Abstract
PURPOSE
The KUNPENG study aimed to evaluate the efficacy and safety of vebreltinib (also known as bozitinib, APL-101, PLB-1001, and CBT-101), a potent and highly selective inhibitor of c-mesenchymal-epithelial transition (MET), in patients with locally advanced or metastatic non–small cell lung cancer (NSCLC) harboring c-Met alterations.
METHODS
This multicenter, multicohort, open-label, single-arm, phase II trial enrolled patients with c-Met dysregulated, locally advanced or metastatic NSCLC from January 2020 to August 2022 across 17 centers. Cohort 1 included patients with MET exon 14 skipping (METex14)–mutant NSCLC who had not previously received MET inhibitors. Participants were administered vebreltinib at a dosage of 200 mg twice a day in 28-day cycles. The primary end point was the objective response rate (ORR), and the key secondary end point was the duration of response (DoR), both evaluated by a blinded independent review committee according to the RECIST version 1.1.
RESULTS
As of August 9, 2022, 52 patients had been enrolled in cohort 1, of whom 35 (67.3%) were treatment-naïve. The ORR reached 75% (95% CI, 61.1 to 86). Among treatment-naïve patients, the ORR was 77.1% (95% CI, 59.9 to 89.6), and in previously treated patients, it was 70.6% (95% CI, 44.0 to 89.7). The disease control rate was 96.2%, with a median DoR of 15.9 months, a median progression-free survival of 14.1 months, and a median overall survival of 20.7 months. The most common treatment-related adverse events were peripheral edema (82.7%), QT prolongation (30.8%), and elevated serum creatinine (28.8%).
CONCLUSION
Vebreltinib has shown promising efficacy and a favorable safety profile in patients with METex14-mutant NSCLC.
Vebreltinib showed promising efficacy and a favorable safety profile for METex14-mutant NSCLC.
INTRODUCTION
The past decade has witnessed substantial progress in the development of targeted therapies for non–small cell lung cancer (NSCLC), specifically tailored to unique genetic aberrations.1-3 Among these, alterations in the mesenchymal-epithelial transition (MET) gene are crucial for oncogenesis, encompassing MET exon 14 skipping (METex14) mutations, gene amplification, gene fusion, and protein overexpression.4 Significantly, METex14 mutations are identified as an independent prognostic factor associated with diminished survival rates in patients with NSCLC.5,6
CONTEXT
Key Objective
Can vebreltinib, a potent and highly selective inhibitor of c-mesenchymal-epithelial transition (MET), provide an effective and safe treatment option for patients with non–small cell lung cancer (NSCLC) with MET exon 14 skipping (METex14) mutations?
Knowledge Generated
Vebreltinib achieved notable objective response rates and a sustained duration of response in patients with METex14-positive NSCLC, regardless of previous treatment, baseline brain metastasis, mutation site, and alteration type. The safety of vebreltinib was acceptable and generally manageable.
Relevance (T.E. Stinchcombe)
Vebreltinib demonstrated activity and an acceptable rate of adverse events. These preliminary results justify additional studies.*
*Relevance section written by JCO Associate Editor Thomas E. Stinchcombe, MD.
In pursuit of more effective treatments for patients with NSCLC harboring the METex14 alteration, spanning both previously treated and treatment-naïve cohorts, a range of tyrosine kinase inhibitors (TKIs) have yielded objective response rates (ORRs) between 49.2% and 68%.7-10 However, these agents are linked with a notable frequency of treatment-related adverse events (TRAEs) of grade 3 or higher, recorded at 34.8% for tepotinib, 37.6% for capmatinib, 46% for savolitinib, and 54% for gumarontinib.7,8,10,11 This underscores the imperative for treatment options that balance efficacy with a manageable safety profile.
Vebreltinib (also known as bozitinib, APL-101, PLB-1001, and CBT-101) stands out as a highly selective c-Met inhibitor. Its preclinical efficacy across various in vivo models heralds its potential as a potent antitumor agent.12 Preliminary phase I study outcomes revealed an ORR of 66.7% alongside a tolerable safety profile in patients with advanced METex14-mutant NSCLC.13 Leveraging these insights, the KUNPENG phase II study is designed to further investigate vebreltinib's therapeutic efficacy and safety in the treatment of patients with locally advanced or metastatic NSCLC harboring METex14 mutation.
METHODS
Study Design and Participants
This multicenter, multicohort, open-label, single-arm, phase II trial evaluated vebreltinib in patients with locally advanced or metastatic NSCLC characterized by c-Met dysregulation. Cohort 1 targeted patients with advanced NSCLC harboring METex14 mutations, naïve to c-Met inhibitor therapies. Cohorts 2 and 3 consisted of patients with c-Met amplification, who were either unsuitable for, had failed, or declined standard chemotherapy, without previous c-Met inhibitor therapy. Cohort 4 focused on patients with METex14 mutations who exhibited resistance after initial benefits from c-Met inhibitors. This manuscript details the results of vebreltinib in cohort 1.
Eligibility criteria included being 18 years or older, having stage IIIB-IV NSCLC confirmed histologically or cytologically, lacking epidermal growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase (ALK) and ROS1 rearrangements, and KRAS mutations; possessing METex14 mutations as verified by next-generation sequencing (NGS); maintaining an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1; demonstrating adequate organ function; and no previous treatment with c-Met inhibitors. Key exclusion criteria were previous therapy targeting MET or hepatocyte growth factor and symptomatic CNS metastases that were neurologically unstable or necessitated escalating steroid doses. The comprehensive inclusion and exclusion criteria are delineated in the Protocol (online only).
Ethical approval for the study was secured from the ethics committees of all participating centers. All participants provided written informed consent. The trial has been registered on ClinicalTrials.gov (identifier: NCT04258033).
Procedure
Participants were administered 200 mg of vebreltinib orally twice a day until disease progression or the emergence of intolerable toxicity. Dose modifications on the basis of adverse events (AEs) were permitted, encompassing reductions to 150 mg twice a day, and further to 100 mg twice a day. Treatment could be halted on the basis of the severity of AEs, with doses below 100 mg twice a day and any dose increases prohibited. Specific guidelines for dose modification are detailed in Appendix Figure A1 (online only). Participants who discontinued treatment for over 4 weeks were withdrawn from the study.
Efficacy assessments were carried out by both a blinded independent review committee (BIRC) and investigators in accordance with the RECIST version 1.1. These evaluations were conducted on the first day of the second cycle, then every two cycles up to cycle 12, and subsequently every three cycles after cycle 13, until disease progression, the commencement of another antitumor therapy, death, or withdrawal of consent. For those exiting the study, survival follow-up was conducted bimonthly via telephone for those not lost to follow-up or deceased. AEs were graded using the National Cancer Institute's Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0. Safety assessments encompassed AE monitoring, ECOG PS, laboratory tests, vital signs, and physical examinations.
A comprehensive NGS panel approach was used to precisely detect METex14 mutations among other genomic alterations. Results derived from tissue and blood samples were predominantly sourced from facilities certified by the Clinical Laboratory Improvement Amendments (CLIA) or the College of American Pathologists (CAP). Samples from non–CLIA- or CAP-certified facilities were subject to a rigorous validation process by a central laboratory. In cases where tissue samples were insufficient, blood samples were obtained for an exhaustive NGS analysis conducted by the central laboratory. Confirmation of MET gene amplification was also achieved through NGS reports from local laboratories.
End Points
The primary end point of the study was the ORR, as determined by the BIRC. The ORR is defined as the percentage of participants who achieved either a complete response (CR) or a confirmed partial response (PR). Secondary end points included the ORR as evaluated by investigators, disease control rate (DCR), duration of response (DoR), time to response (TTR), progression-free survival (PFS), and overall survival (OS).
Statistical Analysis
For cohort 1, the study required a sample size of 48 evaluable patients with METex14-positive NSCLC receiving vebreltinib at 200 mg twice a day to confidently reject the null hypothesis that the ORR was 40% or lower. This calculation was based on achieving an 80% power to detect an actual ORR of 60%, using a one-sided alpha of 2.5%. With an anticipated dropout rate of about 15%, the study aimed to enroll a minimum of 57 participants in this cohort.
The full analysis set (FAS) included all participants who had undergone baseline tumor assessment and received at least one dose (200 mg twice a day) of vebreltinib, with efficacy evaluations derived from this set. The safety set (SS) comprised all participants who were administered a minimum of one dose (200 mg twice a day) of the vebreltinib.
Statistical analyses were executed using SAS version 9.4 (SAS Institute, Cary, NC). The ORR, DCR, and their 95% CIs were determined using the Clopper-Pearson method. The chi-square test with continuity correction and Fisher's exact test were applied to assess differences in ORR among patients with various coexisting genetic alterations. TTR, DoR, PFS, and OS, along with their respective 95% CIs, were estimated using the Kaplan-Meier method. The log-rank test was used to compare PFS among patients with different coexisting genetic alterations.
RESULTS
Baseline Characteristics of Participants
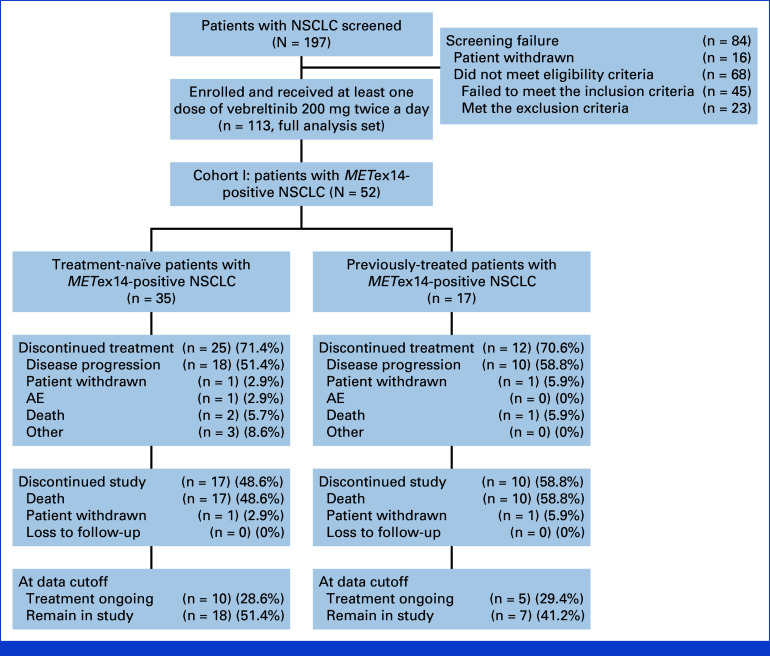
Between January 17, 2020, and August 9, 2022, a total of 197 patients were screened across 17 sites in China. Of these, 16 individuals withdrew consent, and 68 were found ineligible because of not meeting inclusion criteria or meeting exclusion criteria, leading to 113 participants being enrolled in the study, including 52 in cohort 1. Up to the data cutoff date of August 9, 2022, data from these 52 patients were included in the FAS and the SS for analysis.
Within cohort 1, the median age was 71 years, with a range from 51 to 90 years. Males accounted for 55.8% (29 of 52) of the cohort, and the majority (90.4%, or 47 of 52) had an ECOG PS score of 1. A significant proportion (94.2%) identified as Han ethnicity. Among these patients, 35 were treatment-naïve, whereas 17 had received previous systemic antitumor therapy, excluding c-Met inhibitors (Table 1). In the subgroup analysis focusing on patients with brain metastases (n = 5), none underwent surgical intervention for their brain metastases before study entry, while two participants were administered radiation therapy targeting brain metastases (Fig 1).
TABLE 1.
Baseline Characteristics
| Variable | Cohort 1 (N = 52) | Treatment-Naïve (n = 35) | Previously Treated (n = 17) |
|---|---|---|---|
| Age, years | |||
| Mean (standard deviation) | 71.3 (8.3) | 71.9 (9.0) | 70.0 (6.5) |
| Median (min, max) | 71.0 (51.0, 90.0) | 71.0 (53.0, 90.0) | 70.0 (57.0, 80.0) |
| Sex, No. (%) | |||
| Male | 29 (55.8) | 18 (51.4) | 11 (64.7) |
| Female | 23 (44.2) | 17 (48.6) | 6 (35.3) |
| ECOG PS, No. (%) | |||
| 0 | 5 (9.6%) | 5 (14.3) | 0 (0.0) |
| 1 | 47 (90.4%) | 30 (85.7) | 17 (100.0) |
| Smoking, No. (%)a | |||
| Current | 3 (5.8) | 2 (5.9) | 1 (5.9) |
| Former | 16 (30.8) | 10 (29.4) | 6 (35.3) |
| Never | 32 (61.5) | 22 (64.7) | 10 (58.8) |
| Histologic subtype, No. (%) | |||
| Adenocarcinoma | 47 (90.4) | 31 (88.6) | 16 (94.1) |
| Squamous carcinoma | 1 (1.9) | 1 (2.9) | 0 (0.0) |
| Large cell lung cancer | 1 (1.9) | 0 (0.0) | 1 (5.9) |
| NSCLC, not otherwise specified | 3 (5.8) | 3 (8.6) | 0 (0.0) |
| Disease stage, No. (%) | |||
| IIIB | 5 (9.6) | 5 (14.3) | 0 (0.0) |
| IIIC | 4 (7.7) | 3 (8.6) | 1 (5.9) |
| IV | 43 (82.7) | 27 (77.1) | 16 (94.1) |
| Previous systematic antitumor treatment, No. (%) | |||
| Chemotherapy | — | 16 (94.1) | |
| Target therapy | — | 3 (17.6) | |
| Immunotherapy | — | 5 (29.4) |
Abbreviations: ECOG PS, Eastern Cooperative Oncology Group performance status; NSCLC, non–small cell lung cancer.
S04001 missing smoking history information.
FIG 1.
Flow diagram. In the treatment-naïve subgroup, discontinuations labeled as other included one patient who refused medication because of low back pain, one patient who had two medication interruptions in a relatively short interval (one because of severe acute coronary syndrome and one because of increased creatinine), and one patient who was assessed as unfit for further trial participation because of persistently reduced creatinine clearance. AE, adverse event; NSCLC, non–small cell lung cancer; METex14, MET exon 14 skipping.
Efficacy
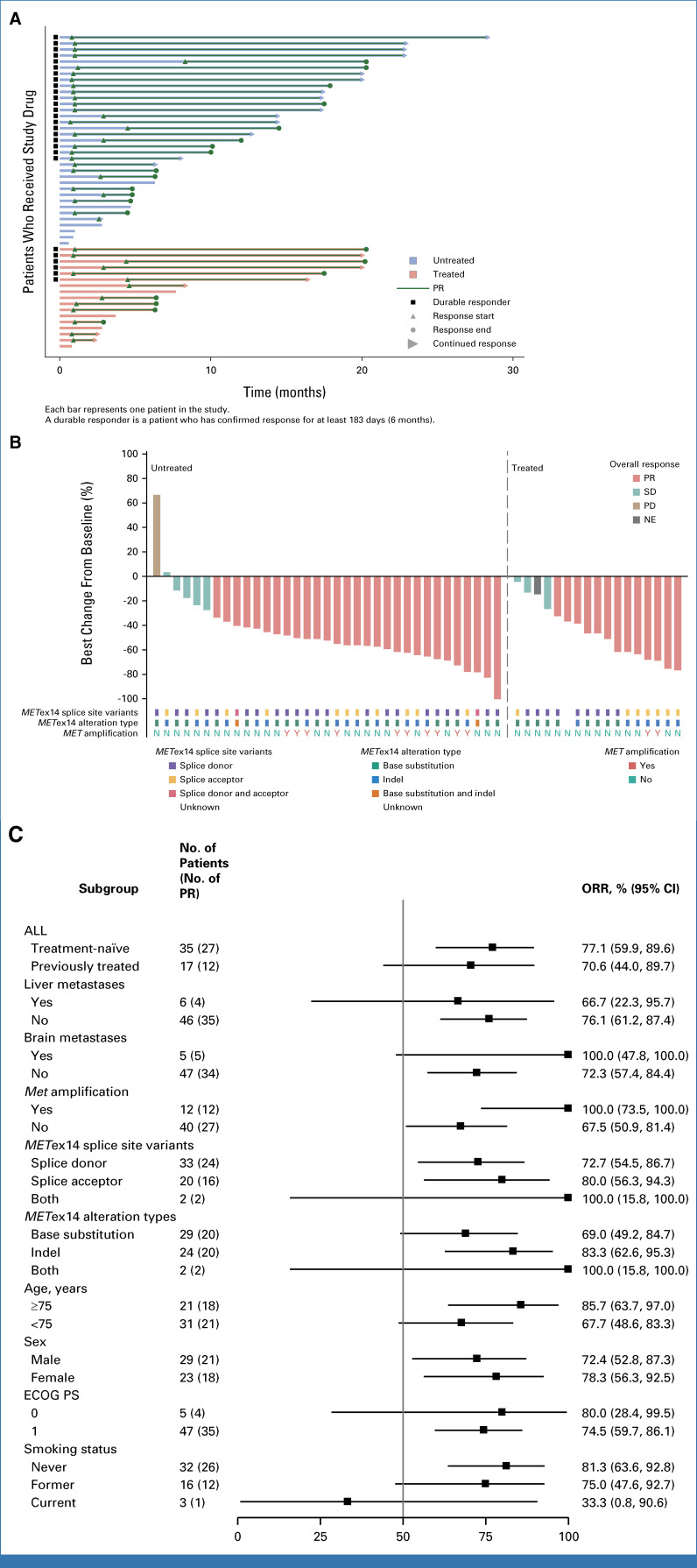
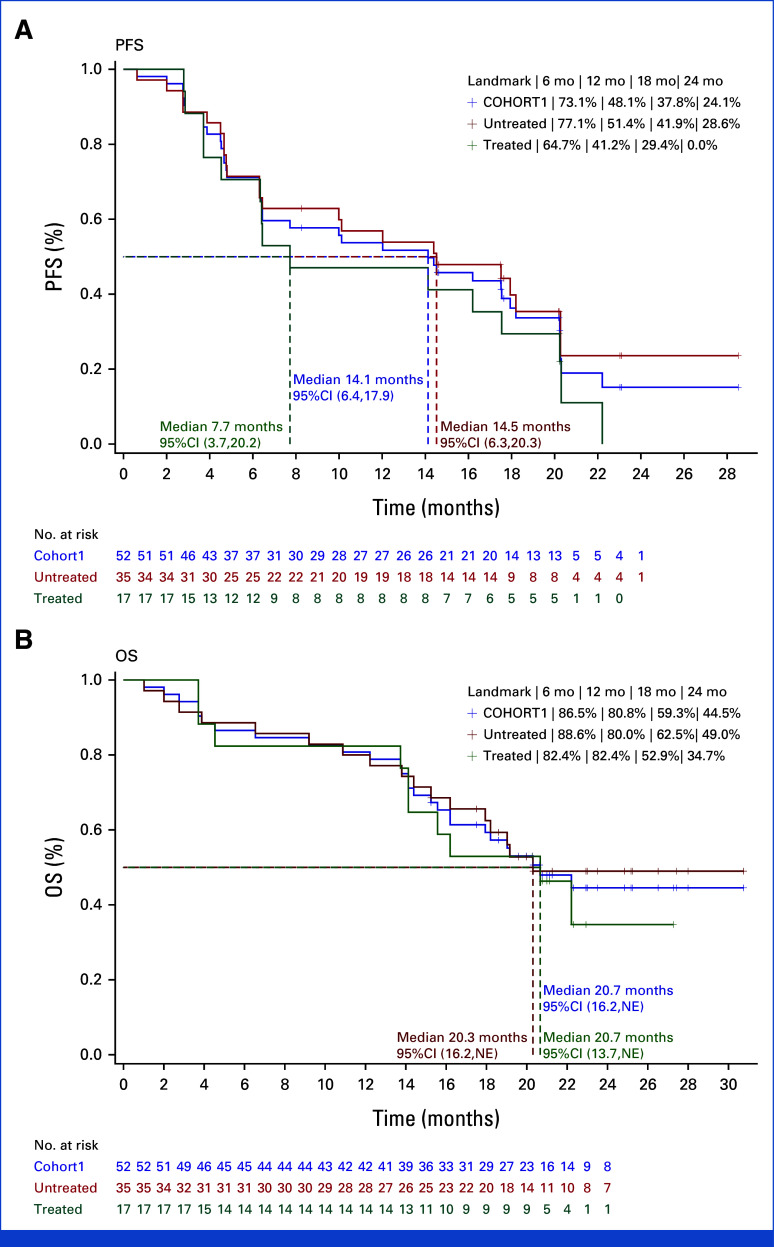
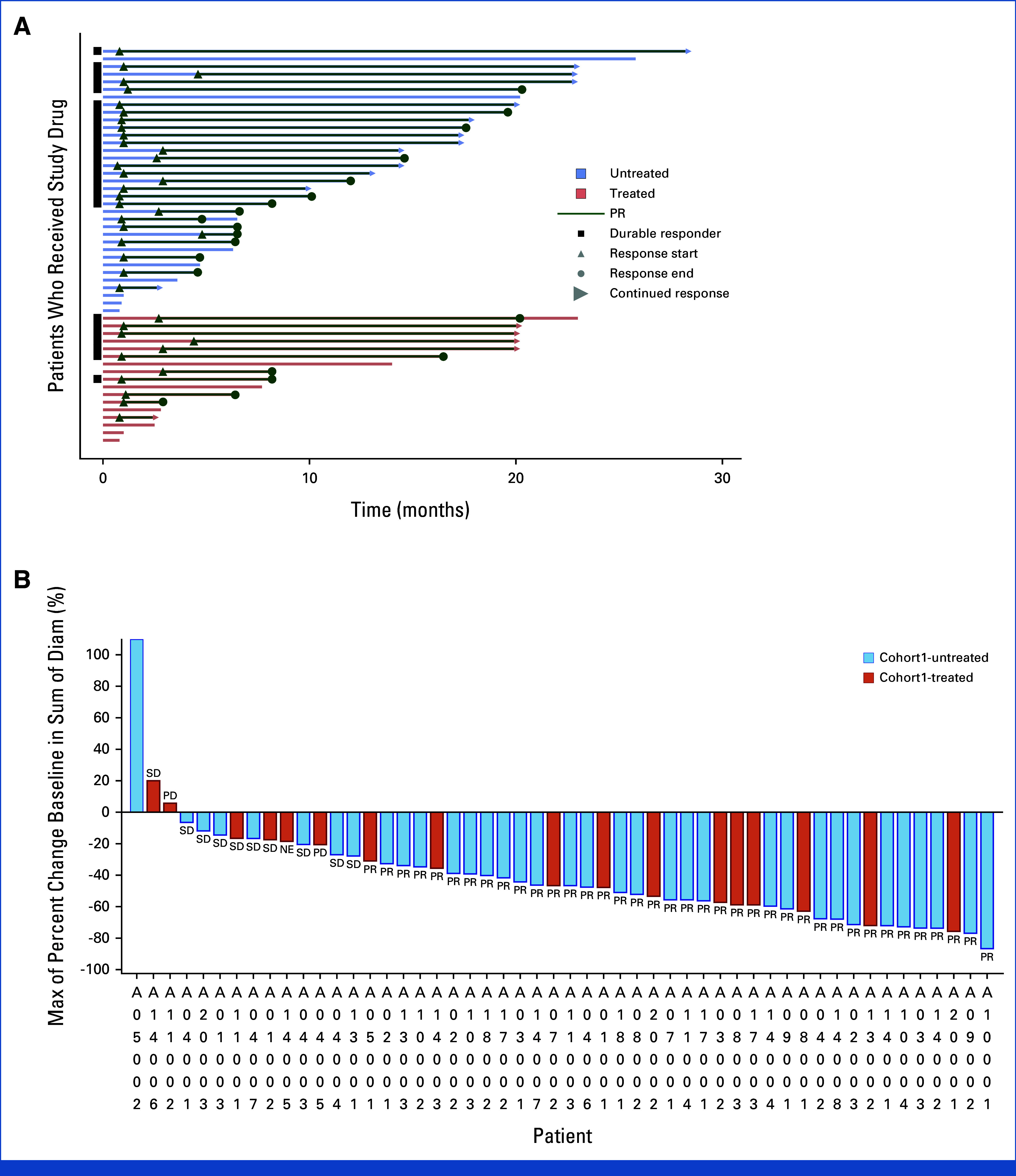
Efficacy assessments conducted by the BIRC revealed that 39 of the 52 participants achieved either a CR or a PR, leading to a confirmed ORR of 75.0% (95% CI, 61.1 to 86.0) and a DCR of 96.2% (95% CI, 86.8 to 99.5) within the FAS. Graphical depictions of the maximum reduction in target lesion size from baseline alongside the duration of vebreltinib treatment for each patient are presented in Figure 2. With a median vebreltinib treatment span of 9.9 months, the median TTR was 1.0 month (95% CI, 1.0 to 2.8), the median DoR was 15.9 months (95% CI, 9.2 to 17.8), and the median PFS was 14.1 months (95% CI, 6.4 to 17.9; Fig 3A). The ORR, as determined by the investigators, was 69.2% (36 of 52 participants), with a median DoR of 15.7 months (Appendix Fig A2 and Table 2).
FIG 2.
Antitumor activity of vebreltinib as assessed by blinded independent review committee. (A) Treatment exposure and response duration of patients. (B) The best percentage changes from baseline in target lesions of patients against MET exon 14 (METex14) splice site variants, alteration types, and MET amplification. Indels are insertion or deletion mutations. A durable responder is defined as a subject with a confirmed response lasting at least 183 days (6 months). (C) Subgroup analysis of ORR assessed by blinded independent review committee. ECOG PS, Eastern Cooperative Oncology Group performance status; METex14, MET exon 14; NE, not evaluable; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
FIG 3.
Kaplan-Meier estimate of (A) PFS assessed by blinded independent review committee and (B) OS. NE, not evaluable; OS, overall survival; PFS, progression-free survival.
TABLE 2.
Efficacy
| Variable | BIRC-Assessed | Investigator-Assessed | ||||
|---|---|---|---|---|---|---|
| Treatment-Naïve (n = 35) | Previously Treated (n = 17) | All (N = 52) | Treatment-Naïve (n = 35) | Previously Treated (n = 17) | All (N = 52) | |
| Objective response rate, No. (%) | 27 (77.1) | 12 (70.6) | 39 (75.0) | 26 (74.3) | 10 (58.8) | 36 (69.2) |
| 95% CI | 59.9 to 89.6 | 44.0 to 89.7 | 61.1 to 86.0 | 56.7 to 87.5 | 32.9 to 81.6 | 54.9 to 81.3 |
| Best overall response, No. (%) | ||||||
| Complete response | 0 | 0 | 0 | 0 | 0 | 0 |
| Partial response | 27 (77.1) | 12 (70.6) | 39 (75.0) | 26 (74.3) | 10 (58.8) | 36 (69.2) |
| Stable disease | 7 (20.0) | 4 (23.5) | 11 (21.2) | 8 (22.9) | 4 (23.5) | 12 (23.1) |
| Progressive disease | 1 (2.9) | 0 | 1 (1.9) | 1 (2.9) | 2 (11.8) | 3 (5.8) |
| Not evaluable | 0 | 1 (5.9) | 1 (1.9) | 0 | 1 (5.9) | 1 (1.9) |
| Disease control rate, No. (%) | 34 (97.1) | 16 (94.1) | 50 (96.2) | 34 (97.1) | 14 (82.4) | 48 (92.3) |
| 95% CI | 85.1 to 99.9 | 71.3 to 99.9 | 86.8 to 99.5 | 85.1 to 99.9 | 56.6 to 96.2 | 81.5 to 97.9 |
| Duration of response, median, months (95% CI) | 16.5 (9.2 to NE) | 15.3 (3.7 to 17.8) | 15.9 (9.2 to 17.8) | 16.8 (5.6 to 19.4) | 15.7 (3.7 to NE) | 15.7 (7.4 to 19.4) |
| Time to response, median, months (95% CI) | 1.0 (1.0 to 1.2) | 1.9 (0.9 to 4.5) | 1.0 (1.0 to 2.8) | 1.0 (1.0 to 2.6) | 2.7 (0.9 to 4.4) | 1.0 (1.0 to 2.7) |
| PFS | ||||||
| Median, months (95%CI) | 14.5 (6.3 to 20.3) | 7.7 (3.7 to 20.2) | 14.1 (6.4 to 17.9) | 12.0 (6.5 to 20.3) | 8.2 (2.9 to NE) | 10.5 (6.5 to 18.2) |
| 6-month PFS (95% CI) | 71.4 (53.4 to 83.5) | 70.6 (43.1 to 86.6) | 71.2 (56.8 to 81.5) | 77.1 (59.5 to 87.9) | 64.7 (37.7 to 82.3) | 73.1 (58.8 to 83.1) |
| 12-month PFS (95% CI) | 56.9 (38.9 to 71.3) | 47.1 (23.0 to 68.0) | 53.7 (39.3 to 66.1) | 51.4 (34.0 to 66.4) | 41.2 (18.6 to 62.6) | 48.1 (34.1 to 60.8) |
Abbreviations: BIRC, blinded independent review committee; NE, not estimate; PFS, progression-free survival.
After a median follow-up period of 19.4 months, the median OS was 20.7 months (95% CI, 16.2 to not estimated [NE]). The survival rates at 6, 12, 18, and 24 months were 86.5%, 80.8%, 59.3%, and 44.5%, respectively (Fig 3B).
Efficacy by Baseline Characteristics
In the cohort of 35 treatment-naïve patients, the ORR confirmed by the BIRC was 77.1% (95% CI, 59.9 to 89.6; Fig 2C). The DCR for this subgroup reached 97.1% (95% CI, 85.1 to 99.9), with these patients achieving a median DoR of 16.5 months (95% CI, 9.2 to NE) and a median PFS of 14.5 months (95% CI, 6.3 to 20.3). For the 17 patients who had undergone previous treatments, the BIRC-confirmed ORR was 70.6% (95% CI, 44.0 to 89.7), with a DCR of 94.1% (95% CI, 71.3 to 99.9). This subgroup exhibited a median DoR of 15.3 months (95% CI, 3.7 to 17.8) and a median PFS of 7.7 months (95% CI, 3.7 to 20.2; Fig 3A and Table 2). The ORR assessed by investigators for the treatment-naïve and previously treated groups was 74.3% (95% CI, 56.7 to 87.5) and 58.8% (95% CI, 32.9 to 81.6), respectively (Fig 2C). The median OS was 20.3 months (95% CI, 16.2 to NE) for treatment-naïve patients and 20.7 months (95% CI, 13.7 to NE) for those previously treated.
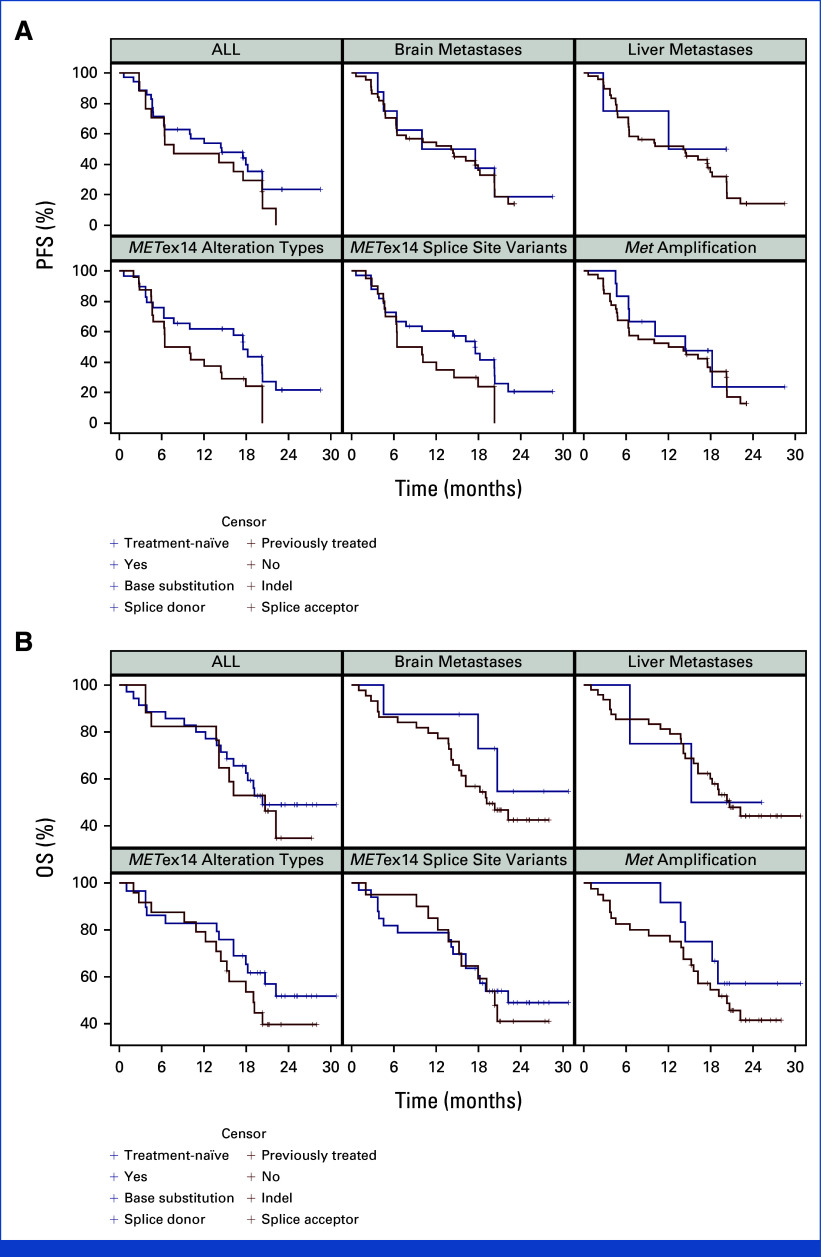
In the specific subgroup of patients with brain metastases (n = 5), both the systemic ORR and DCR achieved a remarkable rate of 100.0% (95% CI, 47.8 to 100.0). These patients had a median DoR of 5.6 months (95% CI, 3.7 to NE) and a median PFS of 6.4 months (95% CI, 4.5 to NE; Appendix Fig A3). Detailed subgroup analyses are provided in Appendix Table A1.
Biomarker Analysis
In our cohort, 12 patients (23.1%) with co-occurring MET amplification showcased an ORR of 100.0% (95% CI, 73.5 to 100.0), a median DoR of 13.5 months (95% CI, 3.7 to NE), and a median PFS of 14.4 months (95% CI, 4.7 to NE; Appendix Table A1). Remarkably, the ORR in patients with concurrent MET amplification was significantly higher compared with those without (P = .024), although the PFS rates were similar (log-rank P = .518).
Among the 52 participants with advanced NSCLC harboring METex14 mutations, mutations at the splice donor site were identified in 33 patients (63.5%), whereas mutations at the splice acceptor site were present in 20 patients (38.5%). Two individuals had both mutation types simultaneously, and the mutation status of one patient remained unspecified. Patients with splice donor site mutations exhibited an ORR of 72.7% (95% CI, 54.5 to 86.7) and a median PFS of 17.5 months (95% CI, 6.3 to 20.3). For those with splice acceptor site mutations, the ORR was 80.0% (95% CI, 56.3 to 94.3), with a median PFS of 8.2 months (95% CI, 4.7 to 17.9). Patients carrying both mutation types demonstrated an ORR of 100.0% (95% CI, 15.8 to 100.0) and a median PFS of 15.1 months (95% CI, 10.0 to NE; Appendix Table A2 and Appendix Fig A3).
Regarding alteration types, 29 patients (55.8%) presented solely with base substitutions, while 24 patients (46.2%) exhibited only base insertions or deletions (indels), and two individuals showed a combination of both base substitutions and indels. The specific genetic alteration of one patient remains undetermined. Among the patients with base substitutions, the ORR was 69.0% (95% CI, 49.2 to 84.7), with a median PFS of 17.5 months (95% CI, 6.3 to 20.3). For those harboring indels, the ORR reached 83.3% (95% CI, 62.6 to 95.3), with a median PFS of 8.2 months (95% CI, 4.7 to 14.5). Patients possessing both alteration types demonstrated outcomes consistent with those observed in the splice donor and splice acceptor site subgroups (Appendix Table A2 and Appendix Fig A3).
Vebreltinib showcased comparable efficacy in patients with METex14 mutations, irrespective of the mutation being at the splice donor or splice acceptor sites (P = .853 for ORR; log-rank P = .100 for PFS), as well as in those with either base substitutions or indels (P = .253 for ORR; log-rank P = .062 for PFS).
Safety
All (100.0%) participants reported at least one AE. A total of 32 individuals (61.5%) encountered AEs of grade 3 or higher severity. During the study, three participants (5.8%) died due to AEs: one due to acute pneumonia, another from pleural effusion, and a third patient deceased at home with causes remaining unspecified. These incidents were not considered related to vebreltinib treatment. Discontinuations of vebreltinib were noted in four patients (7.7%), with dosage reductions required for 11 participants (21.2%) and treatment suspensions in 26 individuals (50.0%). Serious adverse events were reported by 23 participants (44.2%), predominantly involving abnormal liver function and pleural effusion, each affecting four participants (7.7% of the cohort), followed by infectious pneumonia in two participants (3.8%). The rate of TRAEs was 98.1% (51 of 52 participants), with 48.1% (25 participants) experiencing TRAEs of grade 3 or higher. The most common TRAEs included peripheral edema (82.7%), QT prolongation (30.8%), and elevated serum creatinine (28.8%). Grade 3-4 TRAEs most frequently reported were peripheral edema (13.5%), abnormal liver function (9.6%), elevated alanine aminotransferase (7.7%), elevated aspartate aminotransferase (5.8%), anemia (5.8%), and infectious pneumonitis (5.8%; Table 3).
TABLE 3.
TRAE
| Event | Any Grade, No. (%) | Grade 3-4, No. (%) | Grade 3, No. (%) | Grade 4, No. (%) |
|---|---|---|---|---|
| At least one TRAE | 51 (98.1) | 25 (48.1) | 25 (48.1) | 3 (5.8) |
| Peripheral edema | 43 (82.7) | 7 (13.5) | 7 (13.5) | 0 (0.0) |
| QT prolongation | 16 (30.8) | 1 (1.9) | 1 (1.9) | 0 (0.0) |
| Elevated serum creatinine | 15 (28.8) | 1 (1.9) | 1 (1.9) | 0 (0.0) |
| Hypoalbuminemia | 14 (26.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hypoproteinemia | 13 (25.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Anemia | 13 (25.0) | 2 (3.8) | 2 (3.8) | 0 (0.0) |
| Elevated aspartate ALT | 12 (23.1) | 4 (7.7) | 4 (7.7) | 0 (0.0) |
| Weight gain | 12 (23.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Pruritus | 12 (23.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Elevated lipase | 11 (21.2) | 3 (5.8) | 3 (5.8) | 0 (0.0) |
| Hypocalcemia | 9 (17.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Weakness | 8 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Abnormal liver function | 8 (15.4) | 5 (9.6) | 5 (9.6) | 1 (1.9) |
| Ventricular premature contraction | 8 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Elevated AST | 8 (15.4) | 3 (5.8) | 3 (5.8) | 0 (0.0) |
| Elevated bilirubin | 8 (15.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Decreased platelet count | 8 (15.4) | 2 (3.8) | 1 (1.9) | 1 (1.9) |
| Hypokalemia | 7 (13.5) | 1 (1.9) | 0 (0.0) | 1 (1.9) |
| Nausea | 7 (13.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Elevated conjugated bilirubin | 7 (13.5) | 1 (1.9) | 1 (1.9) | 0 (0.0) |
| Sinus tachycardia | 7 (13.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Decreased white cell count | 6 (11.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Hyponatremia | 6 (11.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Elevated amylase | 6 (11.5) | 1 (1.9) | 1 (1.9) | 0 (0.0) |
| Hyperuricaemia | 6 (11.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Vomiting | 6 (11.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Decreased neutrophil count | 4 (7.7) | 1 (1.9) | 1 (1.9) | 0 (0.0) |
| Infectious pneumonia | 2 (3.8) | 1 (1.9) | 1 (1.9) | 0 (0.0) |
| Peripheral swelling | 2 (3.8) | 1 (1.9) | 1 (1.9) | 0 (0.0) |
| Decreased renal creatinine clearance rate | 1 (1.9) | 1 (1.9) | 1 (1.9) | 0 (0.0) |
| GI diseases | 1 (1.9) | 1 (1.9) | 1 (1.9) | 0 (0.0) |
NOTE. TRAEs of any grade considered to be related to study drug by the investigators in at least 10% of patients, or any grade 3 or 4 events reported in any patient are shown. No grade 5 TRAE occurred.
Abbreviation: TRAE, treatment-related adverse event.
DISCUSSION
In this phase II clinical trial, vebreltinib showcased a remarkable ORR of 75.0% in the management of advanced NSCLC with MET exon 14 skipping mutations, alongside a sustained DoR (median, 15.9 months), regardless of previous treatment. This performance is consistent with other MET inhibitors, which have reported ORRs ranging from 42.9% to 68% and DoR spanning 8.3 to 18 months.7,8,10,11 Furthermore, vebreltinib was characterized by a rapid onset of therapeutic action, evidenced by a median TTR of just 1 month. It is pertinent to acknowledge, however, that this rapidity could be partly attributed to the earlier restaging scans used in our study protocol (4 weeks) as opposed to the 6- to 8-week time frame commonly used in other studies.7,9,10
Vebreltinib's potent antitumor efficacy was observed across all patient subsets, including those without previous chemotherapy, underscoring its significant therapeutic promise. In treatment-naïve patients, vebreltinib achieved an ORR of 77.1%, a DCR of 97.2%, and a median DoR of 16.5 months. Remarkably, even in patients who had received previous systemic therapy, vebreltinib maintained robust efficacy, demonstrating an ORR of 70.6%, a DCR of 94.1%, and a median DoR of 15.3 months. These results, showcasing promising efficacy in both treatment-naïve and previously treated patient populations, position vebreltinib as a promising candidate in the therapeutic landscape of NSCLC.
The study revealed that vebreltinib treatment resulted in a median PFS of 14.1 months, outperforming the median PFS observed with gumarontinib (8.5 months),7 tepotinib (11.2 months),11 and savolitinib (6.8 months).10 Additionally, the median OS after vebreltinib treatment was 20.7 months, surpassing the outcomes reported for tepotinib (17.1 months), gumarontinib (17.3 months), and savolitinib (12.5 months).7,9,10 These results highlight vebreltinib's notable antitumor efficacy and its potential to improve survival, marking it as an important advancement in the treatment landscape for NSCLC, especially for those patients with METex14 mutations. Nonetheless, the interpretation of these results necessitates a careful consideration of the variability in study designs and patient demographics across the studies.
In the cohort of patients with brain metastases (n = 5), vebreltinib was associated with a systemic ORR of 100%. Previous findings suggested vebreltinib's capability to cross the blood-brain barrier, as evidenced in phase I studies targeting patients with glioma harboring MET alterations.14 Although this systemic ORR is promising and indirectly supports vebreltinib's potential therapeutic effect on CNS metastases, it is important to note that specific intracranial ORRs were not directly assessed in the current study. Previous research has underscored the prognostic significance of liver metastases in NSCLC, indicating poorer outcomes in patients treated with EGFR TKIs and immune checkpoint inhibitors.15-17 Despite the small sample size, our findings suggest vebreltinib may offer benefits to patients with liver metastases and those age 75 years or older, warranting further investigation to validate these promising outcomes and explore personalized treatment strategies for NSCLC.
In our study, the indel subgroup showed a numerically higher ORR compared with the base substitution subgroup, nevertheless a numerically shorter DoR and PFS. This differs from the study of savolitinib, where despite similar ORRs, the indel subgroup showed a longer PFS.10 Although no significant difference was observed in this study, these discrepancies highlight the complex interplay between genetic alterations and treatment efficacy. Given the small sample size and exploratory nature of our study, these preliminary findings emphasize the importance of additional research to elucidate the implications of different genetic alterations on treatment outcomes.
The occurrence of METex14 mutations alongside other driver mutations is uncommon, yet instances of METex14 mutations coexisting with MET amplification have been documented.18 Savolitinib has demonstrated a numerically superior ORR and extended PFS in patients with concurrent MET amplification,10 while capmatinib's effectiveness seemed unaffected by simultaneous MET amplification in patients with METex14-positive NSCLC.8 In our study, an ORR of 100.0% was observed in 12 patients with coexisting MET amplification, a rate significantly higher than that in patients without, although PFS rates were similar. These results align with those from an initial phase I study of vebreltinib.13 Besides, similar to findings with capmatinib,8 responses to vebreltinib may differ among patients with various METex14 splice site variants and alteration types. Such findings emphasize the need for further studies to clarify the clinical implications of concurrent MET amplification in patients with METex14 mutation-positive NSCLC treated with MET inhibitors.
In this study, the most prevalent TRAEs were peripheral edema (82.7%), QT prolongation (30.8%), and elevated serum creatinine (28.8%). Notably, no grade 5 TRAEs were observed. The significant occurrence of peripheral edema, potentially linked to hypoalbuminemia and hypoproteinemia, warrants particular attention. Although the incidence of QT interval prolongation calls for careful monitoring of potential cardiac toxicity, it is important to highlight that most prolongations were of grade 1 and 2 severity, indicating generally mild effects. Moreover, instances of grade 3 QT prolongation were infrequent, with only one patient necessitating a brief pause in treatment. This individual was able to resume therapy at the initial dose after QT normalization, emphasizing the critical role of regular monitoring and educating patients on the safe use of vebreltinib, especially for those with existing cardiac conditions or taking other QT interval-affecting medications. However, given the study's limited sample size, vebreltinib's safety profile warrants further validation in larger-scale studies. Comparatively, the primary TRAEs associated with capmatinib were edema (51%) and nausea (45%).8 Tepotinib was linked to a notable instance of a grade 5 TRAE.9 The incidence of grade 3 or higher TRAEs for vebreltinib was 48.1%, a figure numerically in line with those reported for savolitinib and gumarontinib, at 46% and 54%, respectively.10 Additionally, TRAEs related to gumarontinib led to permanent discontinuation in 8% of patients. These suggest that vebreltinib may offer a potentially more favorable safety profile for treating patients with METex14-positive NSCLC.
The enhanced ORR observed with vebreltinib is promising, likely because of its highly selective and specific inhibition of tumor cell proliferation and c-Met phosphorylation.12 However, it is critical to acknowledge certain limitations. First, the study's patient population was exclusively from China, although the efficacy of MET inhibitor therapy in Asia may reflect that of the global population.19 Additionally, the study did not collect post-treatment biopsy samples for drug resistance mechanism analysis. The small sample size further constrains the extrapolation of these findings to a broader, more heterogeneous patient population. In response, a global phase I/II study (ClinicalTrials.gov identifier: NCT03175224) is currently evaluating vebreltinib's efficacy in a wider population, aiming to deepen our understanding of its therapeutic value across diverse demographics. Moreover, an ongoing larger-scale phase IIIb study (ClinicalTrials.gov identifier: NCT05989542) is expected to provide additional clinical evidence, potentially overcoming drug resistance challenges and advancing the treatment of NSCLC with METex14 mutations. Fourth, the subgroup analyses were conducted on relatively small cohorts, which necessitates a cautious interpretation of the results. In particular, for the subgroup of patients with brain metastases, the small sample size precluded a specific assessment of intracranial response. This aspect will be addressed in future large-scale studies designed to provide more definitive insights into both systemic and intracranial efficacy.
The KUNPENG study offers evidence of vebreltinib's efficacy and safety for patients with locally advanced or metastatic METex14-positive NSCLC, highlighting its capacity to induce robust and durable responses alongside a favorable safety profile. Vebreltinib emerges as a viable therapeutic candidate for patients with METex14-positive NSCLC.
ACKNOWLEDGMENT
The authors sincerely appreciate all the patients and their families who took part in this trial, as well as the research nurses and study coordinators who contributed to the success of this study. The authors express their gratitude to the investigators who participated in this research. The authors acknowledge Xincheng Liu, MD, PhD, in medicine team of Beijing Pearl Biotechnology Co, Ltd for statistical analysis, visualization, writing review, and editing; and Peony Yu, MD, of Apollomics, Inc. for writing review.
APPENDIX
FIG A1.
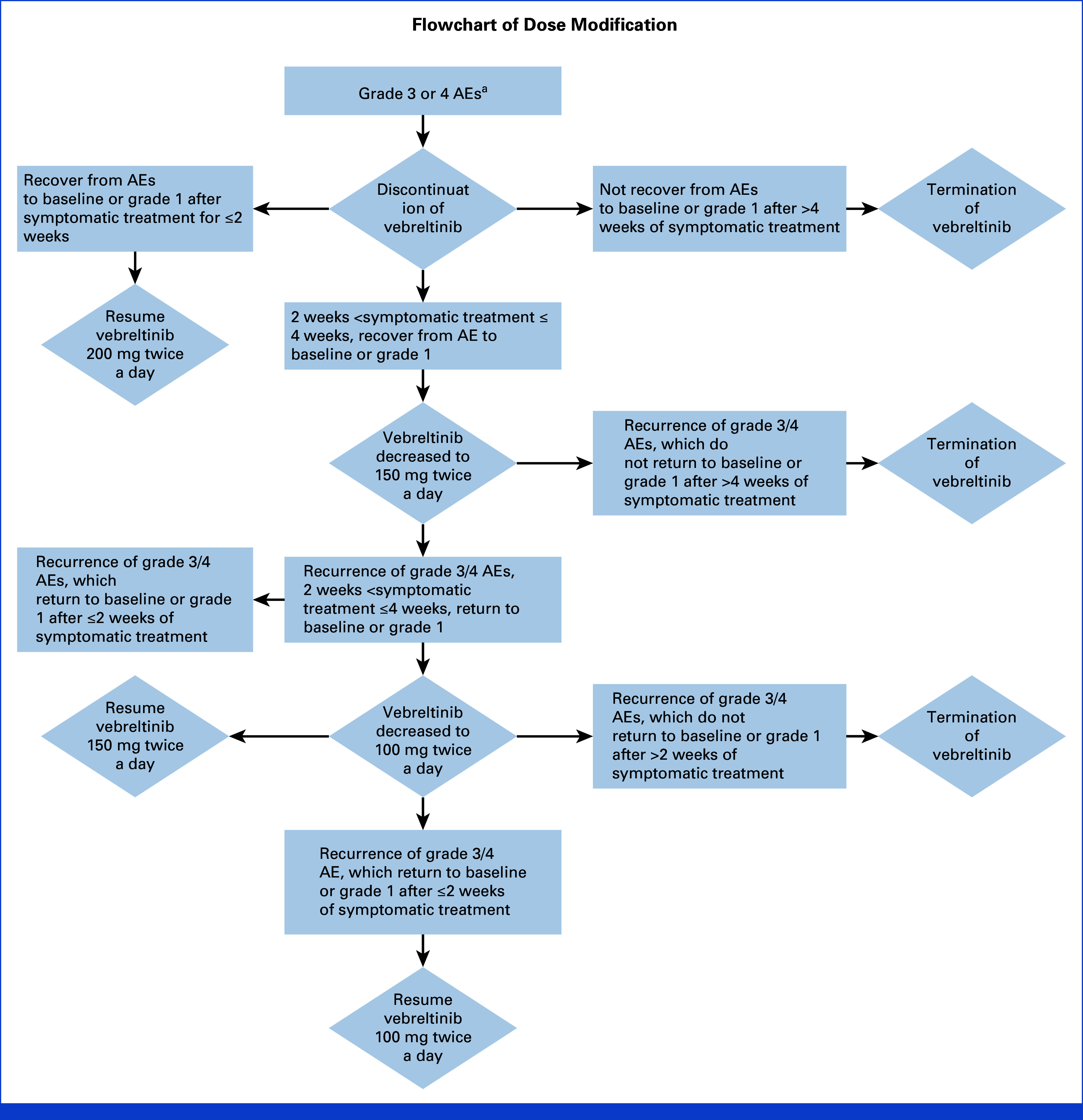
Flowchart of dose modification. aExcept for nausea, vomiting, diarrhea, constipation, or grade 3 and 4 nonhematologic toxicities that are considered manageable by the investigator. AE, adverse event.
FIG A2.
Antitumor activity of vebreltinib as assessed by investigator. (A) Treatment exposure and response duration of patients. (B) The best percentage changes from baseline in target lesions of patients. Each bar represents one subject in the study. A durable responder is a subject who has confirmed response for at least 183 days (6 months). NE, not evaluable; PD, progressive disease; PR, partial response; SD, stable disease.
FIG A3.
Subgroup analysis of (A) progression-free survival assessed by blinded independent review committee and (B) overall survival in patients with different metastases sites and different mutation sites. METex14, MET exon 14; OS, overall survival; PFS, progression-free survival.
TABLE A1.
Subgroup Analysis on the Basis of MET Amplification, Liver Metastases, Brain Metastases, and Age
| Variable | MET Amplification+ (n = 12) | MET Amplification– (n = 40) | With Liver Metastases (n = 6) | With Brain Metastases (n = 5) | ≥75 Years (n = 21) |
|---|---|---|---|---|---|
| Objective response rate, % | 12 (100.0) | 27 (67.5) | 4 (66.7) | 5 (100.0) | 18 (85.7) |
| 95% CI | 73.5 to 100.0 | 50.9 to 81.4 | 22.3 to 95.7 | 47.8 to 100.0 | 63.7 to 97.0 |
| Disease control rate, % | 12 (100.0) | 38 (95.0) | 6 (100.0) | 5 (100.0) | 20 (95.2) |
| 95% CI | 73.5 to 100.0 | 83.1 to 99.4 | 54.1 to 100.0 | 47.8 to 100.0 | 76.2 to 99.9 |
| Duration of response, median, months (95% CI) |
13.5 (3.7 to NE) | 15.9 (9.2 to 19.1) | 9.2 (5.5 to NE) | 5.6 (3.7 to NE) | 19.1 (9.2 to NE) |
| 6-month PFS rate (95% CI) | 66.7 (33.7 to 86.0) | 73.3 (53.7 to 85.7) | 75.0 (12.8 to 96.1) | 40.0 (5.2,75.3) | 78.9 (53.2 to 91.5) |
| 12-month PFS rate (95% CI) | 57.1 (25.4 to 79.6) | 56.5 (37.1 to 72.0) | 25.0 (0.9 to 66.5) | 20.0 (0.8 to 58.2) | 68.4 (42.8 to 84.4) |
| PFS, median, months (95% CI) | 14.4 (4.7 to NE) | 13.1 (6.3 to 17.9) | 8.2 (2.8 to NE) | 6.4 (4.5 to NE) | 18.2 (6.3 to NE) |
| OS, months, median (95% CI) | NR (13.7 to NE) | 20.3 (15.2 to NE) | 14.5 (3.7 to NE) | 17.9 (4.5 to NE) | NR (14.4 to NE) |
Abbreviations: NE, not estimated; NR, not reached; OS, overall survival; PFS, progression-free survival.
TABLE A2.
Subgroup Analysis of Subjects With MET Exon 14 Splice Site Variants and Alteration Types
| Variable | Splice Donor (n = 33) | Splice Acceptor (n = 20) | SD and SA (BS and I, n = 2) | Base Substitution (n = 29) | Indel (n = 24) |
|---|---|---|---|---|---|
| Objective response rate, % | 24 (72.7) | 16 (80.0) | 2 (100.0) | 20 (69.0) | 20 (83.3) |
| 95% CI | 54.5 to 86.7 | 56.3 to 94.3 | 15.8 to 100.0 | 49.2 to 84.7 | 62.6 to 95.3 |
| Disease control rate, % | 31 (93.9) | 20 (100.0) | 2 (100.0) | 27 (93.1) | 24 (100.0) |
| 95% CI | 79.8 to 99.3 | 83.2 to 100.0 | 15.8 to 100.0 | 77.2 to 99.2 | 85.8 to 100.0 |
| Duration of response, months, median (95% CI) |
16.7 (13.5 to NE) | 9.2 (3.9 to 17.1) | 10.6 (9.2 to NE) | 17.3 (15.3 to NE) | 9.2 (3.9 to 13.5) |
| 6-month PFS rate (95% CI) | 80.8 (59.8 to 91.5) | 58.8 (32.5 to 77.8) | 100.0 (100.0 to 100.0) | 86.4 (63.4 to 95.4) | 57.1 (33.8 to 74.9) |
| 12-month PFS rate (95% CI) | 72.5 (50.7 to 85.8) | 29.4 (10.7 to 51.1) | 0.0 (NE to NE) | 76.5 (52.3 to 89.5) | 33.3 (14.9 to 53.1) |
| PFS, months, median (95% CI) | 17.5 (6.3 to 20.3) | 8.2 (4.7 to 17.9) | 15.1 (10.0 to NE) | 17.5 (6.3 to 20.3) | 8.2 (4.7 to 14.5) |
| OS, months, median (95% CI) | 22.2 (16.2 to NE) | 20.3 (13.7 to NE) | NR (17.9 to NE) | NR (16.2 to NE) | 19.0 (13.7 to NE) |
Abbreviations: BS, base substitution; I, indel; NE, not estimated; NR, not reached; OS, overall survival; PFS, progression-free survival; SA, splice acceptor; SD, splice donor.
Lin Wu
Speakers' Bureau: MSD, AstraZeneca, Roche China, Bristol Myers Squibb, Pfizer, Lilly, Innovate Biopharmaceuticals, Hengrui Medicine
Zhe-Hai Wang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yong Song
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
He-Peng Shi
Employment: Beijing Pearl Biotechnology Co, Ltd
Leadership: Beijing Pearl Biotechnology Co, Ltd
Stock and Other Ownership Interests: Beijing Pearl Biotechnology Co, Ltd
Wei-Zhe Xue
Employment: Beijing Pearl Biotechnology Co, Ltd
Stock and Other Ownership Interests: Beijing Pearl Biotechnology Co, Ltd
Di Han
Employment: Beijing Pearl Biotechnology Co, Ltd
Stock and Other Ownership Interests: Beijing Pearl Biotechnology Co, Ltd
Pei-Long Zhang
Employment: Beijing Pearl Biotechnology Co, Ltd
Leadership: Beijing Pearl Biotechnology Co, Ltd
Stock and Other Ownership Interests: Beijing Pearl Biotechnology Co, Ltd
Yi-Long Wu
Honoraria: AstraZeneca, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical, BeiGene Beijing
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), BMS (Inst)
No other potential conflicts of interest were reported.
SUPPORT
Supported by Beijing Pearl Biotechnology Co, Ltd.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Other study data may be shared upon submission of a request to Beijing Pearl Biotechnology Co, Ltd. The data request will be reviewed, and if agreed, the requestors will need to sign a data sharing agreement.
AUTHOR CONTRIBUTIONS
Conception and design: Jin-Ji Yang, He-Peng Shi, Wei-Zhe Xue, Di Han, Pei-Long Zhang, Yi-Long Wu
Financial support: He-Peng Shi
Administrative support: Yi-Long Wu
Provision of study materials or patients: Jin-Ji Yang, Yan Zhang, Lin Wu, Jie Hu, Zhe-Hai Wang, Jing-Hua Chen, Yun Fan, Gen Lin, Qi-Ming Wang, Yu Yao, Jun Zhao, Yuan Chen, Jian Fang, Yong Song, Wei Zhang, Ying Cheng
Collection and assembly of data: Jin-Ji Yang, Yan Zhang, Lin Wu, Jie Hu, Zhe-Hai Wang, Jing-Hua Chen, Yun Fan, Gen Lin, Qi-Ming Wang, Yu Yao, Jun Zhao, Yuan Chen, Jian Fang, Yong Song, Wei Zhang, Ying Cheng, Ren-Hua Guo, Xing-Ya Li, Wei-Zhe Xue, Di Han
Data analysis and interpretation: Wei-Zhe Xue, Di Han, Pei-Long Zhang, Yi-Long Wu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Vebreltinib for Advanced Non–Small Cell Lung Cancer Harboring c-Met Exon 14 Skipping Mutation: A Multicenter, Single-Arm, Phase II KUNPENG Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Lin Wu
Speakers' Bureau: MSD, AstraZeneca, Roche China, Bristol Myers Squibb, Pfizer, Lilly, Innovate Biopharmaceuticals, Hengrui Medicine
Zhe-Hai Wang
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
Yong Song
Consulting or Advisory Role: Hansoh Pharma
Research Funding: Hansoh Pharma (Inst)
He-Peng Shi
Employment: Beijing Pearl Biotechnology Co, Ltd
Leadership: Beijing Pearl Biotechnology Co, Ltd
Stock and Other Ownership Interests: Beijing Pearl Biotechnology Co, Ltd
Wei-Zhe Xue
Employment: Beijing Pearl Biotechnology Co, Ltd
Stock and Other Ownership Interests: Beijing Pearl Biotechnology Co, Ltd
Di Han
Employment: Beijing Pearl Biotechnology Co, Ltd
Stock and Other Ownership Interests: Beijing Pearl Biotechnology Co, Ltd
Pei-Long Zhang
Employment: Beijing Pearl Biotechnology Co, Ltd
Leadership: Beijing Pearl Biotechnology Co, Ltd
Stock and Other Ownership Interests: Beijing Pearl Biotechnology Co, Ltd
Yi-Long Wu
Honoraria: AstraZeneca, Roche, Pfizer, Boehringer Ingelheim, MSD Oncology, Bristol Myers Squibb/China, Hengrui Pharmaceutical, BeiGene Beijing
Consulting or Advisory Role: AstraZeneca, Roche, Boehringer Ingelheim, Takeda
Research Funding: Boehringer Ingelheim (Inst), Roche (Inst), Pfizer (Inst), BMS (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1. Tan AC, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. 2022;40:611–625. doi: 10.1200/JCO.21.01626. [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 3. Suster DI, Mino-Kenudson M. Molecular pathology of primary non-small cell lung cancer. Arch Med Res. 2020;51:784–798. doi: 10.1016/j.arcmed.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 4. Drilon A, Cappuzzo F, Ou SHI, et al. Targeting MET in lung cancer: Will expectations finally Be MET? J Thorac Oncol. 2017;12:15–26. doi: 10.1016/j.jtho.2016.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yeung SF, Tong JHM, Law PPW, et al. Profiling of oncogenic driver events in lung adenocarcinoma revealed MET mutation as independent prognostic factor. J Thorac Oncol. 2015;10:1292–1300. doi: 10.1097/JTO.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 6. Tong JH, Yeung SF, Chan AW, et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin Cancer Res. 2016;22:3048–3056. doi: 10.1158/1078-0432.CCR-15-2061. [DOI] [PubMed] [Google Scholar]
- 7. Yu Y, Zhou J, Li X, et al. Gumarontinib in patients with non-small-cell lung cancer harbouring MET exon 14 skipping mutations: A multicentre, single-arm, open-label, phase 1b/2 trial. EClinicalMedicine. 2023;59:101952. doi: 10.1016/j.eclinm.2023.101952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolf J, Seto T, Han JY, et al. Capmatinib in MET exon 14-mutated or MET-amplified non-small-cell lung cancer. N Engl J Med. 2020;383:944–957. doi: 10.1056/NEJMoa2002787. [DOI] [PubMed] [Google Scholar]
- 9. Paik PK, Felip E, Veillon R, et al. Tepotinib in non-small-cell lung cancer with MET exon 14 skipping mutations. N Engl J Med. 2020;383:931–943. doi: 10.1056/NEJMoa2004407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu S, Fang J, Li X, et al. Once-daily savolitinib in Chinese patients with pulmonary sarcomatoid carcinomas and other non-small-cell lung cancers harbouring MET exon 14 skipping alterations: A multicentre, single-arm, open-label, phase 2 study. Lancet Respir Med. 2021;9:1154–1164. doi: 10.1016/S2213-2600(21)00084-9. [DOI] [PubMed] [Google Scholar]
- 11. Mazieres J, Paik PK, Garassino MC, et al. Tepotinib treatment in patients with MET exon 14-skipping non-small cell lung cancer: Long-term follow-up of the VISION phase 2 nonrandomized clinical trial. JAMA Oncol. 2023;9:1260–1266. doi: 10.1001/jamaoncol.2023.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shih J, Zhong B, Shi H, et al. Bozitinib, a highly selective inhibitor of cMet, demonstrates robust activity in gastric, lung, hepatic and pancreatic in vivo models. Cancer Res. 2017;77:2096. suppl 13; abstr 2096. [Google Scholar]
- 13. Yang J, Zhou Q, Chen H, et al. A phase I study of cMET inhibitor bozitinib in patients with advanced NSCLC harboring cMET alterations. Cancer Res. 2020;80:CT127. suppl 13; abstr CT127. [Google Scholar]
- 14. Hu H, Mu Q, Bao Z, et al. Mutational landscape of secondary glioblastoma guides MET-targeted trial in brain tumor. Cell. 2018;175:1665–1678.e18. doi: 10.1016/j.cell.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 15. Chang YP, Chen YM, Lai CH, et al. The impact of de novo liver metastasis on clinical outcome in patients with advanced non-small-cell lung cancer. PLoS One. 2017;12:e0178676. doi: 10.1371/journal.pone.0178676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jiang T, Cheng R, Zhang G, et al. Characterization of liver metastasis and its effect on targeted therapy in EGFR-mutant NSCLC: A multicenter study. Clin Lung Cancer. 2017;18:631–639 e2. doi: 10.1016/j.cllc.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 17. Tian BW, Han CL, Wang HC, et al. Effect of liver metastasis on the efficacy of immune checkpoint inhibitors in cancer patients: A systemic review and meta-analysis. Clin Exp Metastasis. 2023;40:255–287. doi: 10.1007/s10585-023-10217-7. [DOI] [PubMed] [Google Scholar]
- 18. Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in non-small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. J Clin Oncol. 2016;34:721–730. doi: 10.1200/JCO.2015.63.4600. [DOI] [PubMed] [Google Scholar]
- 19. Sakai H, Morise M, Kato T, et al. Tepotinib in patients with NSCLC harbouring MET exon 14 skipping: Japanese subset analysis from the phase II VISION study. Jpn J Clin Oncol. 2021;51:1261–1268. doi: 10.1093/jjco/hyab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Other study data may be shared upon submission of a request to Beijing Pearl Biotechnology Co, Ltd. The data request will be reviewed, and if agreed, the requestors will need to sign a data sharing agreement.