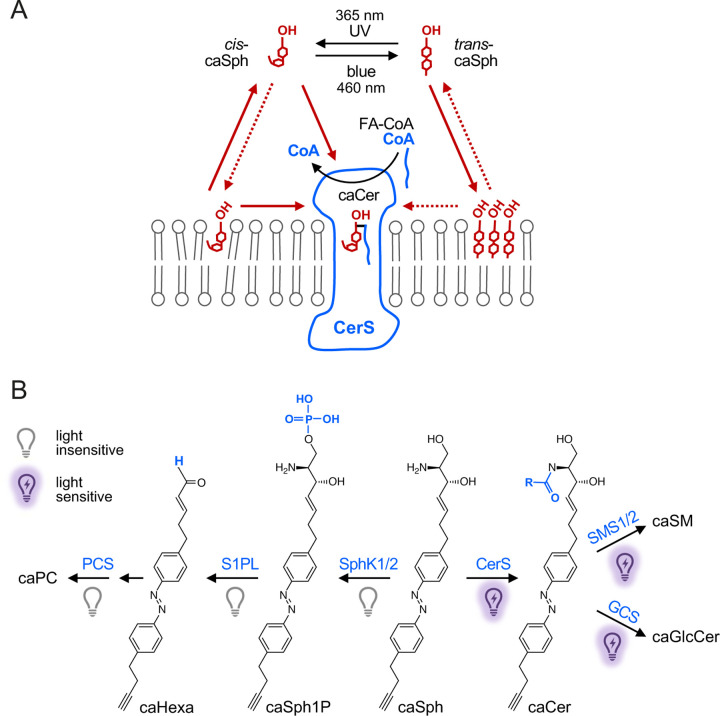
Figure 5. Model of how caSph enables optical control of ceramide biosynthesis.
(A) CerS preferentially metabolizes cis-isomers of caSph over trans-isomers to generate caCer using a CoA-conjugated fatty acid (CoA-FA). This isomer selectivity can be explained in several ways. For instance, a substrate with a bent carbon chain may be better accommodated in the Sph binding pocket of CerS than a substrate with a straight, rigid carbon chain. Alternatively, the flat azobenzene in trans-isomers may drive self-assembly of caSph molecules into tightly packed clusters through intermolecular stacking, thereby restricting their availability for metabolic conversion. Moreover, photo-isomerization affects the affinity of caSph for membranes as the highly-curved cis-isomer is more polar and harder to pack in the lipid bilayer than the straight trans-isomer. Sph may gain access to the active site of CerS by a head-first entry from the cytosol after CoA has left. According to this model, trans-isomers would face a higher energy barrier to reach the Sph binding pocket than cis-isomers because the former are tighter bound to the membrane bilayer. (B) Impact of light on the metabolic fate of caSph in cells. See main text for details.