivity holds both relative to the control condition (nonword processing), which is similar perceptually to the sentence comprehension condition, and relative to a non-linguistic working memory task. We also do not see any evidence of responses to language in the MD network of older adults, similar to young adults. These results suggest that the MD network is not recruited as an additional neural resource to process language in older individuals.
With respect to the functional connectivity data, we see similarly strong within-network synchronization for the language network in the older and younger cohorts, but we do see some evidence of reduced within-network synchronization in the MD network and a small increase in the synchronization between the MD and the language areas. This result is among the weakest results in the current study as it only emerges in one OA cohort during one of the naturalistic conditions in the left hemisphere (rest, but not during story comprehension; this difference may be taken as evidence against the functional importance of this effect for language processing), and the difference is small. This finding aligns with a previous report of robust segregation of the left-hemisphere language network from other networks at rest, despite reduced segregation for other networks (Zhang & Diaz, 2023a). Thus, dedifferentiation does not ubiquitously characterize an aging brain, across networks. Advocates of this hypothesis should therefore offer more specific predictions about the networks for which reduced selectivity and increased overlap should be observed.
Overall, different patterns of age-related change in the language versus the MD networks challenge the hypotheses whereby activation increases with age within specialized regions or across bilateral frontal areas (Cabeza, 2002; Reuter-Lorenz & Cappell, 2008), and the hypotheses whereby networks lose their specificity with age or become more integrated with other networks (Park et al., 2001, 2004; Rajah & D’Esposito, 2005). Instead, these results are most consistent with the brain maintenance hypothesis such that brain networks that support functions that decline with age (e.g., executive functions) show age-related changes in neural measures, but networks that support functions that are well-preserved in aging (e.g., language comprehension) remain young-like neurally.
Limitations
One limitation of the current study is that we used a passive comprehension paradigm (in an effort to eliminate any potential confounds having to do with task demands), and as a result, we do not have a direct measure of comprehension for the materials used in the fMRI language localizer tasks. However, past work has provided ample evidence that healthy older adults do not exhibit difficulties in language comprehension (Burke & Shafto, 2007; Shafto & Tyler, 2014; Thornton & Light, 2006; Wingfield & Stine-Morrow, 2000). In addition, the synchronization measures are independent of particular paradigms, but whether task-based neural measures in the language network (response magnitude, selectivity, and degree of lateralization) would show similar preservation for a language production task remains to be determined. Furthermore, similar to most brain imaging work on aging (cf. Di Biase et al., 2023), this is a cross-sectional study, and we may therefore be missing complex non-linear changes across the lifespan whose detection would require a longitudinal approach, or more dense sampling along the age continuum, along with matching the samples for factors, such as educational level, occupation complexity, and specific generational experiences. Finally, we have here focused on the core frontal and temporal language areas; future work should more comprehensively examine other brain areas that comprise the extended language network, including other cortical areas, cerebellar areas, and subcortical areas (Fedorenko et al., 2024; Wolna, Wright, et al., 2024).
Conclusion
In conclusion, this study demonstrates that aging affects distinct brain networks differently, with neural changes mirroring the preservation or decline of the cognitive functions they support. Whereas the domain-general Multiple Demand network exhibits age-related decline in activity and internal synchronization, mirroring the decline in executive functions, the language-selective network—which supports one of the most well-preserved cognitive functions—remains remarkably stable with age. These results underscore the importance of individual-level functional localization in characterizing age-related differences among adjacent networks, and also highlight the need for more nuanced models of brain aging that consider the distinct trajectories of different brain networks and their associated cognitive functions.
Materials and Methods
Participants
509 participants were recruited from MIT and the greater Boston community. All participants were native English speakers, had normal or corrected-to-normal vision and hearing, and did not report any psychiatric or neurological disorder. These participants were recruited between 2015 and 2021 and divided into two cohorts: 470 young adults (YA, age range: 19–39; average=27.8, SD=4.4) and 39 older adults (OA1, age range: 40–75; average=51.9, SD=10.5). Another cohort of 38 older adults was recruited between 2021 and 2023 (OA2, age range: 44–80; average=63.9, SD=10.3). All participants gave informed consent in accordance with the requirements of MIT’s Committee on the Use of Humans as Experimental Subjects (COUHES) and were paid for their participation.
Experimental design
Each participant completed two localizer tasks designed to identify and measure the response of the two networks of interest: a reading task for the language network and a spatial working memory task for the domain-general multiple-demand (MD) network. The language localizer task included sentences and lists of pronounceable nonwords that participants had to passively read in a blocked design, one word or nonword at a time. A simple button-press task was included at the end of each trial, to help participants remain alert. The materials are available at https://www.evlab.mit.edu/resources. The sentences > nonwords contrast targets brain regions that support high-level linguistic processing, including lexico-semantic, combinatorial syntactic, and semantic processes (Blank et al., 2016; Fedorenko et al., 2012; Fedorenko & Blank, 2020). This task has been shown to be robust to the materials, task, and modality of presentation (Fedorenko et al., 2010, 2011; Mahowald & Fedorenko, 2016; Scott et al., 2017). A slightly different version of the task was used for 2 of the 38 participants in OA2 where participants read sentences and nonwords (similar to the main version), but also lists of words and “Jabberwocky” sentences (morphologically and syntactically intact sentences made up of nonwords). At the end of each trial, participants had to decide whether a probe word/nonword had appeared in the immediately preceding stimulus. The sentences > nonwords contrast has been shown, across numerous studies, to not engage the MD regions, which respond more strongly during the nonwords condition (Diachek et al., 2020; Fedorenko, 2014; Fedorenko et al., 2013). Sample stimuli and trial timing details are presented in Figures 1 and SI-1, and Table SI-5. Each participant completed two runs, with condition order counterbalanced across runs.
In the Multiple Demand localizer task, participants had to keep track of four (easy condition) or eight (hard condition) sequentially presented locations in a 3 × 4 grid (Fedorenko et al., 2013). In both conditions, participants performed a two-alternative forced-choice task at the end of each trial to indicate the set of locations they just saw. The hard > easy contrast has been previously shown to robustly activate MD regions (Blank et al., 2014; Fedorenko et al., 2013), which also have been shown to respond to difficulty manipulations across many diverse tasks (Duncan & Owen, 2000; Fedorenko et al., 2013; Hugdahl et al., 2015). Participants in the YA and OA1 cohorts did one version of this task (Figure 1), and participants in the OA2 cohort did a slightly different version (Figure SI-1); minor differences in the timing/procedure do not appear to affect the activations as we had seen in cases of direct within-individual comparisons (unpublished data from the Fedorenko lab).
Sample stimuli and trial timing details are presented in Figures 1 and SI-1, and Table SI-6. Each participant completed two runs, with condition order counterbalanced across runs.
A subset of the younger adults (YA) (N=83) and a subset of the older adult cohorts (OA1 N=22, OA2 N=38) also completed a resting state scan to examine functional correlations in neural activity among the language regions, among the MD regions, and between the language and the MD networks. The same YA subset and the OA2 cohort completed a second naturalistic cognition paradigm, where they passively listened to a ~5 min-long story. The YA subset listened to a story extracted from the fairy tale Alice in Wonderland and OA2 listened to an edited version of the publicly available story “Elvis Died at the Florida Barber College” (by Roger Dean Kiser; unedited version: www.eastoftheweb.com/short-stories/UBooks/ ElvDie.shtml). Description of the stories can be found in (Blank et al., 2014; Blank & Fedorenko, 2017; Malik-Moraleda et al., 2022). The OA2 cohort responded to four simple yes/no questions at the end of the scan to confirm their attention and comprehension of the story.
MRI data acquisition
YA and OA1 cohorts:
Structural and functional data were collected on the whole-body, 3 Tesla, Siemens Trio scanner with a 32-channel head coil, at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT. T1-weighted structural images were collected in 176 sagittal slices with 1 mm isotropic voxels (TR = 2,530 ms, TE = 3.48 ms). Functional, blood oxygenation level dependent (BOLD), data were acquired using an EPI sequence (with a 90 degree flip angle and using GRAPPA with an acceleration factor of 2), with the following acquisition parameters: 31 4 mm thick near-axial slices acquired in the interleaved order (with 10% distance factor), 2.1 mm x 2.1 mm in-plane resolution, FoV in the phase encoding (A >> P) direction 200 mm and matrix size 96 mm x 96 mm, TR = 2,000 ms and TE = 30 ms. Prospective acquisition correction was used to adjust the positions of the gradients based on the participant’s motion from the previous TR. The first 10 s of each run were excluded to allow for steady state magnetization.
OA2 cohort:
Structural and functional data were collected on a whole-body 3 Tesla Siemens Prisma scanner with a 32-channel head coil at the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research at MIT. T1-weighted, Magnetization Prepared Rapid Gradient Echo (MP-RAGE) structural images were collected in 176 sagittal slices with 1 mm isotropic voxels (TR = 2,530 ms, TE1 = 1.69 ms, TE2 = 3.55 ms, TE3 = 5.41 ms, TE4 = 7.27ms, TI = 1100 ms, flip = 7 degrees). Functional, blood oxygenation level-dependent (BOLD) data were acquired using an SMS EPI sequence with a 80° flip angle and using a slice acceleration factor of 3, with the following acquisition parameters: eighty-one 1.8 mm thick slices acquired in the interleaved order (with 0% distance factor), 2.4 mm × 2.4 mm in-plane resolution, FoV in the phase encoding (A >> P) direction 216 mm and matrix size 216 × 146, TR = 2,000 ms, TE = 32 ms. The first 10 s of each run were excluded to allow for steady-state magnetization.
fMRI Preprocessing
fMRI data were analyzed using SPM12 (release 7487), CONN EvLab module (release 19b), and other custom MATLAB scripts. Each participant’s functional and structural data were converted from DICOM to NIFTI format. All functional scans were coregistered and resampled using B-spline interpolation to the first scan of the first session (Friston et al., 1995). Potential outlier scans were identified from the resulting subject-motion estimates as well as from BOLD signal indicators using default thresholds in CONN preprocessing pipeline (5 standard deviations above the mean in global BOLD signal change, or framewise displacement values above 0.9 mm; Nieto-Castanon, 2020). Functional and structural data were independently normalized into a common space (the Montreal Neurological Institute [MNI] template; IXI549Space) using SPM12 unified segmentation and normalization procedure (Ashburner & Friston, 2005) with a reference functional image computed as the mean functional data after realignment across all timepoints omitting outlier scans. The output data were resampled to a common bounding box between MNI-space coordinates (−90, −126, −72) and (90, 90, 108), using 2 mm isotropic voxels and 4th order spline interpolation for the functional data, and 1mm isotropic voxels and trilinear interpolation for the structural data. Last, the functional data were smoothed spatially with a 4 mm FWHM Gaussian kernel. Data from the two naturalistic paradigms (resting-state and story listening) were further preprocessed by regressing out of each voxel’s time-course principal components of the six subject-specific motion parameters and BOLD signal time-courses extracted from the white matter and cerebrospinal fluid. Residuals were then bandpass filtered (0.0100–0.2500 Hz).
First-level analysis
Responses in individual voxels were estimated using a General Linear Model (GLM) in which each experimental condition was modeled with a boxcar function convolved with the canonical hemodynamic response function (HRF) (fixation was modeled implicitly, such that all timepoints that did not correspond to one of the conditions were assumed to correspond to a fixation period). Temporal autocorrelations in the BOLD signal timeseries were accounted for by a combination of high-pass filtering with a 128 seconds cutoff, and whitening using an AR(0.2) model (first-order autoregressive model linearized around the coefficient a=0.2) to approximate the observed covariance of the functional data in the context of Restricted Maximum Likelihood estimation (ReML). In addition to experimental condition effects, the GLM design included first-order temporal derivatives for each condition (included to model variability in the HRF delays), as well as nuisance regressors to control for the effect of slow linear drifts, subject-specific motion parameters (6 parameters), and potential outlier scans (identified during preprocessing as described above) on the BOLD signal.
Functional localization of the language and MD networks and response estimation
For each participant, functional regions of interest (fROIs) were defined using the Group-constrained Subject-Specific (GSS) approach (Fedorenko et al., 2010), whereby a set of parcels or “search spaces” (i.e., brain areas within which most individuals in prior studies showed activity for the localizer contrast) is combined with each individual participant’s activation map for the same or similar contrast. To define the language fROIs, we used five parcels derived from a group-level representation of data for the sentences > nonwords contrast in 220 independent participants. These parcels were used in much prior work (e.g., Fedorenko et al., 2020; Hu et al., 2023; Malik-Moraleda et al., 2022; Pereira et al., 2018) and included three regions in the left frontal cortex: two located in the inferior frontal gyrus (LH IFG and LH IFGorb), and one located in the middle frontal gyrus (LH MFG); and two regions in the left temporal cortex spanning the entire extent of the lateral temporal lobe (LH AntTemp and LH PostTemp). Additionally, we examined activations in the right hemisphere (RH) homotopes of the language regions. To define the fROIs in the RH, the left hemisphere parcels were mirror-projected onto the RH to create five homotopic parcels. By design, the parcels cover relatively large swaths of cortex in order to be able to accommodate inter-individual variability. Hence the mirrored versions are likely to encompass RH language regions despite possible hemispheric asymmetries in the precise locations of activations (for validation, see Blank et al., 2014; Lipkin et al., 2022; Mahowald & Fedorenko, 2016; Shain et al., 2023). Individual language fROIs were defined by selecting within each parcel the 10% of most localizer-responsive voxels based on the t values for the sentences > nonwords contrast of the language task.
To define the MD fROIs, we used a set of 20 parcels (10 in each hemisphere) derived from a group-level probabilistic activation overlap map for the hard > easy spatial working memory contrast in 197 participants. These parcels have been used in prior work such as (Ivanova et al., 2020). The parcels include the posterior parietal cortex (LH and RH postParietal), middle parietal cortex (LH and RH midParietal), anterior parietal cortex (LH and RH antParietal), superior frontal gyrus (LH and RH supFrontal), precentral gyrus (LH and RH PrecG), IFG pars opercularis (LH and RH IFGop), middle frontal gyrus (LH and RH midFront), middle frontal gyrus, orbital part (LH and RH MidFrontOrb), insula (LH and RH insula) and medial frontal cortex (LH and RH medialFront). Individual MD fROIs were defined by selecting 10% of voxels within each parcel that were most responsive to the hard > easy MD task contrast, as defined by their t values.
For both the language and the MD networks, we estimated the responses of these individually defined fROIs to the sentences and nonwords conditions of the language task, and the easy and hard conditions of the MD task. For extracting the responses of the language fROIs to the language task conditions, and for extracting the responses of the MD fROIs to the MD task conditions, an across-runs cross-validation procedure was used (Nieto-Castañón & Fedorenko, 2012), ensuring independence (Kriegeskorte et al., 2009).
Activation extent, lateralization, and spatial overlap estimation
Each participant’s activation map for each localizer contrast (sentences > nonwords for language and hard > easy spatial working memory for MD) were thresholded at an alpha level 0.05 after FDR correction and binarized. To determine activation extent, the number of voxels with p-values above this threshold was computed at the whole-brain level and within each language and MD parcel. To determine lateralization (which was only done for the language network, as the MD network does not show a strong hemispheric bias), the number of contrast-activated voxels in the right hemisphere (RH) at the FDR q <0.05 significance threshold was subtracted from the number of contrast-activated voxels in the left hemisphere (LH) at the same threshold, and the resulting value was divided by the sum of contrast-activated voxels across hemispheres. Finally, Dice similarity coefficients were first computed between the FDR-corrected activation maps of the two language runs and between the FDR-corrected activation maps of the two MD runs to measure within-network overlap. To measure the overlap between the two networks, a Dice coefficient was computed for each language-MD pair of runs and then averaged across the four pairs.
Estimation of inter-regional correlations within and between networks
For each participant, we first averaged BOLD timeseries across voxels within each language and MD fROI (i.e., top 10% voxels most responsive to the language and MD localizer tasks). Fisher-transformed Pearson correlations were then estimated between averaged timeseries of pairs of language and MD fROIs for each naturalistic paradigm (i.e., resting-state and story listening) for each participant to measure within and between-network synchronization.
Statistical analyses
1. Examining functional response profiles (magnitude and extent) within the language and the MD networks across cohorts
All statistical analyses were performed in R (version 4.2.1). To evaluate the response of the language and MD networks to both the language task (i.e., the size of the sentences > nonwords effect) and the MD task (i.e., the size of the hard > easy effect) within each cohort, analyses were performed at the network, hemisphere, and fROI levels. At the network and hemisphere levels, differences in BOLD response across conditions of the language and MD tasks were evaluated with linear mixed-effect models using the lme4 package (https://cran.r-project.org/web/packages/lme4/index.html); the models included a fixed effect for task condition and a random intercept by participant and fROI (Eq. 1). P-value approximation was performed with the lmerTest package (Kuznetsova et al., 2017). Multiple comparisons across tasks (i.e., language and MD) and hemispheres were corrected using false discovery rate (FDR) correction (Benjamini & Yekutieli, 2001). At the fROI level, repeated measures t-tests were performed on the BOLD response magnitude values extracted for each condition, using FDR for the number of fROIs in each network.
| Eq. 1: |
To evaluate differences in BOLD response magnitude and extent between younger and older adults in each network, analyses were performed using linear mixed-effects regression models at the network and hemisphere levels. Models evaluating differences in response magnitude included a fixed effect for the interaction between cohort and task condition, and random intercepts for participant and fROI (Eq. 2). Models evaluating differences in activation extent included a fixed effect for cohort and random intercepts for participant and parcel (Eq. 3). Multiple comparisons across tasks (i.e., language and MD) and hemispheres were corrected using FDR. Estimates were also obtained at the level of each anatomical parcel using linear regression models and FDR correction for the number of fROIs in each network. Posthoc pairwise cohort contrasts of estimated marginal means were examined with the emmeans package (Lenth et al., 2024) and p-values were adjusted for multiple comparisons using Tukey’s HSD (honest significant difference) correction.
| Eq. 2: |
| Eq. 3: |
Difference in BOLD response lateralization across cohorts was evaluated in the language network using a linear regression model.
MD task accuracy was compared across cohorts using a linear mixed-effects model to investigate the effects of cohort and condition on accuracy, as well as their interaction. The model included cohort, condition, and their interaction as fixed effects, with a random intercept for participant (Accuracy ~ Cohort*Condition + (1|Participant)). To further investigate the differences in accuracy between groups within each condition, we conducted post-hoc pairwise comparisons using Tukey-adjusted estimated marginal means (EMMs) to control for multiple comparisons.
2. Examining inter-regional correlations within and between the language and the MD networks across cohorts
To evaluate differences in inter-regional correlations within and between networks across cohorts, analyses were performed using linear mixed-effects regression models at the hemisphere and network levels. Models included a fixed effect for cohort and random intercepts for fROI pair and participant (Eq. 4). Multiple comparisons across tasks (i.e., resting-state and story listening) and hemispheres were corrected using FDR. Further, to compare the degree of within-network synchronization to the degree of between-network synchronization within each cohort, differences in correlation coefficients within and between the language and the MD networks were evaluated using linear mixed-effects regression models at the network level. Models included a fixed effect for network type (i.e., within language, within MD, between language and MD) and random intercepts for fROI pair and participant (Eq. 5). Multiple comparisons across tasks and cohorts were corrected using FDR.
| Eq. 4: |
| Eq. 5: |
3. Examining spatial overlap between the language and the MD networks across cohorts
To evaluate differences in spatial overlap (measured by Dice coefficient) within and between networks across cohorts, analyses were performed using linear regression models at the network level. Multiple comparisons across tasks were corrected using FDR. To assess the topographical consistency of each task activation map within each cohort, the degree of spatial overlap between runs of the same task was compared to the degree of spatial overlap between language and MD runs using linear regression models at the network level. Multiple comparisons across tasks and cohorts were corrected using FDR.
Supplementary Material
Significance Statement.
All organs, including brains, change as we age. However, the brain is not a uniform structure: it comprises multiple distinct networks, each supporting a different aspect of perception, motor control, and cognition. We examine two cognitive brain networks using fMRI and—across two independent cohorts—find a clear dissociation: the so-called Multiple Demand network, which supports executive functions (e.g., working memory), shows clear age-related decline; however, the language-selective network, which supports comprehension and production, remains young-like on all measures of network function and connectivity, in line with the preservation of linguistic skills in older adults. These findings challenge the notion of generalized brain aging and highlight the importance of dissociable components in the brain and mind.
Acknowledgments
We thank all individuals who participated in this study. We additionally acknowledge present and past members of the Boston University Center for Brain Recovery and the Fedorenko Lab at MIT. We acknowledge the Athinoula A. Martinos Imaging Center at the McGovern Institute for Brain Research, MIT. For technical support during scanning, we thank Steve Shannon, and Atsushi Takahashi. This work was supported by the NIH - National Institute on Deafness and Other Communication Disorders (grant R01DC016950 to SK and EF).
Footnotes
Competing Interest Statement: Dr. Kiran is a scientific advisor for Constant Therapy Health, but there is no overlap between this role and the submitted investigation. The other authors report no conflicts.
References
- Adali T., & Calhoun V. D. (2022). Reproducibility and replicability in neuroimaging data analysis. Current Opinion in Neurology, 35(4), 475–481. 10.1097/WCO.0000000000001081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agcaoglu O., Miller R., Mayer A. R., Hugdahl K., & Calhoun V. D. (2015). Lateralization of resting state networks and relationship to age and gender. NeuroImage, 104, 310–325. 10.1016/j.neuroimage.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amalric M., & Dehaene S. (2019). A distinct cortical network for mathematical knowledge in the human brain. NeuroImage, 189, 19–31. 10.1016/j.neuroimage.2019.01.001 [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Snyder A. Z., Vincent J. L., Lustig C., Head D., Raichle M. E., & Buckner R. L. (2007). Disruption of Large-Scale Brain Systems in Advanced Aging. Neuron, 56(5), 924–935. 10.1016/j.neuron.2007.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonenko D., Brauer J., Meinzer M., Fengler A., Kerti L., Friederici A. D., & Flöel A. (2013). Functional and structural syntax networks in aging. NeuroImage, 83, 513–523. 10.1016/j.neuroimage.2013.07.018 [DOI] [PubMed] [Google Scholar]
- Ashburner J., & Friston K. J. (2005). Unified segmentation. NeuroImage, 26(3), 839–851. 10.1016/j.neuroimage.2005.02.018 [DOI] [PubMed] [Google Scholar]
- Assem M., Blank I. A., Mineroff Z., Ademoğlu A., & Fedorenko E. (2020). Activity in the fronto-parietal multiple-demand network is robustly associated with individual differences in working memory and fluid intelligence. Cortex, 131, 1–16. 10.1016/j.cortex.2020.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baciu M., Boudiaf N., Cousin E., Perrone-Bertolotti M., Pichat C., Fournet N., Chainay H., Lamalle L., & Krainik A. (2016). Functional MRI evidence for the decline of word retrieval and generation during normal aging. Age (Dordrecht, Netherlands), 38(1), 3. 10.1007/s11357-015-9857-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard H. K., Jackson T. B., Symm A. C., Hicks T. H., & Bernard J. A. (2023). Age-related differences in functional network segregation in the context of sex and reproductive stage. Human Brain Mapping, 44(5), 1949–1963. 10.1002/hbm.26184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota D. A., Dolan P. O., & Duchek J. M. (2000). Memory Changes in Healthy Young and Older Adults. In Tulving E. (Ed.), The Oxford Handbook of Memory (pp. 395–410). Oxford University Press. [Google Scholar]
- Bedard A.-C., Nichols S., Barbosa J. A., Schachar R., Logan G. D., & Tannock R. (2002). The development of selective inhibitory control across the life span. Developmental Neuropsychology, 21(1), 93–111. 10.1207/S15326942DN2101_5 [DOI] [PubMed] [Google Scholar]
- Benjamini Y., & Yekutieli D. (2001). The Control of the False Discovery Rate in Multiple Testing under Dependency. The Annals of Statistics, 29(4), 1165–1188. [Google Scholar]
- Blank I., Balewski Z., Mahowald K., & Fedorenko E. (2016). Syntactic processing is distributed across the language system. NeuroImage, 127, 307–323. 10.1016/j.neuroimage.2015.11.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank I., & Fedorenko E. (2017). Domain-General Brain Regions Do Not Track Linguistic Input as Closely as Language-Selective Regions. The Journal of Neuroscience, 37(41), 9999–10011. 10.1523/JNEUROSCI.3642-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank I., Kanwisher N., & Fedorenko E. (2014). A functional dissociation between language and multiple-demand systems revealed in patterns of BOLD signal fluctuations. Journal of Neurophysiology, 112(5), 1105–1118. 10.1152/jn.00884.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossier H., Roels S. P., Seurinck R., Banaschewski T., Barker G. J., Bokde A. L. W., Quinlan E. B., Desrivières S., Flor H., Grigis A., Garavan H., Gowland P., Heinz A., Ittermann B., Martinot J.-L., Artiges E., Nees F., Orfanos D. P., Poustka L., … Moerkerke B. (2020). The empirical replicability of task-based fMRI as a function of sample size. NeuroImage, 212, 116601. 10.1016/j.neuroimage.2020.116601 [DOI] [PubMed] [Google Scholar]
- Braga R. M., DiNicola L. M., Becker H. C., & Buckner R. L. (2020). Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks. Journal of Neurophysiology, 124(5), 1415–1448. 10.1152/jn.00753.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L. (2004). Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron, 44(1), 195–208. 10.1016/j.neuron.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Burke D. M., & Shafto M. A. (2007). Language and Aging. In The Handbook of Aging and Cognition. Psychology Press. [Google Scholar]
- Cabeza R. (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychology and Aging, 17(1), 85–100. 10.1037//0882-7974.17.1.85 [DOI] [PubMed] [Google Scholar]
- Cabeza R., Albert M., Belleville S., Craik F., Duarte A., Grady C., Lindenberger U., Nyberg L., Park D., Reuter-Lorenz P. A., Rugg M. D., Steffener J., & Rajah M. N. (2018). Cognitive neuroscience of healthy aging: Maintenance, reserve, and compensation. Nature Reviews. Neuroscience, 19(11), 701–710. 10.1038/s41583-018-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. L., Samu D., Davis S. W., Geerligs L., Mustafa A., Tyler L. K., & for Cambridge Centre for Aging and Neuroscience. (2016). Robust Resilience of the Frontotemporal Syntax System to Aging. The Journal of Neuroscience, 36(19), 5214–5227. 10.1523/JNEUROSCI.4561-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell K. L., & Tyler L. K. (2018). Language-related domain-specific and domain-general systems in the human brain. Current Opinion in Behavioral Sciences, 21, 132–137. 10.1016/j.cobeha.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda N. J., Kramer A. F., & Gonzalez de Sather J. C. (2001). Changes in executive control across the life span: Examination of task-switching performance. Developmental Psychology, 37(5), 715–730. [PubMed] [Google Scholar]
- Chan M. Y., Park D. C., Savalia N. K., Petersen S. E., & Wig G. S. (2014). Decreased segregation of brain systems across the healthy adult lifespan. Proceedings of the National Academy of Sciences of the United States of America, 111(46), E4997–5006. 10.1073/pnas.1415122111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J. S. X., Ng K. K., Tandi J., Wang C., Poh J.-H., Lo J. C., Chee M. W. L., & Zhou J. H. (2019). Longitudinal Changes in the Cerebral Cortex Functional Organization of Healthy Elderly. Journal of Neuroscience, 39(28), 5534–5550. 10.1523/JNEUROSCI.1451-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor L. T., Spiro A. III, Obler L. K., & Albert M. L. (2004). Change in Object Naming Ability During Adulthood. The Journals of Gerontology: Series B, 59(5), P203–P209. 10.1093/geronb/59.5.P203 [DOI] [PubMed] [Google Scholar]
- Craik F. I. M., & Bialystok E. (2006). Cognition through the lifespan: Mechanisms of change. Trends in Cognitive Sciences, 10(3), 131–138. 10.1016/j.tics.2006.01.007 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. S. (2017). Effects of aging on functional and structural brain connectivity. NeuroImage, 160, 32–40. 10.1016/j.neuroimage.2017.01.077 [DOI] [PubMed] [Google Scholar]
- Dave S., Brothers T. A., Traxler M. J., Ferreira F., Henderson J. M., & Swaab T. Y. (2018). Electrophysiological evidence for preserved primacy of lexical prediction in aging. Neuropsychologia, 117, 135–147. 10.1016/j.neuropsychologia.2018.05.023 [DOI] [PubMed] [Google Scholar]
- De Luca C. R., Wood S. J., Anderson V., Buchanan J.-A., Proffitt T. M., Mahony K., & Pantelis C. (2003). Normative data from the CANTAB. I: Development of executive function over the lifespan. Journal of Clinical and Experimental Neuropsychology, 25(2), 242–254. 10.1076/jcen.25.2.242.13639 [DOI] [PubMed] [Google Scholar]
- Diachek E., Blank I., Siegelman M., Affourtit J., & Fedorenko E. (2020). The Domain-General Multiple Demand (MD) Network Does Not Support Core Aspects of Language Comprehension: A Large-Scale fMRI Investigation. Journal of Neuroscience, 40(23), 4536–4550. 10.1523/JNEUROSCI.2036-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M. T., Rizio A. A., & Zhuang J. (2016). The Neural Language Systems That Support Healthy Aging: Integrating Function, Structure, and Behavior: Neural Language Systems in Aging. Language and Linguistics Compass, 10(7), 314–334. 10.1111/lnc3.12199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Biase M. A., Tian Y. E., Bethlehem R. A. I., Seidlitz J., Alexander-Bloch Aaron. F., Yeo B. T. T., & Zalesky A. (2023). Mapping human brain charts cross-sectionally and longitudinally. Proceedings of the National Academy of Sciences, 120(20), e2216798120. 10.1073/pnas.2216798120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. (2010). The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends in Cognitive Sciences, 14(4), 172–179. 10.1016/j.tics.2010.01.004 [DOI] [PubMed] [Google Scholar]
- Duncan J. (2013). The Structure of Cognition: Attentional Episodes in Mind and Brain. Neuron, 80(1), 35–50. 10.1016/j.neuron.2013.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J., Assem M., & Shashidhara S. (2020). Integrated Intelligence from Distributed Brain Activity. Trends in Cognitive Sciences, 24(10), 838–852. 10.1016/j.tics.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J., & Owen A. M. (2000). Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences, 23(10), 475–483. 10.1016/S0166-2236(00)01633-7 [DOI] [PubMed] [Google Scholar]
- Düzel E., Schütze H., Yonelinas A. P., & Heinze H.-J. (2011). Functional phenotyping of successful aging in long-term memory: Preserved performance in the absence of neural compensation. Hippocampus, 21(8), 803–814. 10.1002/hipo.20834 [DOI] [PubMed] [Google Scholar]
- Federmeier K. D., Kutas M., & Schul R. (2010). Age-related and individual differences in the use of prediction during language comprehension. Brain and Language, 115(3), 149–161. 10.1016/j.bandl.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier K. D., Van Petten C., Schwartz T. J., & Kutas M. (2003). Sounds, Words, Sentences: Age-Related Changes Across Levels of Language Processing. Psychology and Aging, 18(4), 858–872. 10.1037/0882-7974.18.4.858 [DOI] [PubMed] [Google Scholar]
- Fedorenko E. (2014). The role of domain-general cognitive control in language comprehension. Frontiers in Psychology, 5. https://www.frontiersin.org/articles/10.3389/fpsyg.2014.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E. (2021). The early origins and the growing popularity of the individual-subject analytic approach in human neuroscience. Current Opinion in Behavioral Sciences, 40, 105–112. 10.1016/j.cobeha.2021.02.023 [DOI] [Google Scholar]
- Fedorenko E., Behr M. K., & Kanwisher N. (2011). Functional specificity for high-level linguistic processing in the human brain. Proceedings of the National Academy of Sciences of the United States of America, 108(39), 16428–16433. 10.1073/pnas.1112937108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., & Blank I. A. (2020). Broca’s Area Is Not a Natural Kind. Trends in Cognitive Sciences, 24(4), 270–284. 10.1016/j.tics.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Blank I. A., Siegelman M., & Mineroff Z. (2020). Lack of selectivity for syntax relative to word meanings throughout the language network. Cognition, 203, 104348. 10.1016/j.cognition.2020.104348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Duncan J., & Kanwisher N. (2012). Language-Selective and Domain-General Regions Lie Side by Side within Broca’s Area. Current Biology, 22(21), 2059–2062. 10.1016/j.cub.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Duncan J., & Kanwisher N. (2013). Broad domain generality in focal regions of frontal and parietal cortex. Proceedings of the National Academy of Sciences, 110(41), 16616–16621. 10.1073/pnas.1315235110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Hsieh P.-J., Nieto-Castañón A., Whitfield-Gabrieli S., & Kanwisher N. (2010). New Method for fMRI Investigations of Language: Defining ROIs Functionally in Individual Subjects. Journal of Neurophysiology, 104(2), 1177–1194. 10.1152/jn.00032.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., Ivanova A. A., & Regev T. I. (2024). The language network as a natural kind within the broader landscape of the human brain. Nature Reviews Neuroscience, 25(5), 289–312. 10.1038/s41583-024-00802-4 [DOI] [PubMed] [Google Scholar]
- Fedorenko E., & Shain C. (2021). Similarity of Computations Across Domains Does Not Imply Shared Implementation: The Case of Language Comprehension. Current Directions in Psychological Science, 30(6), 526–534. 10.1177/09637214211046955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorenko E., & Thompson-Schill S. L. (2014). Reworking the language network. Trends in Cognitive Sciences, 18(3), 120–126. 10.1016/j.tics.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson H. J., Brunsdon V. E. A., & Bradford E. E. F. (2021). The developmental trajectories of executive function from adolescence to old age. Scientific Reports, 11(1), Article 1. 10.1038/s41598-020-80866-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré P., Jarret J., Brambati S. M., Bellec P., & Joanette Y. (2020). Task-Induced Functional Connectivity of Picture Naming in Healthy Aging: The Impacts of Age and Task Complexity. Neurobiology of Language, 1(2), 161–184. 10.1162/nol_a_00007 [DOI] [Google Scholar]
- Finn E. S., Shen X., Scheinost D., Rosenberg M. D., Huang J., Chun M. M., Papademetris X., & Constable R. T. (2015). Functional connectome fingerprinting: Identifying individuals using patterns of brain connectivity. Nature Neuroscience, 18(11), Article 11. 10.1038/nn.4135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzhugh M. C., Braden B. B., Sabbagh M. N., Rogalsky C., & Baxter L. C. (2019). Age-Related Atrophy and Compensatory Neural Networks in Reading Comprehension. Journal of the International Neuropsychological Society, 25(6), 569–582. 10.1017/S1355617719000274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A. D. (2012). The cortical language circuit: From auditory perception to sentence comprehension. Trends in Cognitive Sciences, 16(5), 262–268. 10.1016/j.tics.2012.04.001 [DOI] [PubMed] [Google Scholar]
- Friston Karl. J., Ashburner J., Frith C. D., Poline J.-B., Heather J. D., & Frackowiak R. S. J. (1995). Spatial registration and normalization of images. Human Brain Mapping, 3(3), 165–189. 10.1002/hbm.460030303 [DOI] [Google Scholar]
- Frost M. A., & Goebel R. (2012). Measuring structural–functional correspondence: Spatial variability of specialised brain regions after macro-anatomical alignment. NeuroImage, 59(2), 1369–1381. 10.1016/j.neuroimage.2011.08.035 [DOI] [PubMed] [Google Scholar]
- Geerligs L., Cam-CAN, & Campbell K. L. (2018). Age-related differences in information processing during movie watching. Neurobiology of Aging, 72, 106–120. 10.1016/j.neurobiolaging.2018.07.025 [DOI] [PubMed] [Google Scholar]
- Geerligs L., Renken R. J., Saliasi E., Maurits N. M., & Lorist M. M. (2015). A Brain-Wide Study of Age-Related Changes in Functional Connectivity. Cerebral Cortex (New York, N.Y.: 1991), 25(7), 1987–1999. 10.1093/cercor/bhu012 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos L., Pereira J. B., Mohanty R., Barroso J., Westman E., & Ferreira D. (2021). Cortical Networks Underpinning Compensation of Verbal Fluency in Normal Aging. Cerebral Cortex, 31(8), 3832–3845. 10.1093/cercor/bhab052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C., Sarraf S., Saverino C., & Campbell K. (2016). Age differences in the functional interactions among the default, frontoparietal control, and dorsal attention networks. Neurobiology of Aging, 41, 159–172. 10.1016/j.neurobiolaging.2016.02.020 [DOI] [PubMed] [Google Scholar]
- Gratton C., & Braga R. M. (2021). Editorial overview: Deep imaging of the individual brain: past, practice, and promise. Current Opinion in Behavioral Sciences, 40, iii–vi. 10.1016/j.cobeha.2021.06.011 [DOI] [Google Scholar]
- Grossman M., Cooke A., DeVita C., Alsop D., Detre J., Chen W., & Gee J. (2002). Age-Related Changes in Working Memory during Sentence Comprehension: An fMRI Study. NeuroImage, 15(2), 302–317. 10.1006/nimg.2001.0971 [DOI] [PubMed] [Google Scholar]
- Hagoort P., & Indefrey P. (2014). The Neurobiology of Language Beyond Single Words. Annual Review of Neuroscience, 37(1), 347–362. 10.1146/annurev-neuro-071013-013847 [DOI] [PubMed] [Google Scholar]
- Hartshorne J. K., & Germine L. T. (2015). When does cognitive functioning peak? The asynchronous rise and fall of different cognitive abilities across the life span. Psychological Science, 26(4), 433–443. 10.1177/0956797614567339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckner M. K., Cieslik E. C., Eickhoff S. B., Camilleri J. A., Hoffstaedter F., & Langner R. (2021). The Aging Brain and Executive Functions Revisited: Implications from Meta-analytic and Functional-Connectivity Evidence. Journal of Cognitive Neuroscience, 33(9), 1716–1752. 10.1162/jocn_a_01616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T., & Gabrieli J. D. E. (2004). Insights into the ageing mind: A view from cognitive neuroscience. Nature Reviews Neuroscience, 5(2), 87–96. 10.1038/nrn1323 [DOI] [PubMed] [Google Scholar]
- Hickok G., & Poeppel D. (2007). The cortical organization of speech processing. Nature Reviews. Neuroscience, 8(5), 393–402. 10.1038/nrn2113 [DOI] [PubMed] [Google Scholar]
- Hiersche K. J., Schettini E., Li J., & Saygin Z. M. (2024). Functional dissociation of the language network and other cognition in early childhood. Human Brain Mapping, 45(9), e26757. 10.1002/hbm.26757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman P., Loginova E., & Russell A. (2018). Poor coherence in older people’s speech is explained by impaired semantic and executive processes. eLife, 7, e38907. 10.7554/eLife.38907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyau E., Boudiaf N., Cousin E., Pichat C., Fournet N., Krainik A., Jaillard A., & Baciu M. (2017). Aging Modulates the Hemispheric Specialization during Word Production. Frontiers in Aging Neuroscience, 9. https://www.frontiersin.org/articles/10.3389/fnagi.2017.00125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J., Small H., Kean H., Takahashi A., Zekelman L., Kleinman D., Ryan E., Nieto-Castañón A., Ferreira V., & Fedorenko E. (2023). Precision fMRI reveals that the language-selective network supports both phrase-structure building and lexical access during language production. Cerebral Cortex, 33(8), 4384–4404. 10.1093/cercor/bhac350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R. J., & Federmeier K. D. (2024). Altered oscillatory neural dynamics related to word prediction in older adult readers. Language, Cognition and Neuroscience, 39(7), 891–908. 10.1080/23273798.2024.2375248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugdahl K., Raichle M. E., Mitra A., & Specht K. (2015). On the existence of a generalized non-specific task-dependent network. Frontiers in Human Neuroscience, 9. 10.3389/fnhum.2015.00430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanova A. A., Srikant S., Sueoka Y., Kean H. H., Dhamala R., O’Reilly U.-M., Bers M. U., & Fedorenko E. (2020). Comprehension of computer code relies primarily on domain-general executive brain regions. eLife, 9, e58906. 10.7554/eLife.58906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S. C., Saykin A. J., Flashman L. A., McAllister T. W., O’Jile J. R., Sparling M. B., Guerin S. J., Moritz C. H., & Mamourian A. C. (2001). Similarities and Differences in Semantic and Phonological Processing with Age Patterns of Functional MRI Activation. Aging, Neuropsychology, and Cognition, 8(4), 307–320. 10.1076/anec.8.4.307.5639 [DOI] [Google Scholar]
- Kamal B. S., Holman C., & de Villers-Sidani E. (2013). Shaping the aging brain: Role of auditory input patterns in the emergence of auditory cortical impairments. Frontiers in Systems Neuroscience, 7. 10.3389/fnsys.2013.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kray J., Li K. Z. H., & Lindenberger U. (2002). Age-related changes in task-switching components: The role of task uncertainty. Brain and Cognition, 49(3), 363–381. 10.1006/brcg.2001.1505 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N., Simmons W. K., Bellgowan P. S. F., & Baker C. I. (2009). Circular analysis in systems neuroscience: The dangers of double dipping. Nature Neuroscience, 12(5), Article 5. 10.1038/nn.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P. B., & Christensen R. H. B. (2017). lmerTest Package: Tests in Linear Mixed Effects Models. Journal of Statistical Software, 82, 1–26. 10.18637/jss.v082.i13 [DOI] [Google Scholar]
- La C., Garcia-Ramos C., Nair V. A., Meier T. B., Farrar-Edwards D., Birn R., Meyerand M. E., & Prabhakaran V. (2016). Age-Related Changes in BOLD Activation Pattern in Phonemic Fluency Paradigm: An Investigation of Activation, Functional Connectivity and Psychophysiological Interactions. Frontiers in Aging Neuroscience, 8. https://www.frontiersin.org/articles/10.3389/fnagi.2016.00110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R. V., Bolker B., Buerkner P., Giné-Vázquez I., Herve M., Jung M., Love J., Miguez F., Riebl H., & Singmann H. (2024). emmeans: Estimated Marginal Means, aka Least-Squares Means (Version 1.10.1) [Computer software]. https://cran.r-project.org/web/packages/emmeans/index.html [Google Scholar]
- Li S.-C., Lindenberger U., & Sikström S. (2001). Aging cognition: From neuromodulation to representation. Trends in Cognitive Sciences, 5(11), 479–486. 10.1016/S1364-6613(00)01769-1 [DOI] [PubMed] [Google Scholar]
- Lipkin B., Tuckute G., Affourtit J., Small H., Mineroff Z., Kean H., Jouravlev O., Rakocevic L., Pritchett B., Siegelman M., Hoeflin C., Pongos A., Blank I. A., Struhl M. K., Ivanova A., Shannon S., Sathe A., Hoffmann M., Nieto-Castañón A., & Fedorenko E. (2022). Probabilistic atlas for the language network based on precision fMRI data from >800 individuals. Scientific Data, 9(1), Article 1. 10.1038/s41597-022-01645-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-F., Kim J., Wilson C., & Bedny M. (2020). Computer code comprehension shares neural resources with formal logical inference in the fronto-parietal network. eLife, 9, e59340. 10.7554/eLife.59340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig C., & Jantz T. (2015). Questions of age differences in interference control: When and how, not if? Brain Research, 1612, 59–69. 10.1016/j.brainres.2014.10.024 [DOI] [PubMed] [Google Scholar]
- Mahowald K., & Fedorenko E. (2016). Reliable individual-level neural markers of high-level language processing: A necessary precursor for relating neural variability to behavioral and genetic variability. NeuroImage, 139, 74–93. 10.1016/j.neuroimage.2016.05.073 [DOI] [PubMed] [Google Scholar]
- Malagurski B., Liem F., Oschwald J., Mérillat S., & Jäncke L. (2020). Functional dedifferentiation of associative resting state networks in older adults – A longitudinal study. NeuroImage, 214, 116680. 10.1016/j.neuroimage.2020.116680 [DOI] [PubMed] [Google Scholar]
- Malik-Moraleda S., Ayyash D., Gallée J., Affourtit J., Hoffmann M., Mineroff Z., Jouravlev O., & Fedorenko E. (2022). An investigation across 45 languages and 12 language families reveals a universal language network. Nature Neuroscience, 25(8), 1014–1019. 10.1038/s41593-022-01114-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik-Moraleda S., Jouravlev O., Taliaferro M., Mineroff Z., Cucu T., Mahowald K., Blank I. A., & Fedorenko E. (2024). Functional characterization of the language network of polyglots and hyperpolyglots with precision fMRI. Cerebral Cortex, 34(3), bhae049. 10.1093/cercor/bhae049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinzer M., Flaisch T., Seeds L., Harnish S., Antonenko D., Witte V., Lindenberg R., & Crosson B. (2012). Same Modulation but Different Starting Points: Performance Modulates Age Differences in Inferior Frontal Cortex Activity during Word-Retrieval. PLOS ONE, 7(3), e33631. 10.1371/journal.pone.0033631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menenti L., Gierhan S. M. E., Segaert K., & Hagoort P. (2011). Shared Language: Overlap and Segregation of the Neuronal Infrastructure for Speaking and Listening Revealed by Functional MRI. Psychological Science, 22(9), 1173–1182. 10.1177/0956797611418347 [DOI] [PubMed] [Google Scholar]
- Mitchell D. J., Mousley A. L. S., Shafto M. A., Cam-CAN, & Duncan J. (2023). Neural Contributions to Reduced Fluid Intelligence across the Adult Lifespan. Journal of Neuroscience, 43(2), 293–307. 10.1523/JNEUROSCI.0148-22.2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti M. M., Parsons L. M., & Osherson D. N. (2012). Thought Beyond Language: Neural Dissociation of Algebra and Natural Language. Psychological Science, 23(8), 914–922. 10.1177/0956797612437427 [DOI] [PubMed] [Google Scholar]
- Mooij S. M. M. de, Henson R. N. A., Waldorp L. J., & Kievit R. A. (2018). Age Differentiation within Gray Matter, White Matter, and between Memory and White Matter in an Adult Life Span Cohort. Journal of Neuroscience, 38(25), 5826–5836. 10.1523/JNEUROSCI.1627-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen L., Meyer A. S., & Humphreys G. W. (2006). Age-related effects on speech production: A review. Language and Cognitive Processes, 21(1–3), 238–290. 10.1080/01690960444000278 [DOI] [Google Scholar]
- Mueller S., Wang D., Fox M. D., Yeo B. T. T., Sepulcre J., Sabuncu M. R., Shafee R., Lu J., & Liu H. (2013). Individual Variability in Functional Connectivity Architecture of the Human Brain. Neuron, 77(3), 586–595. 10.1016/j.neuron.2012.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel I. E., Preuschhof C., Li S.-C., Nyberg L., Bäckman L., Lindenberger U., & Heekeren H. R. (2009). Performance level modulates adult age differences in brain activation during spatial working memory. Proceedings of the National Academy of Sciences, 106(52), 22552–22557. 10.1073/pnas.0908238106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel I. E., Preuschhof C., Li S.-C., Nyberg L., Bäckman L., Lindenberger U., & Heekeren H. R. (2011). Load modulation of BOLD response and connectivity predicts working memory performance in younger and older adults. Journal of Cognitive Neuroscience, 23(8), 2030–2045. 10.1162/jocn.2010.21560 [DOI] [PubMed] [Google Scholar]
- Nielson K. A., Langenecker S. A., & Garavan H. (2002). Differences in the functional neuroanatomy of inhibitory control across the adult life span. Psychology and Aging, 17(1), 56–71. 10.1037/0882-7974.17.1.56 [DOI] [PubMed] [Google Scholar]
- Nieto-Castanon A. (2020). Handbook of functional connectivity Magnetic Resonance Imaging methods in CONN. Hilbert Press. [Google Scholar]
- Nieto-Castañón A., & Fedorenko E. (2012). Subject-specific functional localizers increase sensitivity and functional resolution of multi-subject analyses. NeuroImage, 63(3), 1646–1669. 10.1016/j.neuroimage.2012.06.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Lövdén M., Riklund K., Lindenberger U., & Bäckman L. (2012). Memory aging and brain maintenance. Trends in Cognitive Sciences, 16(5), 292–305. 10.1016/j.tics.2012.04.005 [DOI] [PubMed] [Google Scholar]
- Ojemann G., Ojemann J., Lettich E., & Berger M. (1989). Cortical language localization in left, dominant hemisphere: An electrical stimulation mapping investigation in 117 patients. Journal of Neurosurgery, 71(3), 316–326. 10.3171/jns.1989.71.3.0316 [DOI] [PubMed] [Google Scholar]
- Olulade O. A., Seydell-Greenwald A., Chambers C. E., Turkeltaub P. E., Dromerick A. W., Berl M. M., Gaillard W. D., & Newport E. L. (2020). The neural basis of language development: Changes in lateralization over age. Proceedings of the National Academy of Sciences of the United States of America, 117(38), 23477–23483. 10.1073/pnas.1905590117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozernov-Palchik O., O’Brien A. M., Lee E. J., Richardson H., Romeo R., Lipkin B., Small H., Capella J., Nieto-Castañón A., Saxe R., Gabrieli J. D. E., & Fedorenko E. (2024). Precision fMRI reveals that the language network exhibits adult-like left-hemispheric lateralization by 4 years of age (p. 2024.05.15.594172). bioRxiv. 10.1101/2024.05.15.594172 [DOI] [Google Scholar]
- Park D. C., & Bischof G. N. (2011). Chapter 7—Neuroplasticity, Aging, and Cognitive Function. In Schaie K. W. & Willis S. L. (Eds.), Handbook of the Psychology of Aging (Seventh Edition) (pp. 109–119). Academic Press. 10.1016/B978-0-12-380882-0.00007-3 [DOI] [Google Scholar]
- Park D. C., Polk T. A., Mikels J. A., Taylor S. F., & Marshuetz C. (2001). Cerebral aging: Integration of brain and behavioral models of cognitive function. Dialogues in Clinical Neuroscience, 3(3), 151–165. 10.31887/DCNS.2001.3.3/dcpark [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. C., Polk T. A., Park R., Minear M., Savage A., & Smith M. R. (2004). Aging reduces neural specialization in ventral visual cortex. Proceedings of the National Academy of Sciences of the United States of America, 101(35), 13091–13095. 10.1073/pnas.0405148101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D. C., & Reuter-Lorenz P. (2009). The Adaptive Brain: Aging and Neurocognitive Scaffolding. Annual Review of Psychology, 60(Volume 60, 2009), 173–196. 10.1146/annurev.psych.59.103006.093656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle J. E., Chandrasekaran K., Powers J., Smith E. E., & Grossman M. (2013). Age-related vulnerability in the neural systems supporting semantic processing. Frontiers in Aging Neuroscience, 5. 10.3389/fnagi.2013.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira F., Lou B., Pritchett B., Ritter S., Gershman S. J., Kanwisher N., Botvinick M., & Fedorenko E. (2018). Toward a universal decoder of linguistic meaning from brain activation. Nature Communications, 9(1), 963. 10.1038/s41467-018-03068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistono A., Guerrier L., Péran P., Rafiq M., Giméno M., Bézy C., Pariente J., & Jucla M. (2021). Increased functional connectivity supports language performance in healthy aging despite gray matter loss. Neurobiology of Aging, 98, 52–62. 10.1016/j.neurobiolaging.2020.09.015 [DOI] [PubMed] [Google Scholar]
- Poldrack R. A., Baker C. I., Durnez J., Gorgolewski K. J., Matthews P. M., Munafò M. R., Nichols T. E., Poline J.-B., Vul E., & Yarkoni T. (2017). Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nature Reviews Neuroscience, 18(2), Article 2. 10.1038/nrn.2016.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C. J. (2012). A review and synthesis of the first 20years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage, 62(2), 816–847. 10.1016/j.neuroimage.2012.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quillen I. A., Yen M., & Wilson S. M. (2021). Distinct Neural Correlates of Linguistic and Non-Linguistic Demand. Neurobiology of Language, 2(2), 202–225. 10.1162/nol_a_00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah M. N., & D’Esposito M. (2005). Region-specific changes in prefrontal function with age: A review of PET and fMRI studies on working and episodic memory. Brain: A Journal of Neurology, 128(Pt 9), 1964–1983. 10.1093/brain/awh608 [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P. A., & Cappell K. A. (2008). Neurocognitive aging and the compensation hypothesis. Current Directions in Psychological Science, 17(3), 177–182. 10.1111/j.1467-8721.2008.00570.x [DOI] [Google Scholar]
- Ryskin R., Levy R. P., & Fedorenko E. (2020). Do domain-general executive resources play a role in linguistic prediction? Re-evaluation of the evidence and a path forward. Neuropsychologia, 136, 107258. 10.1016/j.neuropsychologia.2019.107258 [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R., Bartrés-Faz D., & Junqué C. (2015). Reorganization of brain networks in aging: A review of functional connectivity studies. Frontiers in Psychology, 6, 663. 10.3389/fpsyg.2015.00663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samu D., Campbell K. L., Tsvetanov K. A., Shafto M. A., & Tyler L. K. (2017). Preserved cognitive functions with age are determined by domain-dependent shifts in network responsivity. Nature Communications, 8(1), Article 1. 10.1038/ncomms14743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaie K. W., & Willis S. L. (2017). History of Cognitive Aging Research. In Pachana N. A. (Ed.), Encyclopedia of Geropsychology (pp. 1068–1086). Springer. 10.1007/978-981-287-082-7_99 [DOI] [Google Scholar]
- Schill J., Simonyan K., Corsten M., Mathys C., Thiel C., & Witt K. (2023). Graph-theoretical insights into the effects of aging on the speech production network. Cerebral Cortex (New York, N.Y.: 1991), 33(5), 2162–2173. 10.1093/cercor/bhac198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky M. T., Wang Y., Pu M., & Leventhal A. G. (2000). Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nature Neuroscience, 3(4), 384–390. 10.1038/73957 [DOI] [PubMed] [Google Scholar]
- Schneider-Garces N. J., Gordon B. A., Brumback-Peltz C. R., Shin E., Lee Y., Sutton B. P., Maclin E. L., Gratton G., & Fabiani M. (2010). Span, CRUNCH, and beyond: Working memory capacity and the aging brain. Journal of Cognitive Neuroscience, 22(4), 655–669. 10.1162/jocn.2009.21230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott T., Gallée J., & Fedorenko E. (2017). A new fun and robust version of an fMRI localizer for the frontotemporal language system. Cognitive Neuroscience, 8(3), 167–176. 10.1080/17588928.2016.1201466 [DOI] [PubMed] [Google Scholar]
- Shafto M. A., & Tyler L. K. (2014). Language in the aging brain: The network dynamics of cognitive decline and preservation. Science, 346(6209), 583–587. 10.1126/science.1254404 [DOI] [PubMed] [Google Scholar]
- Shain C., Paunov A., Chen X., Lipkin B., & Fedorenko E. (2023). No evidence of theory of mind reasoning in the human language network. Cerebral Cortex (New York, N.Y.: 1991), 33(10), 6299–6319. 10.1093/cercor/bhac505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashidhara S., Mitchell D. J., Erez Y., & Duncan J. (2019). Progressive Recruitment of the Frontoparietal Multiple-demand System with Increased Task Complexity, Time Pressure, and Reward. Journal of Cognitive Neuroscience, 31(11), 1617–1630. 10.1162/jocn_a_01440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. E., Geva A., Jonides J., Miller A., Reuter-Lorenz P., & Koeppe R. A. (2001). The neural basis of task-switching in working memory: Effects of performance and aging. Proceedings of the National Academy of Sciences, 98(4), 2095–2100. 10.1073/pnas.98.4.2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spieler D. H., Balota D. A., & Faust M. E. (1996). Stroop performance in healthy younger and older adults and in individuals with dementia of the Alzheimer’s type. Journal of Experimental Psychology. Human Perception and Performance, 22(2), 461–479. 10.1037//0096-1523.22.2.461 [DOI] [PubMed] [Google Scholar]
- Thornton R., & Light L. L. (2006). Language Comprehension and Production in Normal Aging. In Handbook of the psychology of aging, 6th ed (pp. 261–287). Elsevier. 10.1016/B978-012101264-9/50015-X [DOI] [Google Scholar]
- Turner G. R., & Spreng R. N. (2012). Executive functions and neurocognitive aging: Dissociable patterns of brain activity. Neurobiology of Aging, 33(4), 826.e1-826.e13. 10.1016/j.neurobiolaging.2011.06.005 [DOI] [PubMed] [Google Scholar]
- Tyler L. K., Shafto M. A., Randall B., Wright P., Marslen-Wilson W. D., & Stamatakis E. A. (2010). Preserving Syntactic Processing across the Adult Life Span: The Modulation of the Frontotemporal Language System in the Context of Age-Related Atrophy. Cerebral Cortex, 20(2), 352–364. 10.1093/cercor/bhp105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varangis E., Habeck C. G., Razlighi Q. R., & Stern Y. (2019). The Effect of Aging on Resting State Connectivity of Predefined Networks in the Brain. Frontiers in Aging Neuroscience, 11. https://www.frontiersin.org/articles/10.3389/fnagi.2019.00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varangis E., Razlighi Q., Habeck C. G., Fisher Z., & Stern Y. (2019). Between-network Functional Connectivity Is Modified by Age and Cognitive Task Domain. Journal of Cognitive Neuroscience, 31(4), 607–622. 10.1162/jocn_a_01368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaeghen P. (2003). Aging and vocabulary score: A meta-analysis. Psychology and Aging, 18(2), 332–339. 10.1037/0882-7974.18.2.332 [DOI] [PubMed] [Google Scholar]
- Wang D., Buckner R. L., Fox M. D., Holt D. J., Holmes A. J., Stoecklein S., Langs G., Pan R., Qian T., Li K., Baker J. T., Stufflebeam S. M., Wang K., Wang X., Hong B., & Liu H. (2015). Parcellating cortical functional networks in individuals. Nature Neuroscience, 18(12), Article 12. 10.1038/nn.4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss-Croft L. J., & Baldeweg T. (2015). Maturation of language networks in children: A systematic review of 22years of functional MRI. NeuroImage, 123, 269–281. 10.1016/j.neuroimage.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Wingfield A., & Stine-Morrow E. A. L. (2000). Language and speech. In The handbook of aging and cognition, 2nd ed (pp. 359–416). Lawrence Erlbaum Associates Publishers. [Google Scholar]
- Wolna A., Szewczyk J., Diaz M., Domagalik A., Szwed M., & Wodniecka Z. (2024). Domain-general and language-specific contributions to speech production in a second language: An fMRI study using functional localizers. Scientific Reports, 14(1), 57. 10.1038/s41598-023-49375-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolna A., Wright A., Lipkin B., & Fedorenko E. (2024). The extended language network. bioRxiv. [Google Scholar]
- Woolgar A., Parr A., Cusack R., Thompson R., Nimmo-Smith I., Torralva T., Roca M., Antoun N., Manes F., & Duncan J. (2010). Fluid intelligence loss linked to restricted regions of damage within frontal and parietal cortex. Proceedings of the National Academy of Sciences, 107(33), 14899–14902. 10.1073/pnas.1007928107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright P., Randall B., Marslen-Wilson W. D., & Tyler L. K. (2011). Dissociating Linguistic and Task-related Activity in the Left Inferior Frontal Gyrus. Journal of Cognitive Neuroscience, 23(2), 404–413. 10.1162/jocn.2010.21450 [DOI] [PubMed] [Google Scholar]
- Yang Y., Liang Z., Li G., Wang Y., Zhou Y., & Leventhal A. G. (2008). Aging affects contrast response functions and adaptation of middle temporal visual area neurons in rhesus monkeys. Neuroscience, 156(3), 748–757. 10.1016/j.neuroscience.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Zhang H., Bai X., & Diaz M. T. (2021). The intensity and connectivity of spontaneous brain activity in a language network relate to aging and language. Neuropsychologia, 154, 107784. 10.1016/j.neuropsychologia.2021.107784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., & Diaz M. T. (2023a). Resting State Network Segregation Modulates Age-Related Differences in Language Production. Neurobiology of Language, 4(2), 382–403. 10.1162/nol_a_00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., & Diaz M. T. (2023b). Task difficulty modulates age-related differences in functional connectivity during word production. Brain and Language, 240, 105263. 10.1016/j.bandl.2023.105263 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.