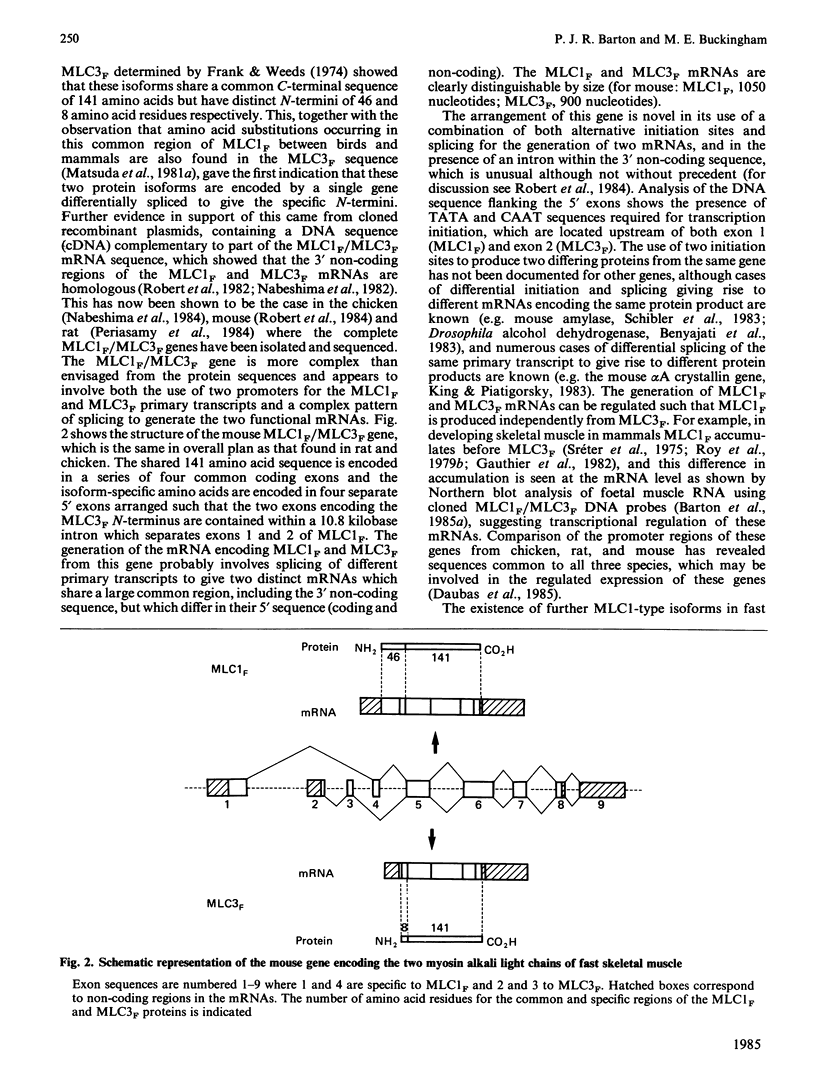
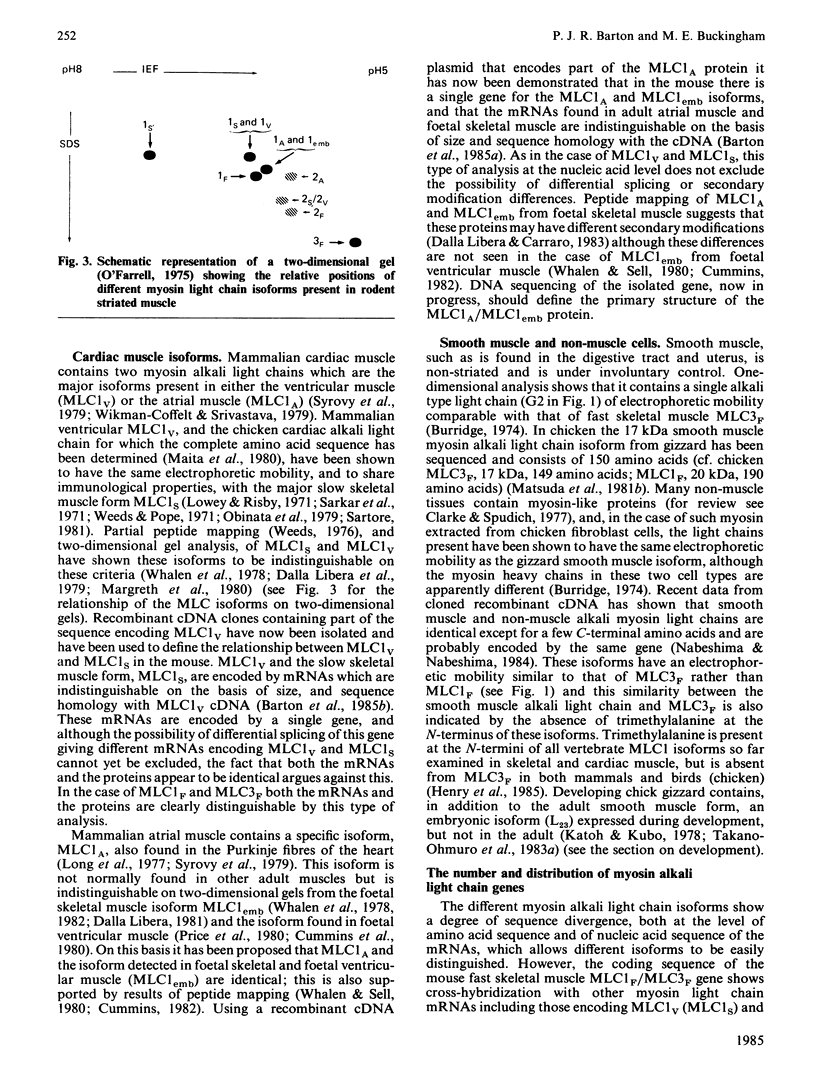
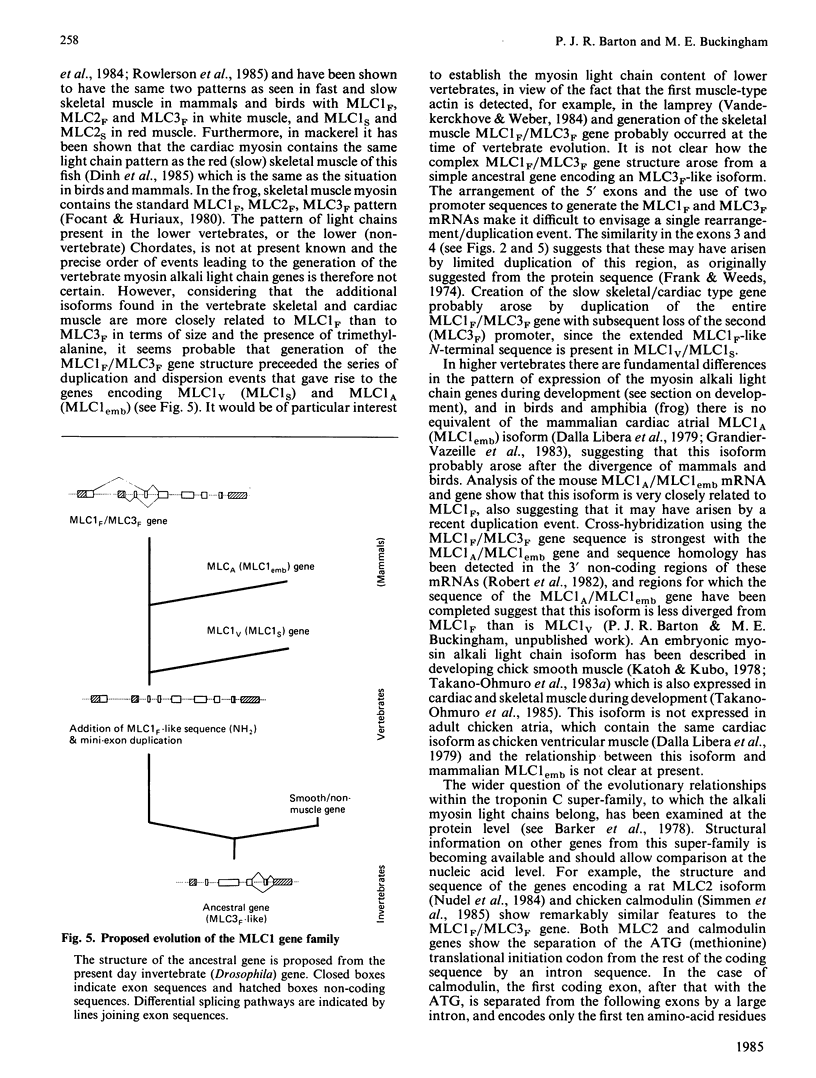
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandman E. Myosin components of the latissimus dorsi and the pectoralis major muscles in the dystrophic chicken. Muscle Nerve. 1984 May;7(4):312–326. doi: 10.1002/mus.880070410. [DOI] [PubMed] [Google Scholar]
- Barton P. J., Cohen A., Robert B., Fiszman M. Y., Bonhomme F., Guénet J. L., Leader D. P., Buckingham M. E. The myosin alkali light chains of mouse ventricular and slow skeletal muscle are indistinguishable and are encoded by the same gene. J Biol Chem. 1985 Jul 15;260(14):8578–8584. [PubMed] [Google Scholar]
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bernards R., Little P. F., Annison G., Williamson R., Flavell R. A. Structure of the human G gamma-A gamma-delta-beta-globin gene locus. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4827–4831. doi: 10.1073/pnas.76.10.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein S. I., Mogami K., Donady J. J., Emerson C. P., Jr Drosophila muscle myosin heavy chain encoded by a single gene in a cluster of muscle mutations. 1983 Mar 31-Apr 6Nature. 302(5907):393–397. doi: 10.1038/302393a0. [DOI] [PubMed] [Google Scholar]
- Billeter R., Heizmann C. W., Howald H., Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. Eur J Biochem. 1981 May 15;116(2):389–395. doi: 10.1111/j.1432-1033.1981.tb05347.x. [DOI] [PubMed] [Google Scholar]
- Biral D., Damiani E., Margreth A., Scarpini E. Myosin subunit composition in human developing muscle. Biochem J. 1984 Dec 15;224(3):923–931. doi: 10.1042/bj2240923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biral D., Damiani E., Volpe P., Salviati G., Margreth A. Polymorphism of myosin light chains. An electrophoretic and immunological study of rabbit skeletal-muscle myosins. Biochem J. 1982 Jun 1;203(3):529–540. doi: 10.1042/bj2030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blau H. M., Chiu C. P., Webster C. Cytoplasmic activation of human nuclear genes in stable heterocaryons. Cell. 1983 Apr;32(4):1171–1180. doi: 10.1016/0092-8674(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Brown W. E., Salmons S., Whalen R. G. The sequential replacement of myosin subunit isoforms during muscle type transformation induced by long term electrical stimulation. J Biol Chem. 1983 Dec 10;258(23):14686–14692. [PubMed] [Google Scholar]
- Buckingham M. E. Actin and myosin multigene families: their expression during the formation of skeletal muscle. Essays Biochem. 1985;20:77–109. [PubMed] [Google Scholar]
- Bugaisky L. B., Siegel E., Whalen R. G. Myosin isozyme changes in the heart following constriction of the ascending aorta of a 25-day old rat. FEBS Lett. 1983 Sep 19;161(2):230–234. doi: 10.1016/0014-5793(83)81014-x. [DOI] [PubMed] [Google Scholar]
- Bullard B., Dabrowska R., Winkelman L. The contractile and regulatory proteins of insect flight muscle. Biochem J. 1973 Oct;135(2):277–286. doi: 10.1042/bj1350277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K. A comparison of fibroblast and smooth muscle myosins. FEBS Lett. 1974 Sep 1;45(1):14–17. doi: 10.1016/0014-5793(74)80799-4. [DOI] [PubMed] [Google Scholar]
- Butler-Browne G. S., Whalen R. G. Myosin isozyme transitions occurring during the postnatal development of the rat soleus muscle. Dev Biol. 1984 Apr;102(2):324–334. doi: 10.1016/0012-1606(84)90197-0. [DOI] [PubMed] [Google Scholar]
- Caravatti M., Minty A., Robert B., Montarras D., Weydert A., Cohen A., Daubas P., Buckingham M. Regulation of muscle gene expression. The accumulation of messenger RNAs coding for muscle-specific proteins during myogenesis in a mouse cell line. J Mol Biol. 1982 Sep;160(1):59–76. doi: 10.1016/0022-2836(82)90131-0. [DOI] [PubMed] [Google Scholar]
- Carlsson S. A., Luger O., Ringertz N. R., Savage R. E. Phenotypic expression in chick erythrocyte x rat myoblast hybrids and in chick myoblast x rat myoblast hybrids. Exp Cell Res. 1974 Mar 15;84(1):47–55. doi: 10.1016/0014-4827(74)90378-4. [DOI] [PubMed] [Google Scholar]
- Carmon Y., Czosnek H., Nudel U., Shani M., Yaffe D. DNAase I sensitivity of genes expressed during myogenesis. Nucleic Acids Res. 1982 May 25;10(10):3085–3098. doi: 10.1093/nar/10.10.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Crow M. T., Olson P. S., Stockdale F. E. Myosin light-chain expression during avian muscle development. J Cell Biol. 1983 Mar;96(3):736–744. doi: 10.1083/jcb.96.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins P., Price K. M., Littler W. A. Foetal myosin light chain in human ventricle. J Muscle Res Cell Motil. 1980 Sep;1(3):357–366. doi: 10.1007/BF00711936. [DOI] [PubMed] [Google Scholar]
- Cummins P. Transitions in human atrial and ventricular myosin light-chain isoenzymes in response to cardiac-pressure-overload-induced hypertrophy. Biochem J. 1982 Jul 1;205(1):195–204. doi: 10.1042/bj2050195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnek H., Nudel U., Mayer Y., Barker P. E., Pravtcheva D. D., Ruddle F. H., Yaffe D. The genes coding for the cardiac muscle actin, the skeletal muscle actin and the cytoplasmic beta-actin are located on three different mouse chromosomes. EMBO J. 1983;2(11):1977–1979. doi: 10.1002/j.1460-2075.1983.tb01687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnek H., Nudel U., Shani M., Barker P. E., Pravtcheva D. D., Ruddle F. H., Yaffe D. The genes coding for the muscle contractile proteins, myosin heavy chain, myosin light chain 2, and skeletal muscle actin are located on three different mouse chromosomes. EMBO J. 1982;1(11):1299–1305. doi: 10.1002/j.1460-2075.1982.tb01314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Libera L., Carraro U. The suggested identity of myosin light chain of cardiac atrial muscle and embryonic skeletal muscle may be excluded by proteolytic mapping. Cell Biol Int Rep. 1983 Apr;7(4):271–273. doi: 10.1016/0309-1651(83)90061-9. [DOI] [PubMed] [Google Scholar]
- Daubas P., Caput D., Buckingham M., Gros F. A comparison between the synthesis of contractile proteins and the accumulation of their translatable mRNAs during calf myoblast differentiation. Dev Biol. 1981 May;84(1):133–143. doi: 10.1016/0012-1606(81)90377-8. [DOI] [PubMed] [Google Scholar]
- Daubas P., Robert B., Garner I., Buckingham M. A comparison between mammalian and avian fast skeletal muscle alkali myosin light chain genes: regulatory implications. Nucleic Acids Res. 1985 Jul 11;13(13):4623–4643. doi: 10.1093/nar/13.13.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenthal S., Parker V. P., Davidson N. Developmental variations in the splicing pattern of transcripts from the Drosophila gene encoding myosin alkali light chain result in different carboxyl-terminal amino acid sequences. Proc Natl Acad Sci U S A. 1985 Jan;82(2):449–453. doi: 10.1073/pnas.82.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenthal S., Parker V. P., Mattox W. W., Davidson N. Drosophila melanogaster has only one myosin alkali light-chain gene which encodes a protein with considerable amino acid sequence homology to chicken myosin alkali light chains. Mol Cell Biol. 1984 May;4(5):956–965. doi: 10.1128/mcb.4.5.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimons R. B., Hoh J. F. Myosin isoenzymes in fast-twitch and slow-twitch muscles of normal and dystrophic mice. J Physiol. 1983 Oct;343:539–550. doi: 10.1113/jphysiol.1983.sp014908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicker P. F., Wallimann T., Vibert P. Electron microscopy of scallop myosin. Location of regulatory light chains. J Mol Biol. 1983 Sep 25;169(3):723–741. doi: 10.1016/s0022-2836(83)80167-3. [DOI] [PubMed] [Google Scholar]
- Focant B., Huriaux F. Preparation of frog myosin. Isolation and characterization of the light chains. J Muscle Res Cell Motil. 1980 Mar;1(1):61–72. doi: 10.1007/BF00711925. [DOI] [PubMed] [Google Scholar]
- Frank G., Weeds A. G. The amino-acid sequence of the alkali light chains of rabbit skeletal-muscle myosin. Eur J Biochem. 1974 May 15;44(2):317–334. doi: 10.1111/j.1432-1033.1974.tb03489.x. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Gambke B., Lyons G. E., Haselgrove J., Kelly A. M., Rubinstein N. A. Thyroidal and neural control of myosin transitions during development of rat fast and slow muscles. FEBS Lett. 1983 Jun 13;156(2):335–339. doi: 10.1016/0014-5793(83)80524-9. [DOI] [PubMed] [Google Scholar]
- Gauthier G. F., Burke R. E., Lowey S., Hobbs A. W. Myosin isozymes in normal and cross-reinnervated cat skeletal muscle fibers. J Cell Biol. 1983 Sep;97(3):756–771. doi: 10.1083/jcb.97.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier G. F., Lowey S., Benfield P. A., Hobbs A. W. Distribution and properties of myosin isozymes in developing avian and mammalian skeletal muscle fibers. J Cell Biol. 1982 Feb;92(2):471–484. doi: 10.1083/jcb.92.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandier-Vazeille X., Tetaert D., Hemon B., Biserte G. Phylogenetic studies of cardiac myosins from amphibia to mammals. Comp Biochem Physiol B. 1983;76(2):263–270. doi: 10.1016/0305-0491(83)90068-8. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Kedes L., Hickey R. J., Skoultchi A. I. Expression of human cardiac actin in mouse L cells: a sarcomeric actin associates with a nonmuscle cytoskeleton. Cell. 1984 Mar;36(3):709–715. doi: 10.1016/0092-8674(84)90351-9. [DOI] [PubMed] [Google Scholar]
- Heilig A., Pette D. Changes in transcriptional activity of chronically stimulated fast twitch muscle. FEBS Lett. 1983 Jan 24;151(2):211–214. doi: 10.1016/0014-5793(83)80071-4. [DOI] [PubMed] [Google Scholar]
- Henry G. D., Trayer I. P., Brewer S., Levine B. A. The widespread distribution of alpha-N-trimethylalanine as the N-terminal amino acid of light chains from vertebrate striated muscle myosins. Eur J Biochem. 1985 Apr 1;148(1):75–82. doi: 10.1111/j.1432-1033.1985.tb08809.x. [DOI] [PubMed] [Google Scholar]
- Ingram R. S., Scott R. W., Tilghman S. M. alpha-Fetoprotein and albumin genes are in tandem in the mouse genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4694–4698. doi: 10.1073/pnas.78.8.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izant J. G., Weintraub H. Inhibition of thymidine kinase gene expression by anti-sense RNA: a molecular approach to genetic analysis. Cell. 1984 Apr;36(4):1007–1015. doi: 10.1016/0092-8674(84)90050-3. [DOI] [PubMed] [Google Scholar]
- Jakob H., Buckingham M. E., Cohen A., Dupont L., Fiszman M., Jacob F. A skeletal muscle cell line isolated from a mouse teratocarcinoma undergoes apparently normal terminal differentiation in vitro. Exp Cell Res. 1978 Jul;114(2):403–408. doi: 10.1016/0014-4827(78)90499-8. [DOI] [PubMed] [Google Scholar]
- John H. A. The myosin of developing and dystrophic skeletal muscle. FEBS Lett. 1974 Mar 1;39(3):278–282. doi: 10.1016/0014-5793(74)80130-4. [DOI] [PubMed] [Google Scholar]
- Johnson M. A., Mastaglia F. L., Montgomery A. G., Pope B., Weeds A. G. Changes in myosin light chains in the rat soleus after thyroidectomy. FEBS Lett. 1980 Feb 11;110(2):230–235. doi: 10.1016/0014-5793(80)80080-9. [DOI] [PubMed] [Google Scholar]
- Katoh N., Kubo S. Light chains of chicken embryonic gizzard myosin. Biochim Biophys Acta. 1978 Aug 21;535(2):401–411. doi: 10.1016/0005-2795(78)90105-8. [DOI] [PubMed] [Google Scholar]
- Keller L. R., Emerson C. P., Jr Synthesis of adult myosin light chains by embryonic muscle cultures. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1020–1024. doi: 10.1073/pnas.77.2.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick-Jones J., Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory light chains in myosins. J Mol Biol. 1976 Jul 15;104(4):747–775. doi: 10.1016/0022-2836(76)90180-7. [DOI] [PubMed] [Google Scholar]
- King C. R., Piatigorsky J. Alternative RNA splicing of the murine alpha A-crystallin gene: protein-coding information within an intron. Cell. 1983 Mar;32(3):707–712. doi: 10.1016/0092-8674(83)90056-9. [DOI] [PubMed] [Google Scholar]
- Ko J., Horiuchi S., Yamaguchi M. Myosin from abdominal flexor muscle in a crayfish, Procambarus clarki Girard. J Biochem. 1979 Feb;85(2):541–548. doi: 10.1093/oxfordjournals.jbchem.a132362. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Biochemistry of actomyosin-dependent cell motility (a review). Proc Natl Acad Sci U S A. 1978 Feb;75(2):588–599. doi: 10.1073/pnas.75.2.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinwand L. A., Fournier R. E., Nadal-Ginard B., Shows T. B. Multigene family for sarcomeric myosin heavy chain in mouse and human DNA: localization on a single chromosome. Science. 1983 Aug 19;221(4612):766–769. doi: 10.1126/science.6879174. [DOI] [PubMed] [Google Scholar]
- Levine M., Hafen E., Garber R. L., Gehring W. J. Spatial distribution of Antennapedia transcripts during Drosophila development. EMBO J. 1983;2(11):2037–2046. doi: 10.1002/j.1460-2075.1983.tb01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libera L. D., Sartore S., Schiaffino S. Comparative analysis of chicken atrial and ventricular myosins. Biochim Biophys Acta. 1979 Dec 14;581(2):283–294. doi: 10.1016/0005-2795(79)90248-4. [DOI] [PubMed] [Google Scholar]
- Long L., Fabian F., Mason D. T., Wikman-Coffelt J. A new cardiac myosin characterized from the canine atria. Biochem Biophys Res Commun. 1977 Jun 6;76(3):626–635. doi: 10.1016/0006-291x(77)91547-9. [DOI] [PubMed] [Google Scholar]
- Lowey S., Benfield P. A., LeBlanc D. D., Waller G. S. Myosin isozymes in avian skeletal muscles. I. Sequential expression of myosin isozymes in developing chicken pectoralis muscles. J Muscle Res Cell Motil. 1983 Dec;4(6):695–716. doi: 10.1007/BF00712161. [DOI] [PubMed] [Google Scholar]
- Lowey S., Risby D. Light chains from fast and slow muscle myosins. Nature. 1971 Nov 12;234(5324):81–85. doi: 10.1038/234081a0. [DOI] [PubMed] [Google Scholar]
- Maita T., Umegane T., Kato Y., Matsuda G. Amino-acid sequence of the L-1 light chain of chicken cardiac-muscle myosin. Eur J Biochem. 1980 Jun;107(2):565–575. doi: 10.1111/j.1432-1033.1980.tb06064.x. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Maita T., Kato Y., Chen J. I., Umegane T. Amino acid sequences of the cardiac L-2A, L-2B and gizzard 17 000-Mr light chains of chicken muscle myosin. FEBS Lett. 1981 Dec 7;135(2):232–236. doi: 10.1016/0014-5793(81)80789-2. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Bandman E., Strohman R. C. Regional differences in the expression of myosin light chains and tropomyosin subunits during development of chicken breast muscle. Dev Biol. 1983 Feb;95(2):484–491. doi: 10.1016/0012-1606(83)90050-7. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Spector D., Strohman R. C. Denervated skeletal muscle displays discoordinate regulation for the synthesis of several myofibrillar proteins. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1122–1125. doi: 10.1073/pnas.81.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloul D., Aloni B., Calvo J., Yaffe D., Nudel U. Developmentally regulated expression of chimeric genes containing muscle actin DNA sequences in transfected myogenic cells. EMBO J. 1984 May;3(5):983–990. doi: 10.1002/j.1460-2075.1984.tb01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Caravatti M., Buckingham M. E. A fetal skeletal muscle actin mRNA in the mouse and its identity with cardiac actin mRNA. Cell. 1982 Aug;30(1):185–192. doi: 10.1016/0092-8674(82)90024-1. [DOI] [PubMed] [Google Scholar]
- Minty A. J., Alonso S., Guénet J. L., Buckingham M. E. Number and organization of actin-related sequences in the mouse genome. J Mol Biol. 1983 Jun 15;167(1):77–101. doi: 10.1016/s0022-2836(83)80035-7. [DOI] [PubMed] [Google Scholar]
- Moerman D. G., Plurad S., Waterston R. H., Baillie D. L. Mutations in the unc-54 myosin heavy chain gene of Caenorhabditis elegans that alter contractility but not muscle structure. Cell. 1982 Jul;29(3):773–781. doi: 10.1016/0092-8674(82)90439-1. [DOI] [PubMed] [Google Scholar]
- Montarras D., Fiszman M. Y. A new muscle phenotype is expressed by subcultured quail myoblasts isolated from future fast and slow muscles. J Biol Chem. 1983 Mar 25;258(6):3883–3888. [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Alternative transcription and two modes of splicing results in two myosin light chains from one gene. Nature. 1984 Mar 22;308(5957):333–338. doi: 10.1038/308333a0. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y., Fujii-Kuriyama Y., Muramatsu M., Ogata K. Molecular cloning and nucleotide sequences of the complementary DNAs to chicken skeletal muscle myosin two alkali light chain mRNAs. Nucleic Acids Res. 1982 Oct 11;10(19):6099–6110. doi: 10.1093/nar/10.19.6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T., Yanagisawa T., Yamaguchi M. Studies on the subunits of myosin from muscle layer of Ascaris lumbricoides suum. Biochim Biophys Acta. 1975 Dec 15;412(2):229–240. doi: 10.1016/0005-2795(75)90037-9. [DOI] [PubMed] [Google Scholar]
- Nudel U., Calvo J. M., Shani M., Levy Z. The nucleotide sequence of a rat myosin light chain 2 gene. Nucleic Acids Res. 1984 Sep 25;12(18):7175–7186. doi: 10.1093/nar/12.18.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Obinata T., Masaki T., Takano-Ohmuro H., Tanaka T., Shimizu N. Coexistence of cardiac-type and fast skeletal-type myosin light chains in embryonic chicken cardiac muscle. J Biochem. 1983 Sep;94(3):1025–1028. doi: 10.1093/oxfordjournals.jbchem.a134401. [DOI] [PubMed] [Google Scholar]
- Obinata T., Masaki T., Takano H. Immunochemical comparison of myosin light chains from chicken fast white, slow red, and cardiac muscle. J Biochem. 1979 Jul;86(1):131–137. [PubMed] [Google Scholar]
- Obinata T., Masaki T., Takano H. Types of myosin light chains present during the development of fast skeletal muscle in chick embryo. J Biochem. 1980 Jan;87(1):81–88. doi: 10.1093/oxfordjournals.jbchem.a132755. [DOI] [PubMed] [Google Scholar]
- Pelloni-muller G., Ermini M., Jenny E. Myosin light chains of developing fast and slow rabbit skeletal muscle. FEBS Lett. 1976 Aug 1;67(1):68–74. doi: 10.1016/0014-5793(76)80872-1. [DOI] [PubMed] [Google Scholar]
- Periasamy M., Strehler E. E., Garfinkel L. I., Gubits R. M., Ruiz-Opazo N., Nadal-Ginard B. Fast skeletal muscle myosin light chains 1 and 3 are produced from a single gene by a combined process of differential RNA transcription and splicing. J Biol Chem. 1984 Nov 10;259(21):13595–13604. [PubMed] [Google Scholar]
- Pette D., Henriksson J., Emmerich M. Myofibrillar protein patterns of single fibres from human muscle. FEBS Lett. 1979 Jul 1;103(1):152–155. doi: 10.1016/0014-5793(79)81270-3. [DOI] [PubMed] [Google Scholar]
- Pope B. J., Wagner P. D., Weeds A. G. Heterogeneity of myosin heavy chains in subfragment-1 isoenzymes rabbit skeletal myosin. J Mol Biol. 1977 Jan 25;109(3):470–473. doi: 10.1016/s0022-2836(77)80024-7. [DOI] [PubMed] [Google Scholar]
- Price K. M., Littler W. A., Cummins P. Human atrial and ventricular myosin light-chains subunits in the adult and during development. Biochem J. 1980 Nov 1;191(2):571–580. doi: 10.1042/bj1910571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert B., Barton P., Minty A., Daubas P., Weydert A., Bonhomme F., Catalan J., Chazottes D., Guénet J. L., Buckingham M. Investigation of genetic linkage between myosin and actin genes using an interspecific mouse back-cross. Nature. 1985 Mar 14;314(6007):181–183. doi: 10.1038/314181a0. [DOI] [PubMed] [Google Scholar]
- Robert B., Daubas P., Akimenko M. A., Cohen A., Garner I., Guenet J. L., Buckingham M. A single locus in the mouse encodes both myosin light chains 1 and 3, a second locus corresponds to a related pseudogene. Cell. 1984 Nov;39(1):129–140. doi: 10.1016/0092-8674(84)90198-3. [DOI] [PubMed] [Google Scholar]
- Robert B., Weydert A., Caravatti M., Minty A., Cohen A., Daubas P., Gros F., Buckingham M. cDNA recombinant plasmid complementary to mRNAs for light chains 1 and 3 of mouse skeletal muscle myosin. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2437–2441. doi: 10.1073/pnas.79.8.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy R. K., Mabuchi K., Sarkar S., Mis C., Sreter F. A. Changes in tropomyosin subunit pattern in chronic electrically stimulated rabbit fast muscles. Biochem Biophys Res Commun. 1979 Jul 12;89(1):181–187. doi: 10.1016/0006-291x(79)90961-6. [DOI] [PubMed] [Google Scholar]
- Roy R. K., Sreter F. A., Sarkar S. Changes in tropomyosin subunits and myosin light chains during development of chicken and rabbit striated muscles. Dev Biol. 1979 Mar;69(1):15–30. doi: 10.1016/0012-1606(79)90271-9. [DOI] [PubMed] [Google Scholar]
- Rubinstein N. A., Kelly A. M. Myogenic and neurogenic contributions to the development of fast and slow twitch muscles in rat. Dev Biol. 1978 Feb;62(2):473–485. doi: 10.1016/0012-1606(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Rushbrook J. I., Somes R. G., Jr Myosin light chain-1: genetic analysis of three variants found in fast white chicken muscle and investigation of linkage with the muscular dystrophy gene. Biochem Genet. 1985 Feb;23(1-2):17–27. doi: 10.1007/BF00499109. [DOI] [PubMed] [Google Scholar]
- Rushbrook J. I., Yuan A. I., Stracher A. Two major allelic forms of myosin light chain-1 in strains of normal and dystrophic chickens. Muscle Nerve. 1982 Sep;5(7):505–514. doi: 10.1002/mus.880050705. [DOI] [PubMed] [Google Scholar]
- Rutz R., Hauschka S. Clonal analysis of vertebrate myogenesis. VII. Heritability of muscle colony type through sequential subclonal passages in vitro. Dev Biol. 1982 May;91(1):103–110. doi: 10.1016/0012-1606(82)90013-6. [DOI] [PubMed] [Google Scholar]
- Salviati G., Betto R., Danieli Betto D. Polymorphism of myofibrillar proteins of rabbit skeletal-muscle fibres. An electrophoretic study of single fibres. Biochem J. 1982 Nov 1;207(2):261–272. doi: 10.1042/bj2070261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G., Betto R., Danieli Betto D., Zeviani M. Myofibrillar-protein isoforms and sarcoplasmic-reticulum Ca2+-transport activity of single human muscle fibres. Biochem J. 1984 Nov 15;224(1):215–225. doi: 10.1042/bj2240215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S., Sreter F. A., Gergely J. Light chains of myosins from white, red, and cardiac muscles. Proc Natl Acad Sci U S A. 1971 May;68(5):946–950. doi: 10.1073/pnas.68.5.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartore S. Immunological cross-reactivity between chicken slow skeletal and ventricular muscle myosin. Biochim Biophys Acta. 1981 Jan 30;667(1):143–156. doi: 10.1016/0005-2795(81)90075-1. [DOI] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Harvey E. V. Localization of a light-chain binding site on smooth muscle myosin revealed by light-chain overlay of sodium dodecyl sulfate-polyacrylamide electrophoretic gels. J Biol Chem. 1984 Nov 25;259(22):14203–14207. [PubMed] [Google Scholar]
- Simmen R. C., Tanaka T., Ts'ui K. F., Putkey J. A., Scott M. J., Lai E. C., Means A. R. The structural organization of the chicken calmodulin gene. J Biol Chem. 1985 Jan 25;260(2):907–912. [PubMed] [Google Scholar]
- Sivaramakrishnan M., Burke M. The free heavy chain of vertebrate skeletal myosin subfragment 1 shows full enzymatic activity. J Biol Chem. 1982 Jan 25;257(2):1102–1105. [PubMed] [Google Scholar]
- Sréter F. A., Bálint M., Gergely J. Structural and functional changes of myosin during development: comparison with adult fast, slow and cardiac myosin. Dev Biol. 1975 Oct;46(2):317–325. doi: 10.1016/0012-1606(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Gergely J. The effect of cross reinnervation on the synthesis of myosin light chains. Biochem Biophys Res Commun. 1974 Jan;56(1):84–89. doi: 10.1016/s0006-291x(74)80318-9. [DOI] [PubMed] [Google Scholar]
- Stockdale F. E., Baden H., Raman N. Slow muscle myoblasts differentiating in vitro synthesize both slow and fast myosin light chains. Dev Biol. 1981 Feb;82(1):168–171. doi: 10.1016/0012-1606(81)90438-3. [DOI] [PubMed] [Google Scholar]
- Stockdale F. E., Raman N., Baden H. Myosin light chains and the developmental origin of fast muscle. Proc Natl Acad Sci U S A. 1981 Feb;78(2):931–935. doi: 10.1073/pnas.78.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strohman R. C., Micou-Eastwood J., Glass C. A., Matsuda R. Human fetal muscle and cultured myotubes derived from it contain a fetal-specific myosin light chain. Science. 1983 Sep 2;221(4614):955–957. doi: 10.1126/science.6879193. [DOI] [PubMed] [Google Scholar]
- Syrovy I., Delcayre C., Swynghedauw B. Comparison of ATPase activity and light subunits in myosins from left and right ventricles and atria in seven mammalian species. J Mol Cell Cardiol. 1979 Oct;11(10):1129–1135. doi: 10.1016/0022-2828(79)90398-5. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Tonomura Y. Developmental changes in the structure and kinetic properties of myosin adenosinetriphosphatase of rabbit skeletal fast muscle. J Biochem. 1975 Dec;78(6):1123–1133. doi: 10.1093/oxfordjournals.jbchem.a131008. [DOI] [PubMed] [Google Scholar]
- Takano-Ohmuro H., Hirose G., Mikawa T. Separation and identification of Drosophila myosin light chains. J Biochem. 1983 Sep;94(3):967–974. doi: 10.1093/oxfordjournals.jbchem.a134440. [DOI] [PubMed] [Google Scholar]
- Takano-Ohmuro H., Obinata T., Mikawa T., Masaki T. Changes in myosin isozymes during development of chicken gizzard muscle. J Biochem. 1983 Mar;93(3):903–908. doi: 10.1093/jb/93.3.903. [DOI] [PubMed] [Google Scholar]
- Trayer H. R., Trayer I. P. Differential binding of rabbit fast muscle myosin light chain isoenzymes to regulated actin. FEBS Lett. 1985 Jan 28;180(2):170–173. doi: 10.1016/0014-5793(85)81065-6. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Actin amino-acid sequences. Comparison of actins from calf thymus, bovine brain, and SV40-transformed mouse 3T3 cells with rabbit skeletal muscle actin. Eur J Biochem. 1978 Oct 16;90(3):451–462. doi: 10.1111/j.1432-1033.1978.tb12624.x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Chordate muscle actins differ distinctly from invertebrate muscle actins. The evolution of the different vertebrate muscle actins. J Mol Biol. 1984 Nov 5;179(3):391–413. doi: 10.1016/0022-2836(84)90072-x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Volpe P., Damiani E., Margreth A., Pellegrini G., Scarlato G. Fast to slow change of myosin in nemaline myopathy: electrophoretic and immunologic evidence. Neurology. 1982 Jan;32(1):37–41. doi: 10.1212/wnl.32.1.37. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Hydrolysis of ATP and reversible binding to F-actin by myosin heavy chains free of all light chains. Nature. 1981 Aug 6;292(5823):560–562. doi: 10.1038/292560a0. [DOI] [PubMed] [Google Scholar]
- Watabe S., Dinh T. N., Ochiai Y., Hashimoto K. Immunochemical specificity of myosin light chains from mackerel ordinary and dark muscles. J Biochem. 1983 Nov;94(5):1409–1419. [PubMed] [Google Scholar]
- Watabe S., Hashimoto K., Watanabe S. The pH-dependency of myosin ATPases from yellowtail ordinary and dark muscles. J Biochem. 1983 Dec;94(6):1867–1875. doi: 10.1093/oxfordjournals.jbchem.a134540. [DOI] [PubMed] [Google Scholar]
- Weeds A. G. Light chains from slow-twitch muscle myosin. Eur J Biochem. 1976 Jun 15;66(1):157–173. doi: 10.1111/j.1432-1033.1976.tb10436.x. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Pope B. Chemical studies on light chains from cardiac and skeletal muscle myosins. Nature. 1971 Nov 12;234(5324):85–88. doi: 10.1038/234085a0. [DOI] [PubMed] [Google Scholar]
- Weeds A. G., Trentham D. R., Kean C. J., Buller A. J. Myosin from cross-reinnervated cat muscles. Nature. 1974 Jan 18;247(5437):135–139. doi: 10.1038/247135a0. [DOI] [PubMed] [Google Scholar]
- Weydert A., Daubas P., Caravatti M., Minty A., Bugaisky G., Cohen A., Robert B., Buckingham M. Sequential accumulation of mRNAs encoding different myosin heavy chain isoforms during skeletal muscle development in vivo detected with a recombinant plasmid identified as coding for an adult fast myosin heavy chain from mouse skeletal muscle. J Biol Chem. 1983 Nov 25;258(22):13867–13874. [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Identification of a novel form of myosin light chain present in embryonic muscle tissue and cultured muscle cells. J Mol Biol. 1978 Dec 15;126(3):415–431. doi: 10.1016/0022-2836(78)90049-9. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Sell S., Gros F. Transitions in contractile protein isozymes during muscle cell differentiation. Biochimie. 1979;61(5-6):625–632. doi: 10.1016/s0300-9084(79)80160-1. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Butler-Browne G. S., Schwartz K., Bouveret P., Pinset-Härstöm I. Three myosin heavy-chain isozymes appear sequentially in rat muscle development. Nature. 1981 Aug 27;292(5826):805–809. doi: 10.1038/292805a0. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M., Eriksson A., Thornell L. E. Myosin subunit types in skeletal and cardiac tissues and their developmental distribution. Dev Biol. 1982 Jun;91(2):478–484. doi: 10.1016/0012-1606(82)90055-0. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M. Myosin from fetal hearts contains the skeletal muscle embryonic light chain. Nature. 1980 Aug 14;286(5774):731–733. doi: 10.1038/286731a0. [DOI] [PubMed] [Google Scholar]
- Wikman-Coffelt J., Srivastava S. Differences in atrial and ventricular myosin light chain LC1. FEBS Lett. 1979 Oct 1;106(1):207–212. doi: 10.1016/0014-5793(79)80729-2. [DOI] [PubMed] [Google Scholar]
- Winstanley M. A., Small D. A., Trayer I. P. Differential binding of myosin subfragment one species to immobilized ADP, and actin: the influence of the alkali light chains. Eur J Biochem. 1979 Aug 1;98(2):441–446. doi: 10.1111/j.1432-1033.1979.tb13204.x. [DOI] [PubMed] [Google Scholar]
- Wright W. E. Induction of myosin light chain synthesis in heterokaryons between normal diploid cells. In Vitro. 1982 Oct;18(10):851–858. doi: 10.1007/BF02796326. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Cellular aspects of muscle differentiation in vitro. Curr Top Dev Biol. 1969;4:37–77. doi: 10.1016/s0070-2153(08)60480-9. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]


