Abstract
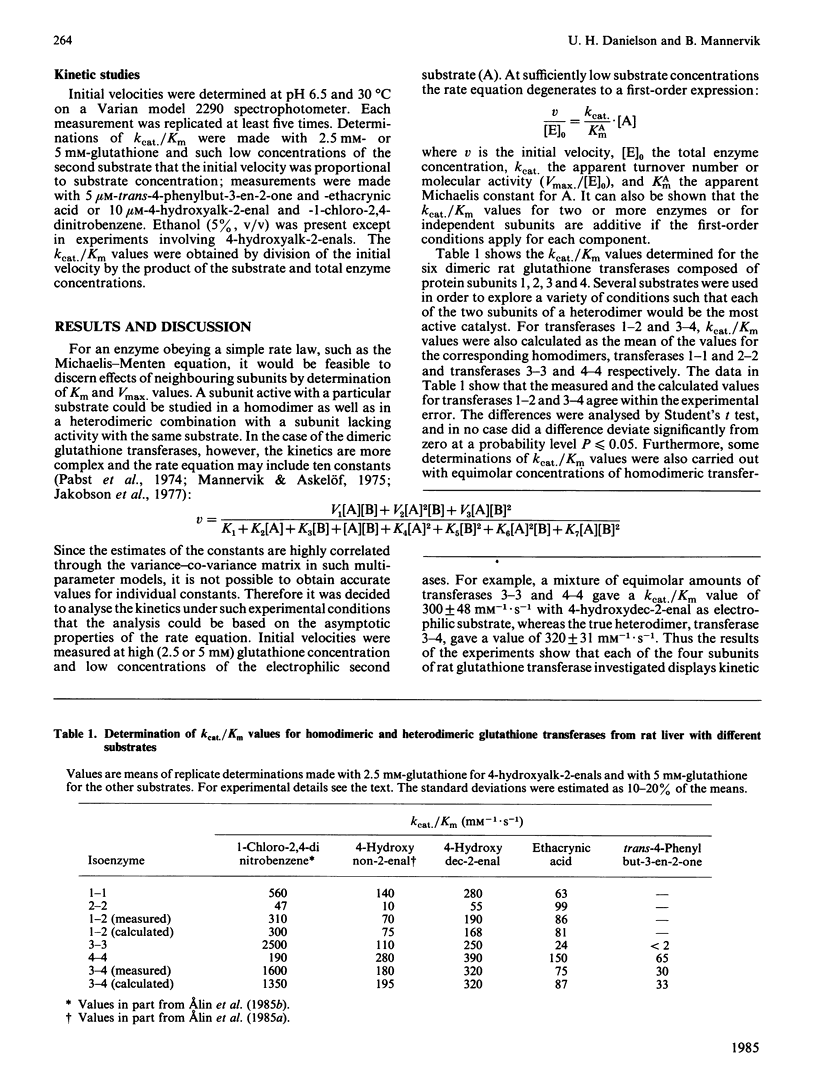
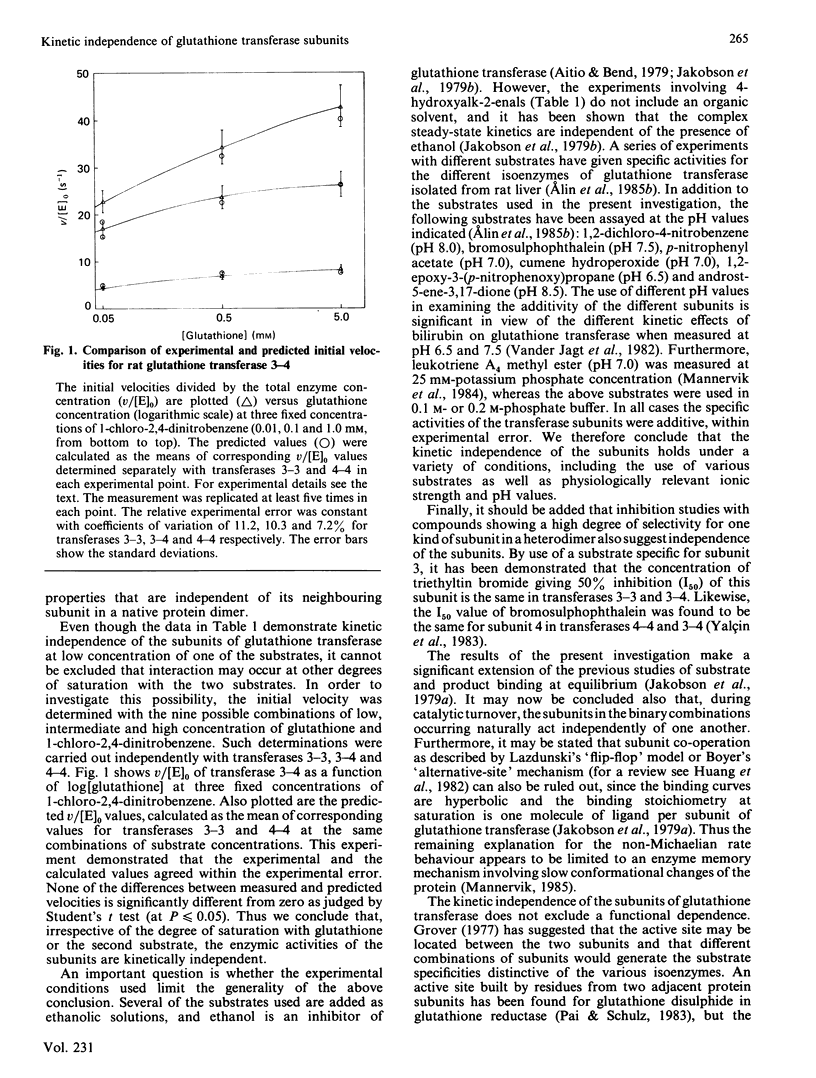
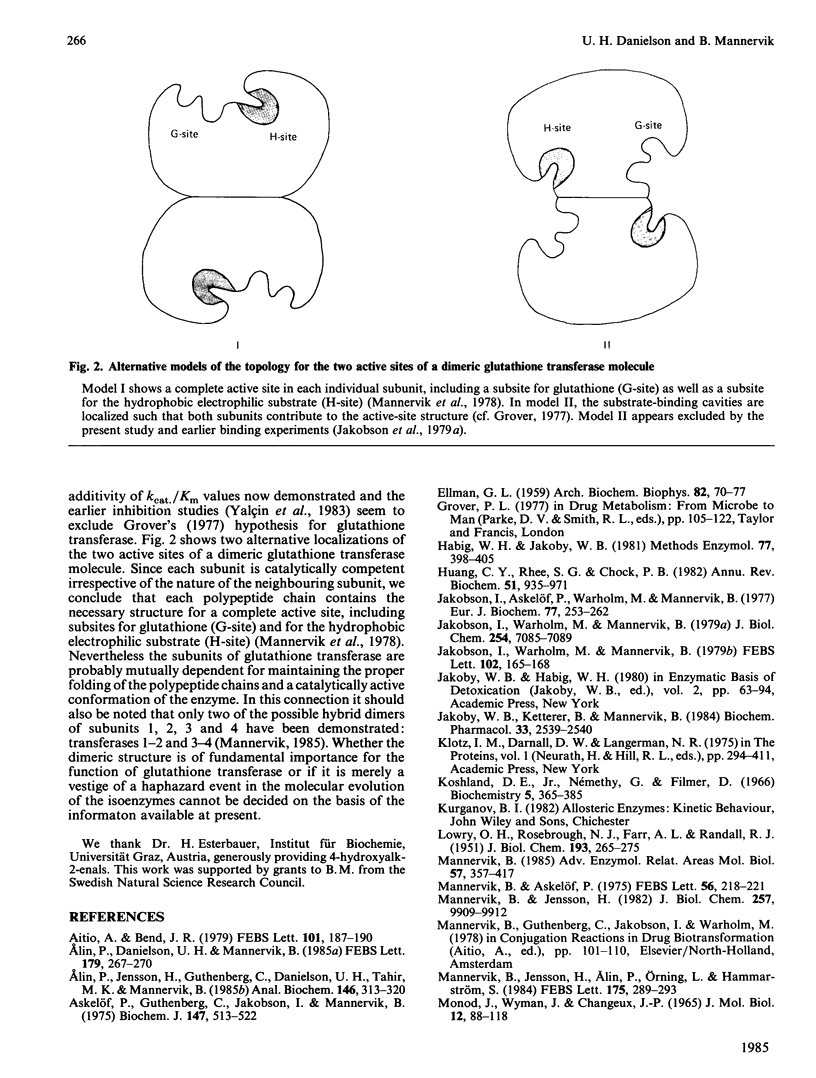
The steady-state kinetics of the dimeric glutathione transferases deviate from Michaelis-Menten kinetics, but have hyperbolic binding isotherms for substrates and products of the enzymic reaction. The possibility of subunit interactions during catalysis as an explanation for the rate behaviour was investigated by use of rat isoenzymes composed of subunits 1, 2, 3 and 4, which have distinct substrate specificities. The kinetic parameter kcat./Km was determined with 1-chloro-2,4-dinitrobenzene, 4-hydroxyalk-2-enals, ethacrynic acid and trans-4-phenylbut-3-en-2-one as electrophilic substrates for six isoenzymes: rat glutathione transferases 1-1, 1-2, 2-2, 3-3, 3-4 and 4-4. It was found that the kcat./Km values for the heterodimeric transferases 1-2 and 3-4 could be predicted from the kcat./Km values of the corresponding homodimers. Likewise, the initial velocities determined with transferases 3-3, 3-4 and 4-4 at different degrees of saturation with glutathione and 1-chloro-2,4-dinitrobenzene demonstrated that the kinetic properties of the subunits are additive. These results show that the subunits of glutathione transferase are kinetically independent.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitio A., Bend J. R. Inhibition of rat liver glutathione S-transferase activity by aprotic solvents. FEBS Lett. 1979 May 1;101(1):187–190. doi: 10.1016/0014-5793(79)81323-x. [DOI] [PubMed] [Google Scholar]
- Alin P., Danielson U. H., Mannervik B. 4-Hydroxyalk-2-enals are substrates for glutathione transferase. FEBS Lett. 1985 Jan 7;179(2):267–270. doi: 10.1016/0014-5793(85)80532-9. [DOI] [PubMed] [Google Scholar]
- Alin P., Jensson H., Guthenberg C., Danielson U. H., Tahir M. K., Mannervik B. Purification of major basic glutathione transferase isoenzymes from rat liver by use of affinity chromatography and fast protein liquid chromatofocusing. Anal Biochem. 1985 May 1;146(2):313–320. doi: 10.1016/0003-2697(85)90545-7. [DOI] [PubMed] [Google Scholar]
- Askelöf P., Guthenberg C., Jakobson I., Mannervik B. Purification and characterization of two glutathione S-aryltransferase activities from rat liver. Biochem J. 1975 Jun;147(3):513–522. doi: 10.1042/bj1470513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Huang C. Y., Rhee S. G., Chock P. B. Subunit cooperation and enzymatic catalysis. Annu Rev Biochem. 1982;51:935–971. doi: 10.1146/annurev.bi.51.070182.004443. [DOI] [PubMed] [Google Scholar]
- Jagt D. L., Wilson S. P., Dean V. L., Simons P. C. Bilirubin binding to rat liver ligandins (glutathione S-transferases A and B). Relationship between bilirubin binding and transferase activity. J Biol Chem. 1982 Feb 25;257(4):1997–2001. [PubMed] [Google Scholar]
- Jakobson I., Askelöf P., Warholm M., Mannervik B. A steady-state-kinetic random mechanism for glutathione S-transferase A from rat liver. A model involving kinetically significant enzyme-product complexes in the forward reaction. Eur J Biochem. 1977 Jul 15;77(2):253–262. doi: 10.1111/j.1432-1033.1977.tb11664.x. [DOI] [PubMed] [Google Scholar]
- Jakobson I., Warholm M., Mannervik B. The binding of substrates and a product of the enzymatic reaction to glutathione S-transferase A. J Biol Chem. 1979 Aug 10;254(15):7085–7089. [PubMed] [Google Scholar]
- Jakobsson I., Warholm M., Mannervik B. The effect of ethanol on the steady-state kinetics of glutathione S-transferase A from rat liver. FEBS Lett. 1979 Jun 1;102(1):165–168. doi: 10.1016/0014-5793(79)80951-5. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ketterer B., Mannervik B. Glutathione transferases: nomenclature. Biochem Pharmacol. 1984 Aug 15;33(16):2539–2540. doi: 10.1016/0006-2952(84)90621-x. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Askelöf P. Absence of a ping-pong pathway in the kinetic mechanism of glutathione S-transferase A from rat liver. Evidence based on quantitative comparison of the asymptotic properties of experimental data and alternative rat equations. FEBS Lett. 1975 Aug 15;56(2):218–221. doi: 10.1016/0014-5793(75)81095-7. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H., Alin P., Orning L., Hammarström S. Transformation of leukotriene A4 methyl ester to leukotriene C4 monomethyl ester by cytosolic rat glutathione transferases. FEBS Lett. 1984 Oct 1;175(2):289–293. doi: 10.1016/0014-5793(84)80753-x. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Jensson H. Binary combinations of four protein subunits with different catalytic specificities explain the relationship between six basic glutathione S-transferases in rat liver cytosol. J Biol Chem. 1982 Sep 10;257(17):9909–9912. [PubMed] [Google Scholar]
- Mannervik B. The isoenzymes of glutathione transferase. Adv Enzymol Relat Areas Mol Biol. 1985;57:357–417. doi: 10.1002/9780470123034.ch5. [DOI] [PubMed] [Google Scholar]
- Pabst M. J., Habig W. H., Jakoby W. B. Glutathione S-transferase A. A novel kinetic mechanism in which the major reaction pathway depends on substrate concentration. J Biol Chem. 1974 Nov 25;249(22):7140–7147. [PubMed] [Google Scholar]
- Pai E. F., Schulz G. E. The catalytic mechanism of glutathione reductase as derived from x-ray diffraction analyses of reaction intermediates. J Biol Chem. 1983 Feb 10;258(3):1752–1757. [PubMed] [Google Scholar]
- Yalçin S., Jensson H., Mannervik B. A set of inhibitors for discrimination between the basic isozymes of glutathione transferase in rat liver. Biochem Biophys Res Commun. 1983 Jul 29;114(2):829–834. doi: 10.1016/0006-291x(83)90856-2. [DOI] [PubMed] [Google Scholar]


