Abstract
Background
Screening for co-infections with HIV, HSV-2 and Chlamydia trachomatis (CT) among high-risk human papilloma virus (hr-HPV) positive women remains essential in alleviating high morbidity of cervical cancer (CC). The aim of this study was to determine the prevalence of cervical intraepithelial neoplasia (CIN) among women referred for CC screening at a referral hospital in Kisumu County, Kenya; and to establish the role of co-infection on CIN.
Method
In a cross-sectional study, we collected HPV, HIV, HSV-2 and CT data, cervical cytology results, and demographic information from 517 referrals. Blood samples were obtained for HIV and HSV-2 tests; urine for CT test and cervical swabs for hr-HPV test.
Results
The overall prevalence of CIN was 18.4% (95/517) with CIN1 observed in 56(29.6%), CIN2 in 27(`14.3%), CIN3 + in 12(6.3%) and normal biopsy in 94(49.7%) of the patients out of which high grade CIN2 and above (CIN2+) was 7.54% (39/517) equivalent to 32.5 per 100,000 women per year. HPV/HIV co-infection (infected vs. uninfected: OR 2.79; 95% CI 1.56–5.10, p < 0.001); HPV/HSV-2 co-infection (infected vs. uninfected: OR 2.41, 95% CI: 1.12–5.46, p < 0.024); HPV/CT co-infection (infected vs. uninfected: OR 3.83; 95% CI 1.84–8.51, p < 0.001) were found to be significantly associated with CIN.
Conclusion
Overall prevalence of CIN was high in the region although high-grade CIN2 + remained relatively lower as reported earlier. Age factor, widowhood and co-infections with HIV, HSV-2 or Chlamydia trachomatis were associated with increased risk of testing positive for CIN.
Keywords: Co-infections, Chlamydia trachomatis, HPV, HSV-2, HIV, CIN, Kenya
Introduction
Cervical intraepithelial neoplasia (CIN), the precursor of cervical cancer remain the greatest threat to the reproductive health of many women especially those living in low income, high HIV burden countries in sub Saharan Africa[1]. Globally, the disease is ranked 4th in both incidence and cancer-related mortality amongst women with an estimated 660,000 new cases and 350,000 deaths annually, and accounts for about 13.1% of all new female cancers globally [1]. However, Kenya among other East African countries remains the most affected with an estimated age-standardized incidence and mortality rates of 40.1 and per 100,000 respectively [2]
Despite improved preventive strategies including public mobilization for early screening, reduced exposure to infectious agents through community awareness campaign, enhanced immunization to targeted age group, and a concerted campaign to reduce risky behaviors that promotes the spread of the disease [3], Kenya has continued to experience higher incidence and mortality of 5236 and 3211 respectively, annually [1] with prevalence in Kisumu estimated at 8.2% [4]. Besides, infection with HIV confers susceptibility to human papilloma virus (HPV), the causative agent of cervical cancer; and women infected with HIV are at high risk of contracting the disease [5]. Women infected with HIV experience a decline in both the number and function of CD4+ T cells leading to high rate of contracting HPV and reduction of chance for spontaneous clearance [6]. Although Kisumu continue to register high burden of HIV [7], the prevalence of CIN in the region remain scanty.
More importantly, high risk human papilloma virus (hr-HPV), particularly HPV types 16 and 18, remain the major causative agent of most malignant and premalignant lesions of the cervix, presenting nearly 99% cases of cervical cancer [3]. The virus penetrates the basal layers of epithelial cells through micro-abrasion of the transformation zone of the cervix using two oncogenes E6 and E7 [8], but which also interact with and inhibit various cell cycle-regulating protein such as retinoblastoma gene product pRB, and p53 protein leading to cervical dyskaryosis following a series of proteolytic degradations. In Kenya, about 9.1% women in the general population are estimated to harbor cervical HPV types 16 /18 infection that also contribute to more than 63% of all invasive cervical cancers in the Country [1]. Besides, higher prevalence of 9.9% have been reported among HIV-infected women in Kisumu [9] suggesting the likelihood of higher burden of CIN in the region.
Moreover, co-infections with herpes simplex virus type 2 (HSV-2) serve as an independent predictor for cervical cancer owing to its role in facilitating HIV acquisition and transmission among sexually active community [10]. Precisely, the virus evolved strategies that counteract caspase activation and apoptosis by encoding anti-apoptotic viral proteins such as ribonucleotide reductase large subunit (R1) [11] leading to the persistence of HPV within the cervix. Although high prevalence of HIV / HSV-2 co-infection have been reported among the local fishing community [12], the role of HSV-2 / HPV co-infection in CIN remain scanty. Equally, Chlamydia trachomatis (CT) like other intracellular pathogens have been shown to possess the potential of altering gene expression and protein production in cervical basal cells leading to induction of host genome duplicate that results in aneuploidy and chromosome instability [13]. As Chlamydia trachomatis induced DNA double-strand breaks, it simultaneously inhibits proper DNA damage response and repair mechanisms rendering host cells prone to loss of genetic integrity and transformation [14]. Although higher prevalence of HIV / Chlamydia trachomatis co-infection have been reported in the region [15] the role of co-infections on CIN is scanty.
Elsewhere, studies have shown that hormonal contraceptives use increase the risks of developing cervical cancer especially when used for longer period of time [16]. Precisely, the upstream regulatory region (URR) of HPV 16 genome that mediate transcriptional control of HPV genes contains promoter elements that are activated by persistent interaction with steroid hormones which binds to specific glucocorticoid-response receptors within the HPV and enhance the expression of E6 and E7; which in turn bind to and degrade p53 gene product, leading to a apoptotic failure and the development of CIN [17]. Locally, adolescent girls and young women form the majority of contraceptives users with higher preference being injectable and implants [18]. We examined the influence of hormonal contraceptives on CIN prevalence in the region.
Method
Study design:
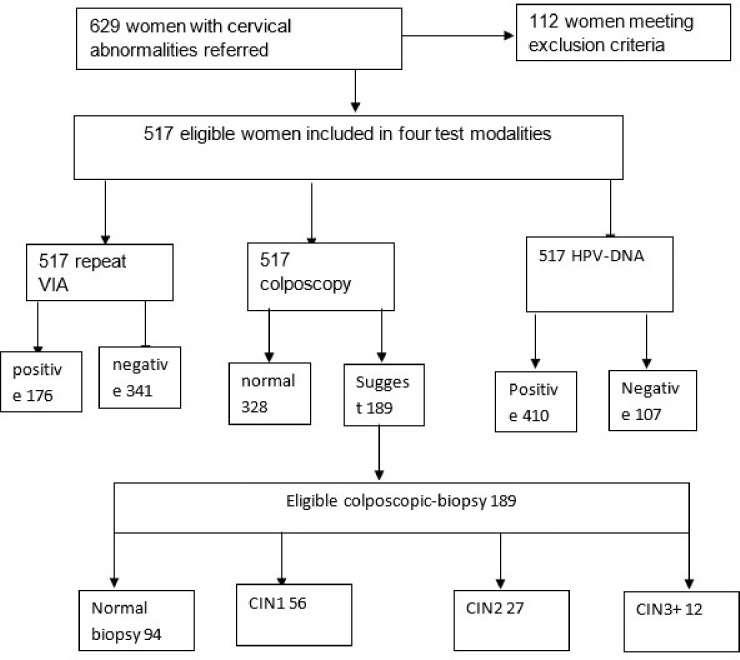
In a cross-sectional study, 517 women aged 25 to 65 years referred to Jaramogi Oginga Odinga Teaching and Referral Hospital (JOOTRH) from peripheral facilities with vaginal or cervix abnormalities between the years 2021 to 2023 were consecutively enrolled in the study after excluding 112 due to insufficient cytological samples (Fig. 1). Eligibility included: (1) women with a history of sexual activity, (2) initial visual inspection with acetic acid positive result from referral facilities and (3) informed consent. The exclusion criteria included women: (1) who were pregnant; (2) who had vaginal medication 2 days prior to the screening day (3) those with hysterectomy, muco-purulent discharge, active virginal bleeding. Nurses in the cervical screening clinic identified potential participants who attended the clinic and explained to them details of the study. Written informed consent was obtained in Kiswahili or English before study enrollment. Clinical examination involved a gynecological examination with inspection of the cervix uteri and collection of specimens by a gynecologist in a separate room. Cervical sample were collected for HPV DNA testing in PreservCyt® Solution (Hologic) using the Cervex-Brush® (Rover). The sample was then stored at ambient temperature until tested. Finally, the cervix was examined after the application of 5% acetic acid (VIA) and the findings recorded in datasheet. All women were informed about the VIA result immediately, but for laboratory results, they were booked to collect after one month in the next clinic review visit.
Figure 1.
Flowchart of participants in the study
Laboratory testing:
HIV infection was tested using Determine HIV-1/2 and confirmed with First Response HIV1/2 card tests; HSV-2 was tested using HerpeSelect-2 enzyme immunoassay; HPV tested using Gene Xpert HPV assay (Cepheid, Sunnyvale, California, United States [US]). Gene Xpert HPV is based on a multiplex real-time PCR targeting E6 and E7 oncogenes of 14 HR-HPV genotypes. The amplification was performed in five fluorescent channels that identifies five groups namely; HPV16, HPV18/45, HPV31/33/35/52/58, HPV51/59, and HPV39/56/66/68, and the results interpreted using GeneXpert software version 4.8 (Cepheid). Chlamydia trachomatis was tested using Gene Xpert CT/NG assay (Cepheid, Sunnyvale, California, United States [US]) with primers and probes for the detection of specific chromosomal sequence (serovariants D-K) expressed by the ompA gene of the bacteria in urine sample; while biopsy was tested using hematoxylin and eosin stains.
Data collection
As enrollment continues, information was collected from participants on socio-demographic status and relevant sexual and reproductive health issues including HIV and ART status, contraceptive use, types and duration and the number of children. Study information was collected electronically except consent forms, a copy of which was issued to the participants after signing. The consent forms with participants’ signatures and national ID numbers were collected and secured in locked file cabinets. Labels of laboratory sample were handwritten and contained a computer-generated subject identifier and sample date.
Statistical analysis
Continuous variables were summarized using mean and standard deviation. Categorical variables were summarized using percentages. Prevalence of CIN, HPV, HIV and HSV-2 positivity were calculated overall. A chi-square test was used to compare proportions within-group and logistic regression to estimate associations between demographic characteristics and co-infections and the prevalence of CIN.
Ethics considerations
The study approval was obtained from the local review board at Maseno University and JOOTRH Kisumu, Kenya.
Results
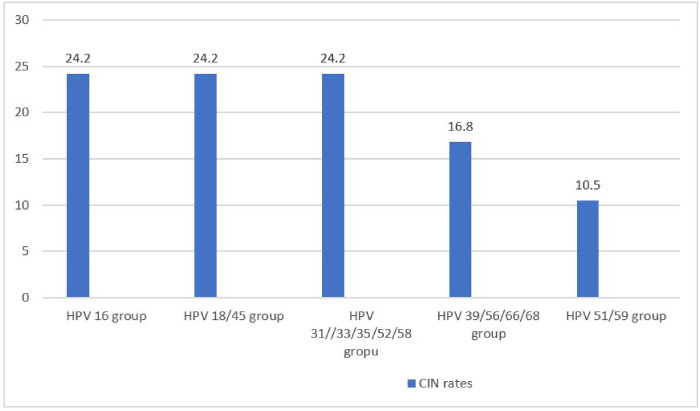
Of the 517 eligible participants, the mean age standard deviation was 34.65 ± 7.45, age range 22–57 years with a median (IQR) of 33 (28, 39) of whom majority 456(88%) were married with children ranging 3 to 4, and implant (34.4%) and injectable (39.3%) were the most preferred contraceptives with a duration median (IQR) of 11 (7, 5) years (Table 1). Among the participants examined, 123(24%), 63(12%), 48(8.9%) and 410(79%), tested positive for HIV, CT, HSV-2 and hr-HPV infections respectively. All colposcopy suggestive of CIN were further subjected to colposcopically guided biopsy for histology examination. Altogether, 189 biopsy specimens were processed and examined, out of which CIN1 were 56(29.6%); CIN2 were 27(14.3%); CIN3 + were 12(6.3%); while normal cervix were 94(49.7%). Overall, the prevalence of CIN was 18.4% (95/517) of which high grade CIN2 and above (CIN2+) was 7.54% (39/517) equivalent to 32.5 per 100,000 women per year. Of the 95 women positive for CIN lesions, hr-HPV subgoup16 and subgroup 18/45 were the most common (Fig. 2)
Table 1.
Demographic and clinical characteristics of study participants
| Histological diagnosis | ||||||
|---|---|---|---|---|---|---|
| Variable | Overall, N = 5171 | CIN1, N = 561 | CIN2, N = 271 | CIN3+, N = 121 | normal, N = 941 | unavailable, N = 3281 |
| Age | 33 (28, 39) | 38(34,46) | 46(39,48) | 43(39,4) | 37(32,42) | 30 (27, 36) |
| Marital status | ||||||
| Married | 456 (88%) | 46 (82%) | 18 (67%) | 10 (83%) | 89 (95%) | 293 (89%) |
| Single | 35 (6.8%) | 1 (1.8%) | 0 (0%) | 1(8.3%) | 3 (3.2%) | 30 (9.1%) |
| Widow | 26 (5.0%) | 9 (16%) | 9 (33%) | 1(8.3%) | 2 (2.1%) | 5 (1.5%) |
| Parity | 3.0(3.0,4.0) | 4.0(3.0,4) | 4.0(4.0,5) | 4.0(3.0,5.0) | 4.0(3.0,5) | 3.0(3.0, 4.0) |
| Contraceptive's type | ||||||
| Hormonal-IUD | 29 (5.6%) | 4 (7.1%) | 3 (11%) | 0 (0%) | 2 (2.1%) | 20 (6.1%) |
| Implant Jadele / Norplant | 179 (35%) | 24 (43%) | 8 (30%) | 3 (25%) | 36 (38%) | 108 (33%) |
| injectable /DepProvera | 202 (39%) | 18 (32%) | 6 (22%) | 5 (42%) | 43 (46%) | 130 (40%) |
| None | 50 (9.7%) | 9 (16%) | 10 (37%) | 3 (25%) | 9 (9.6%) | 19 (5.8%) |
| Oral pill | 57 (11%) | 1 (1.8%) | 0 (0%) | 1 (8.3%) | 4 (4.3%) | 51 (16%) |
| Duration (Yrs.) | 11 (7, 15) | 13(11,17) | 13 (0, 17) | 15 (8,18) | 13(10,19) | 10 (7, 13) |
| VIA | ||||||
| negative | 341 (66%) | 35 (63%) | 11(41%) | 5 (42%) | 58 (62%) | 232 (71%) |
| positive | 176 (34%) | 21 (37%) | 16(59%) | 7 (58%) | 36 (38%) | 96 (29%) |
| HPV test | ||||||
| negative | 107 (21%) | 3 (5.4%) | 2 (7.4%) | 1 (8.3%) | 12(12.8) | 89 (27%) |
| positive | 410 (79%) | 53(94.6) | 25(92.6) | 11(91.7%) | 82 (87%) | 239 (73%) |
| HIV status | ||||||
| negative | 394 (76%) | 22 (39%) | 12(44%) | 6 (50%) | 63 (67%) | 291 (89%) |
| positive | 123 (24%) | 34 (61%) | 15(56%) | 6 (50%) | 31 (33%) | 37 (11%) |
| HSV-2 | ||||||
| negative | 471(91%) | 40(71%) | 23(85%) | 9(75%) | 83(88%) | 316(96%) |
| positive | 46(8.9%) | 16(29%) | 4(15%) | 3(25%) | 11(12%) | 12(3.7%) |
| Chlamydia | ||||||
| Negative | 454 (88%) | 35 (63%) | 20(74%) | 8 (67%) | 83 (88%) | 308 (94%) |
| Positive | 63 (12%) | 21 (38%) | 7 (26%) | 4 (33%) | 11 (12%) | 20 (6.1%) |
CIN1, CIN2, CIN3: Cervical intraepithelial neoplasia 1, 2 or3, HSV2: herpes simplex virus 2,
Median (IQR); n (%).
Figure 2.
Distribution rate of CIN among hr-HPV subgroups (n = 95)
Correlates associated with CIN positivity
In a Univariate analysis, the study found women aged 30 years and below (OR 0.55; 95% CI 0.22–1.31, p < 0.001) were 0.5 times less likely to test positive for CIN as compared to women above. 30 years. Besides, widows (OR 11.4; 95% CI 3.18–73.2, p < 0.001) were 11.4 times more likely to test positive for CIN, while single women [OR 0.80; 95% CI 0.10–4.96, p = 0.300) were 0.80 times less likely to test positive for CIN as compared to married women. Women co-infected with HIV (OR = 2.79; 95% CI 1.56–5.10, p < 0.001); were 2.8 times more likely to test positive for CIN as compared to HIV uninfected counter parts. Additionally, women co-infected with HSV-2 (OR = 2.41, 95% CI: 1.12–5.46, p < 0.024); were 2.4 times more likely to test positive for CIN as compared to HSV-2 uninfected counter parts. Women co-infected with Chlamydia trachomatis (OR = 3.83; 95% CI 1.84–8.51, p < 0.001); were 3.8 times more likely to test positive for CIN as compared to uninfected counter parts (Table 2)
Table 2.
Correlates associated with CIN positivity by demographic and clinical characteristics
| Univariate | Multivariable | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | Event N | OR1 | 95% CI1 | p-value | OR1 | 95% CI1 | p-value |
| Age category | 189 | 95 | 0.18 | |||||
| > 30 years | ref. | – | ref. | – | ||||
| ≤ 30 years | 0.55 | 0.22–1.31 | 0.20 | 0.08–0.47 | < 0.001 | |||
| Marital status | 189 | 95 | < 0.001 | |||||
| Married | ref. | – | ref. | – | ||||
| Single | 0.80 | 0.10–4.96 | 2.55 | 0.35–12.3 | 0.303 | |||
| Widow | 11.4 | 3.18–73.2 | 12.3 | 3.70–46.5 | < 0.001 | |||
| parity | 189 | 95 | 0.460 | |||||
| > 4 children | ref. | – | ||||||
| ≤ 4 children | 0.79 | 0.42–1.47 | ||||||
| Contraceptives types | 189 | 95 | 0.017 | |||||
| Hormonal-IUD | ref. | – | ref. | – | ||||
| Implant Jadele / Norplant | 0.28 | 0.04–1.24 | 1.79 | 0.57–6.43 | 0.302 | |||
| injectable / Depoprovera | 0.19 | 0.03–0.86 | 1.24 | 0.39–4.42 | 0.700 | |||
| None | 0.70 | 0.09–3.60 | 1.35 | 0.33–5.85 | 0.711 | |||
| Oral pill | 0.14 | 0.01–1.27 | 0.80 | 0.10–4.92 | 0.801 | |||
| Contraceptive duration | 189 | 95 | 0.700 | |||||
| > 20 | ref. | – | ||||||
| ≤ 20 years | 0.86 | 0.38–1.92 | ||||||
| HIV status | 189 | 95 | < 0.001 | |||||
| negative | ref. | – | ref. | – | ||||
| positive | 2.79 | 1.56–5.10 | 4.45 | 2.53–7.92 | < 0.001 | |||
| HSV2 | 189 | 95 | 0.024 | |||||
| Negative | ref. | – | ref. | – | ||||
| Positive | 2.41 | 1.12–5.46 | 5.67 | 2.61–12.4 | < 0.001 | |||
| Chlamydia | 189 | 95 | < 0.001 | |||||
| Negative | ref. | – | ref. | – | ||||
| Positive | 3.83 | 1.84–8.51 | 6.03 | 3.00–12.2 | < 0.001 | |||
Regression analysis.
OR = Odds Ratio; CI = Confidence Interval; ref = reference; HSV-2, Herpes simplex virus-2; HPV, Human papilloma virus.
DISCUSSION
The present study was set to determine the prevalence and determinants of CIN among women in Kisumu County, Kenya. Although continuous monitoring and reporting on prevalence of CIN is essential for estimating the risk of developing cervical cancer and optimizing screening strategy for early detections and treatment [3] this is the first study to comprehensively examine the carriage rate of CIN exclusively in HIV endemic region of western Kenya.
Findings of the study revealed that the overall prevalence of cervical intraepithelial neoplasia (CIN) was 18.4% (95/517) of which high grade CIN2 and above (CIN2+) was 7.54% (39/517) equitable to approximately 32.5 per 100,000 women per year; relatively lower than the national incidence of 40.1 per 100, 000 women per year [2],as well as neighbourhood Uganda (56.2 per 100,000 women per year) [19] and Tanzania (54.0 per 100, 000 women per year) [20]. Although previous estimate had reported higher prevalence of 27.9% severe dysplasia in the same region [21] as well as 21.4% prevalence in Nairobi County [22], our finding was within the range of 3.7% – 22.6% reported around Africa [23] and comparable to 8.2% prevalence report earlier by Mungo et al in the same region [4] suggesting the disease could be widespread locally. This finding is a reflection of high HPV prevalence of 9.1% in the general population [1] as well as 51.1% prevalence among sexually active men of Kisumu County [24] majorly attributed to HPV subtypes 16 and 18 [25]. In this study, hr-HPV subgroup 16 and 18/45 were the most prevalent and major cause of CIN in the region contributing to nearly half of all new cases detected. Higher prevalence of HPV genotype 16 have been reported earlier in the same region [5] as well as in Nairobi [22] thus affirming subtype 16 as key player in CIN prevalence locally.
Analysis of risk determinants revealed that women aged 30 years and below were less likely to test positive for CIN compared to their counterpart above 30 years. However, widows were ten times more likely to test positive compared to their married counterpart. Studies have shown that being married is associated with early diagnosis of CIN and a more favorable prognosis for cervical cancer [26] possibly owing to partner support in regular clinic visit for screening and treatment. In this study, we found age factor and widowhood significantly associated with higher Odds of developing CIN. Similar observations have been recorded by Orang’o and Pinheiro [5, 26] although early onset of CIN among teenagers have also been observed in the region, possibly attributed to changes in diet, lifestyle, obesity, environment and the microbiome all of which interact with genomic and genetic susceptibilities [27]. However, parity and hormonal contraceptive use were found not associated with CIN.
Further analysis of risk factors revealed that women co-infected with HIV were twice more likely to test positive for CIN compared to their HIV-uninfected counterparts suggesting that, co-infection with HIV is independently and significantly associated with higher Odds of developing CIN. Similar observation have been reported by Perez-Gonzalez et al and Sosso et al all noted that HPV infection was common among people living with HIV (PLWH) who were at greater risk of developing CIN [6, 28]. Indeed, PLWH have been shown to experience increased risk of persistent HPV infection including high viral load essential for CIN aetiology [29] more so when co-infection involve multiple HPV genotypes [22]. Few studies have explored the relationship between HSV-2 co-infection and the severity of cervical lesion. In our study, women co-infected with HSV-2 were twice more likely to test positive for CIN compared to their HSV-2-uninfected counterparts suggesting that co-infection with HSV-2 is significantly associated with higher Odds of developing CIN. It’s good to note that HSV-2 co-infection has been incriminated with the initiation of oncogenic processes that are eventually picked by HPV in driving cervical cancer development [30]. Our finding corroborated a systematic review and a meta-analysis by Zhang et al [31] showing that women co-infected with HSV-2 were 3 times more likely to test positive for CIN. Elsewhere, in another systematic review, higher frequency of HSV-2 was observed among women experiencing invasive cervical cancer [30] suggesting a possible association that could potentially be attributed to shared immunological compromise, genetic predisposition, or lifestyle factors. In other studies, it has been suggested that genital HSV-2 infection possibly act in conjunction with HPV infection to increase modestly the risk of cervical intraepithelial neoplasia [32] of which investigation is still in progress.
Women co-infected with Chlamydia trachomatis (CT) were three times more likely to test positive for CIN compared to their CT-uninfected counterparts suggesting that co-infection with CT is equally associated with higher Odds of developing CIN. Again, this was in concurrence with a finding by Lu et al., showing prevalence of 21.8% low grade squamous intraepithelial lesions (LSIL) and 10.8% high grade squamous intraepithelial lesions (HSIL) among women co-infected with CT / HPV [33], and consistent with another study from neighborhood Uganda recording a significant association between CT / HPV co-infection and the development of LSIL [23]. More importantly, studies have shown that co-infection with CT supports HPV persistence by suppressing the functions of Langerhans cells (LCs) pathways which are involved in the regulation of immune responses. Besides, the infection impairs LC functions by reducing the antigen-presenting ability and density of LCs; alter T-cell subsets, resulting in fewer CD4 + and CD8 + T cells and more infiltrating Tregs; decreases the CD4+/CD8 + T cell ratio to below 1; and induces greater T lymphocytes’ apoptosis, hence impairing cell-mediated immunity and accelerating the progression of CIN [33]. In Rome Italy, women co-infected with CT / HPV were found to experience high frequency of high grade cervical lesions compared to their counterparts infected with HPV only [34] suggesting the important role played by CT in cervical carcinogenesis.
It is also good to note that previous local studies have mainly focused on the relationship between cervical cytology results and sexually transmitted pathogens majorly HIV and HPV [5, 22]. Conversely, cervical cytology results do not adequately represent the true picture of the cervix. Instead, cervical biopsy provides the gold standard for assessing cervical abnormalities, highlighting the need for assessing the relationship between sexually transmitted pathogens, HPV infection, and histological findings. In our study, we found a strong association between HIV, CT and HSV-2 co-infection with hr-HPV and prevalence of CIN. This study had some limitations. First, the participants were recruited from a gynecological clinic having been referred from peripheral facilities with either vaginal or cervical abnormalities. This facility-based recruitment coupled with structural inefficiency in some rural setting that conveyed referrals limit the generalization of the study findings.
Conclusion
The overall prevalence of CIN was high in the region although high-grade CIN2 and above (CIN2+) remained relatively lower as reported earlier. Age factor, widowhood and co-infections with HIV, HSV-2 or Chlamydia trachomatis were associated with increased risk of testing positive for CIN, but not parity or hormonal contraceptives use.
Acknowledgments
We thank our study participants and staffs of JOOTRH, Kenya for their participation in sample collection and providing institutional approval of the study. We equally appreciate Sustainable Development for Health on HIV (SD4H) program of Maseno University for supporting review of manuscript through organized workshop mentorship program; more so in statistical data analysis as provided by Prof. Lucas Othuon. More thanks to Dr. Thomas Ongalo and Mr. Felix Humwa for additional statistical analysis.
Funding
Research reported in this publication was supported in part by Fogarty International Center of the National Institute of Health (NIH) under Award Number D43TW011306. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health (NIMH). The NIMH had no role in the design and conduct of the study; collection, management, analysis, interpretation of data, review or approval of the manuscript; and decision to submit the manuscript for publication.
Abbreviations
- CI
confidence interval
- SD
standard deviation
- VL
viral load
- CT
Chlamydia Trachomatis
- CC
Cervical Cancer
- CIN 1, 2, 3
Cervical Intraepithelial Neoplasia (Grade 1, Grade 2, Grade 3)
- ICO-IARC
Catalan Institute of Oncology / International Agency for Research on Cancer
- HPV
Human Papilloma Virus
- HIV
Human Immunodeficiency virus
- HR-HPV
High Risk Human Papilloma Virus
- JOOTRH
Jaramogi Oginga Odinga Teaching and Referral Hospital
Footnotes
Competing interests
The authors declare no competing interests
Supplementary Information
The dataset used or analyzed during the current study are available from the corresponding author on reasonable request
Ethics approval and consent to participate
The institutional review board of JOOTRH provided ethics approval of the study number ERC.IB/VOL.1/602. Written informed consent was obtained from all participants.
Consent for publication
Consent was not needed as we used de-indentified data.
Contributor Information
Calleb George Onyango, Maseno University.
Lilian Ogonda, Maseno University.
Bernard Guyah, Maseno University.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request
References
- 1.ICO/IARC. Human Papillomavirus and Related Diseases Report [Internet]. 2023; http://www.hpvcentre.net/
- 2.Hassan MA, Itsura P, Odongo BE. Colposcopic and Histopathologic Comparative Interpretations Among Patients Undergoing Evaluation for Cervical Dysplasia in Western Kenya. EMJ Repro Health [Internet] 2024. [cited 2024 Aug 17]; https://www.emjreviews.com/reproductive-health/article/colposcopic-and-histopathologic-comparative-interpretations-among-patients-undergoing-evaluation-for-cervical-dysplasia-in-western-kenya/
- 3.Mwenda V, Mburu W, Bor JP, Nyangasi M, Arbyn M, Weyers S et al. Cervical cancer programme, Kenya, 2011–2020: lessons to guide elimination as a public health problem. ecancer [Internet] 2022. [cited 2024 Aug 14];16. https://ecancer.org/en/journal/article/1442-cervical-cancer-programme-kenya-2011-2020-lessons-to-guide-elimination-as-a-public-health-problem [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mungo C, Omoto J, Gwer S, Wameyo A, Kays M, Ganda G. Toward Cervical Cancer Elimination: Evaluation of Access to Diagnostic Services After Referral to a Specialist Gynecologist Clinic at a Major Referral Hospital in Kisumu, Kenya. JCO Global Oncol. 2020;6:35–35. [Google Scholar]
- 5.Orang’o EO, Were E, Rode O, Muthoka K, Byczkowski M, Sartor H, et al. Novel concepts in cervical cancer screening: a comparison of VIA, HPV DNA test and p16INK4a/Ki-67 dual stain cytology in Western Kenya. Infect Agents Cancer. 2020;15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pérez-González A, Cachay E, Ocampo A, Poveda E. Update on the Epidemiological Features and Clinical Implications of Human Papillomavirus Infection (HPV) and Human Immunodeficiency Virus (HIV) Coinfection. Microorganisms. 2022;10:1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.UNAIDS. The Urgency of Now: AIDS at a Crossroads — 2024 global AIDS update. 2024.
- 8.García C, Hernández-García D, Valencia C, Rojo-León V, Pérez-Estrada JR, Werner M, et al. E6/E7 oncogenes in epithelial suprabasal layers and estradiol promote cervical growth and ear regeneration. Oncogenesis. 2017;6:e374–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chachage M, Parikh AP, Mahenge A, Bahemana E, Mnkai J, Mbuya W, et al. High-risk human papillomavirus genotype distribution among women living with and at risk for HIV in Africa. AIDS. 2023;37:625–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li M, Nyabigambo A, Navvuga P, Nuwamanya E, Nuwasiima A, Kaganda P, et al. Acceptability of cervical cancer screening using visual inspection among women attending a childhood immunization clinic in Uganda. Papillomavirus Res. 2017;4:17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu X, He S. The interplay between human herpes simplex virus infection and the apoptosis and necroptosis cell death pathways. Virol J. 2016;13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macharia VM, Ngugi C, Lihana R, Ngayo MO, Transmitted. HIV-1 Drug resistance and the Role of Herpes Simplex Virus – 2 Coinfection among Fishermen along the Shores of Lake Victoria, Kisumu, Kenya. J HIV Retrovirus [Internet] 2016. [cited 2024 Aug 14];02. http://hiv.imedpub.com/transmitted-hiv1-drug-resistance-and-the-role-of-herpes-simplex-virus-2-coinfection-among-fishermen-along-the-shores-of-lake-victo.php?aid=17345 [Google Scholar]
- 13.Arcia Franchini AP, Iskander B, Anwer F, Oliveri F, Fotios K, Panday P et al. The Role of Chlamydia Trachomatis in the Pathogenesis of Cervical Cancer. Cureus [Internet] 2022. [cited 2024 Aug 14]; https://www.cureus.com/articles/70826-the-role-of-chlamydia-trachomatis-in-the-pathogenesis-of-cervical-cancer [DOI] [PMC free article] [PubMed]
- 14.Mi Y, Gurumurthy RK, Zadora PK, Meyer TF, Chumduri C. Chlamydia trachomatis Inhibits Homologous Recombination Repair of DNA Breaks by Interfering with PP2A Signaling. Volume 9. mBio; 2018. pp. e01465–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nyakambi M, Waruru A, Oladokun A. Prevalence of genital Chlamydia trachomatis among women of reproductive age attending outpatient clinic at Kisumu County Referral Hospital, Kenya, 2021. J Public Health Afr. 2022;13:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roura E, Travier N, Waterboer T, De Sanjosé S, Bosch FX, Pawlita M, et al. The Influence of Hormonal Factors on the Risk of Developing Cervical Cancer and Pre-Cancer: Results from the EPIC Cohort. PLoS ONE. 2016;11:e0147029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadducci A, Cosio S, Fruzzetti F. Estro-progestin Contraceptives and Risk of Cervical Cancer: A Debated Issue. Anticancer Res. 2020;40:5995–6002. [DOI] [PubMed] [Google Scholar]
- 18.MoH. Kenya Demographic and Health Survey 2022. Key Indicators Report. 2023. [Google Scholar]
- 19.Kabanda R, Kiconco A, Ronald A, Beyer KMM, John SA. Correlates of intention to screen for cervical cancer among adult women in Kyotera District, Central Uganda: a community based cross-sectional study. BMC Women’s Health. 2024;24:296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okyere J, Ayebeng C, Dosoo AK, Dickson KS. Cervical cancer screening among women with comorbidities: evidence from the 2022 Tanzania demographic and health survey. BMC Public Health. 2024;24:1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khozaim K, Orang’o E, Christoffersen-Deb A, Itsura P, Oguda J, Muliro H, et al. Successes and challenges of establishing a cervical cancer screening and treatment program in western Kenya. Intl J Gynecol Obste. 2014;124:12–8. [DOI] [PubMed] [Google Scholar]
- 22.Kangethe JM, Gichuhi S, Odari E, Pintye J, Mutai K, Abdullahi L et al. Confronting the human papillomavirus–HIV intersection: Cervical cytology implications for Kenyan women living with HIV. South. Afr. j. HIV med. [Internet] 2023. [cited 2024 Aug 14];24. https://sajhivmed.org.za/index.php/hivmed/article/view/1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ssedyabane F, Amnia DA, Mayanja R, Omonigho A, Ssuuna C, Najjuma JN, et al. HPV-Chlamydial Coinfection, Prevalence, and Association with Cervical Intraepithelial Lesions: A Pilot Study at Mbarara Regional Referral Hospital. J Cancer Epidemiol. 2019;2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith JS, Backes DM, Hudgens MG, Bailey RC, Veronesi G, Bogaarts M, et al. Prevalence and risk factors of human papillomavirus infection by penile site in uncircumcised Kenyan men. Intl J Cancer. 2010;126:572–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Onyango CG, Ogonda L, Guyah B, Shiluli C, Ganda G, Orang’o OE, et al. Novel biomarkers with promising benefits for diagnosis of cervical neoplasia: a systematic review. Infect Agents Cancer. 2020;15:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El Ibrahimi S, Pinheiro PS. The effect of marriage on stage at diagnosis and survival in women with cervical cancer: Marriage and cervical cancer stage and survival. Psycho-oncology. 2017;26:704–10. [DOI] [PubMed] [Google Scholar]
- 27.Ugai T, Sasamoto N, Lee HY, Ando M, Song M, Tamimi RM, et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat Rev Clin Oncol. 2022;19:656–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sosso SM, Tchouaket MCT, Fokam J, Simo RK, Semengue ENJ, Sando Z, et al. Human papillomavirus positivity and cervical lesions in relation to HIV infection: a comparative assessment in the Cameroonian female population. J Public Health Afr. 2023;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon S, Wusiman A, Boily MC, Kariisa M, Mabeya H, Luchters S, et al. Epidemiology of HPV Genotypes among HIV Positive Women in Kenya: A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0163965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sausen D, Shechter O, Gallo E, Dahari H, Borenstein R. Herpes Simplex Virus, Human Papillomavirus, and Cervical Cancer: Overview, Relationship, and Treatment Implications. Cancers. 2023;15:3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H, Cai S, Xia Y, Lin Y, Zhou G, Yu Y, et al. Association between human herpesvirus infection and cervical carcinoma: a systematic review and meta-analysis. Virol J. 2023;20:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Y, Cao X, Zheng Y, Tang J, Cai W, Wang H, et al. Relationship between cervical disease and infection with human papillomavirus types 16 and 18, and herpes simplex virus 1 and 2. J Med Virol. 2012;84:1920–7. [DOI] [PubMed] [Google Scholar]
- 33.Lu Y, Wu Q, Wang L, Ji L. Chlamydia trachomatis enhances HPV persistence through immune modulation. BMC Infect Dis. 2024;24:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancini F, Vescio F, Mochi S, Accardi L, di Bonito P, Ciervo A. HPV and Chlamydia trachomatis coinfection in women with Pap smear abnormality: baseline data of the HPV Pathogen ISS study. Le Infezioni in Medicina 2018;2. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request