Abstract
Introduction
Non-tuberculous mycobacterial (NTM) disease is an underdiagnosed condition that usually manifests as pulmonary infection. Extrapulmonary manifestations are rare and can be easily overlooked or misdiagnosed as tuberculosis or malignancy.
Case report
Herein, we present four cases of extrapulmonary NTM disease in immunocompetent patients. Patient 1 had bone marrow suppression secondary to NTM infection. Patient 2 was diagnosed with Mycobacterium abscessus meningitis, brain abscess and arachnoiditis. Patient 3 had pleural effusion, and fluid cytology revealed Mycobacterium fortuitum. Patient 4 was a 30-year-old male with cervical lymphadenopathy due to NTM. Two patients (case 2 and case 4) were initially diagnosed with tuberculosis but showed no response to anti-tubercular drugs. One patient (case 3) died within seven days of initiation of treatment. The rest of the patients (cases 1 and 2) showed clinical improvement with antimicrobial therapy for NTM species. Case 4 responded well to surgical excision without the need for antibiotics.
Conclusions
Clinicians should be vigilant about the possibility of NTM disease. Early diagnosis is vital to prevent poor outcomes, particularly in the setting of disseminated infections.
Keywords: Non-tuberculous mycobacteria, lymphadenitis, disseminated, immunocompetent
Introduction
Extrapulmonary non-tuberculous mycobacterial (NTM) infections are rare and predominantly seen in immunocompromised individuals. With the increased expertise in laboratory facilities in developing countries like India, NTM is increasingly recognized as an important pathogen in immunocompetent hosts.1 NTM is a common pulmonary pathogen and causes disease in patients who have pre-existing chronic lung pathology like cystic fibrosis, bronchiectasis and previously treated pulmonary tuberculosis.2 The reported incidences of pulmonary and extrapulmonary NTM disease in India range between 0.7% to 34% in different geographical areas.3-5 Many cases with acid-fast bacilli (AFB) positivity and negative GeneXpert are erroneously treated as tuberculosis. Moreover, in case of incomplete response, many of these cases were falsely assumed as drug-resistant tuberculosis,6,7 instead of searching for alternative diagnoses such as NTM.
There are very few clinical studies which elucidate the course and natural history of extrapulmonary NTM disease. Instead, most of the published data is focused on microbiological aspects of NTM infections, especially pulmonary disease. We herein report four interesting cases of extrapulmonary NTM infections involving various organs that can imitate various inflammatory and malignant diseases. These cases may help in understanding the wide canvas of NTM infections. Our study highlights the clinical relevance of having NTM as a possible etiology in appropriate clinical scenarios to prevent morbidity and mortality associated with the disease.
Case reports
Case 1
A 40-year-old female with a known case of systemic lupus erythematosus presented to the outpatient department with a low-grade fever for the past two months. She was on hydroxychloroquine and methotrexate therapy for approximately 12 months but had discontinued one month back. A general physical examination showed only mild pallor. The rest of the systemic examination was unremarkable. On evaluation, a complete hemogram showed pancytopenia with hemoglobin (Hb) of 5.6 g/dL (normal range: 12-16 g/dL), total leukocyte count (TLC) of 2840/μL (4000-11000/μL) and platelet count of 75 × 103/μL (1.5-4.5 × 103/μL). Peripheral blood film showed normocytic normochromic anemia with a reticulocyte count of 1.52%. Serum vitamin B12 levels were 695 pg/mL (211-911 pg/mL). Serum iron (57 μg/dL), total iron binding capacity (248 μg/dL), and ferritin (189.8 ng/mL) were within normal limits. Erythrocyte sedimentation rate (ESR, 33 mm) and high-sensitivity C-reactive protein (hs-CRP, 111.14 mg/L, normal <1) were elevated. Rheumatoid factor was elevated (26.7 IU/mL, normal <14 IU/mL) and ANA was positive. Dengue, Chikungunya, HIV serology, HBsAg and anti-HCV were negative. Ultrasound of the abdomen was unremarkable except for mild hepatomegaly. Though she had stopped methotrexate one month back, still the possibility of drug-induced bone marrow suppression was considered due to the prolonged half-life of methotrexate. Bone marrow examination showed normal cellular marrow with all hematopoietic components. The patient was started on hydroxychloroquine. Bone marrow aspirate for mycobacterial culture, which was sent during hospital admission, showed the growth of AFB in an automated culture system after 16 days of incubation (Figure 1). MPT-64 antigen test was negative from culture, which denotes the growth of NTM species. PCR was performed for species identification but could not be detected, and the patient was started on clarithromycin, ethambutol and rifampicin (with the possibility of slow-grower NTM like Mycobacterium avium complex). At three months of follow-up, her complete blood counts were normalized, and she remains symptom free.
Figure 1.
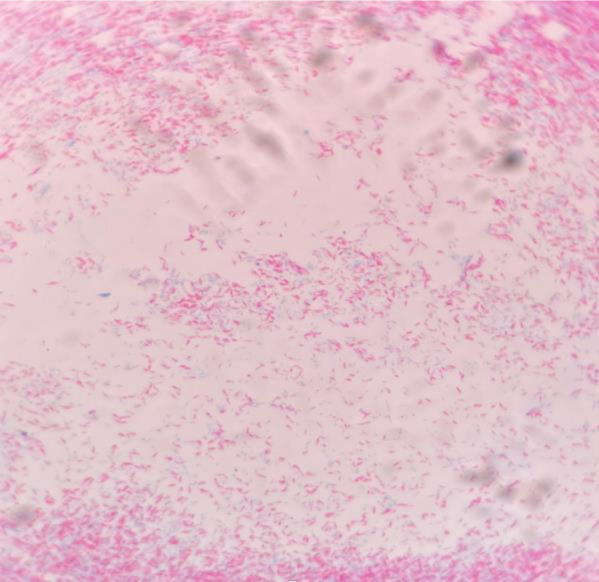
Ziehl-Neelsen stain showing acid fast bacilli from a culture-positive isolate of non-tuberculous mycobacteria (×1000)
Case 2
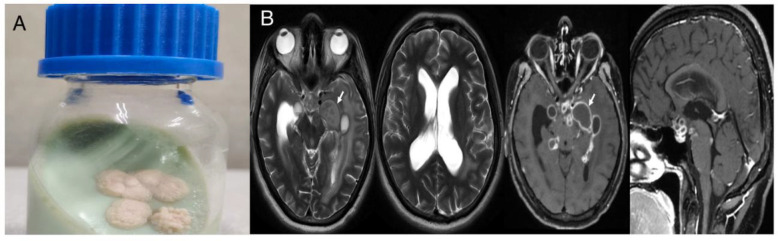
A 22-year-old male presented to the emergency room with altered sensorium and difficulty walking for one week. On examination, he was confused and disoriented. The power of limbs could not be assessed. However, deep tendon reflexes were exaggerated. On further investigation, the complete hemogram was normal, and ESR was 33 mm with an hs-CRP of 16.49 mg/L (normal <1.0 mg/dL). The rest of the biochemical parameters were within normal limits. Cerebrospinal fluid (CSF) examination showed glucose of 111 mg/dL (40-80 mg/dL), protein of 340 mg/dL (20-40 mg/dL), adenosine deaminase (ADA) of 15 U/L (<5 U/L) and cytology showed 116 WBC/mm3 with 90% lymphocytes. GeneXpert and CSF cultures for Mycobacterium were negative. The MRI brain was suggestive of cytotoxic edema in the left ventrolateral thalami, and an MRI of the spine showed focal adhesive arachnoiditis of the spinal cord at T4-T10 levels. The patient was initiated on antitubercular drugs (isoniazid, rifampicin, ethambutol and pyrazinamide) and steroids, with a suspicion of tubercular meningoencephalitis with arachnoiditis. The patient was discharged on day 10 with some clinical improvement. After one month, he was again admitted with similar complaints. Repeat CSF examination revealed sugar of 83 mg/dL, protein of 293 mg/dL and 460 WBC/mm3 with 98% lymphomononuclear cells. He was managed symptomatically, and a repeat GeneXpert for MTB again came back negative. The mycobacterial culture showed growth of AFB in an automated culture system. In addition, the MPT-64 Ag test was negative from culture. Mycobacterium abscessus grew finally from the culture after five days of incubation (Figure 2A). Repeat neuroimaging showed multiple conglomerated tiny ring-enhancing lesions in the basal cistern and left the parietal-temporal region with moderate perilesional oedema with communicating hydrocephalus (Figure 2B). Based on these findings, Mycobacterium abscessus meningitis, brain abscess with hydrocephalus and arachnoiditis was kept as final diagnosis. The patient was started on i.v. linezolid, amikacin, imipenem and tablet clarithromycin (based on susceptibility report). Corticosteroids were stopped because of their debatable role in NTM CNS disease (the patient had already completed four weeks of corticosteroid therapy). Neurosurgical reference was taken, and VP shunting was performed for hydrocephalus. The patient continues to show clinical improvement at three months of follow-up, and is scheduled to continue antibiotics (linezolid and clarithromycin) for the next 12 months.
Figure 2.
(A) Growth of Mycobacterium abscessus after 5 days of incubation on Löwenstein-Jensen medium at 37°C. (B) MRI of the brain showing multiple basal cisternal granulomas with abscess, leptomeningeal enhancement and communicating hydrocephalus.
Case 3
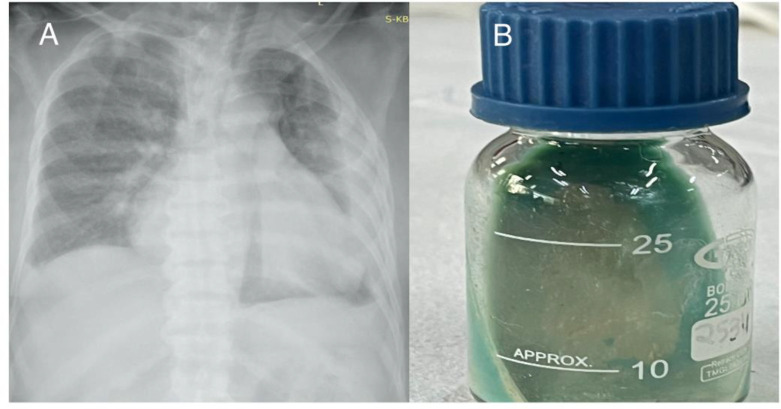
A 67-year-old male presented in emergency with the complaint of shortness of breath and low-grade fever for the last 2 months. He had a history of hypertension and chronic kidney disease (KDIGO Stage 5D). On physical examination, the left basal lung field was dull on percussion, and auscultation revealed decreased air entry up to half of the left lung field. Chest X-ray was suggestive of left-sided pleural effusion (Figure 3A). Ultrasound-guided thoracocentesis was performed. Pleural fluid analysis showed sugar of 91 mg/dL, protein of 3.07 g/dL, ADA of 4 U/L and cytology showed TLC of 18 cells/mm3 with predominant lymphocytes. Pleural fluid Ziehl Neelsen stain (ZN stain) was negative for AFB. Pleural fluid was exudative on the basis of Lights criteria. Mycobacterial culture of the pleural fluid showed growth of Mycobacterium fortuitum in an automated culture system after three days of incubation (Figure 3B). The patient was started on i.v. imipenem, tablet clarithromycin and levofloxacin (based on drug susceptibility and disease severity and NTM species), but succumbed due to renal failure and NTM infection after six days of hospitalization.
Figure 3.
(A) Chest X-ray suggestive of left-sided pleural effusion. (B) Growth of Mycobacterium fortuitum after 3 days of incubation on Löwenstein-Jensen medium at 37°C.
Case 4
A 30-year-old male presented with complaints of high-grade fever and exertional breathlessness for 15 days. He had a history of chronic kidney disease (stage 5D) and hypertension. General examination shows mild pallor, bilateral pitting pedal edema and cervical lymphadenopathy. The rest of the physical examination was unremarkable. Complete blood count was suggestive of moderate anemia (Hb 8.5 mg/dL, 13.5-17 mg/dL) and raised TLC of 13740/μL (4000-11000/μL). Hs-CRP (114.14 mg/L, <1 mg/L) and ESR (80 mm in 1 hour) were also raised. In addition, serum ferritin was also elevated (453.3 ng/mL, normal 20-250 ng/mL). The ultrasound of the abdomen showed multiple mesenteric lymph nodes. USG-guided FNAC (final needle aspiration cytology) of mesenteric lymph nodes was performed, which revealed granulomatous lymphadenitis. The patient was advised antitubercular therapy (isoniazid, rifampicin, pyrazinamide and ethambutol) in view of tubercular lymphadenitis. After seven days, his cervical lymph node biopsy came, which was suggestive of necrotizing granulomatous lymphadenitis. The patient was discharged on antitubercular therapy (ATT) after ten days of hospitalization. After 25 days of incubation, mycobacterial culture showed the growth of AFB in an automated culture system. MPT-64 antigen test was negative from culture, which denotes the growth of NTM. Further species identification could not be performed. In view of NTM lymphadenitis and no other organ involvement, surgical removal of lymph nodes was discussed. At the end of six weeks, the patient underwent surgical resection of lymph nodes, and ATT was stopped. He remains symptom-free at three months of follow-up. The clinical characteristics and outcomes of all four patients is summarized in Table 1.
Table 1. Clinical characteristics and outcomes in patients with extrapulmonary NTM infections.
| Patient characteristics | Case 1 | Case 2 | Case 3 | Case 4 |
|---|---|---|---|---|
| Age/gender | 40/F | 22/M | 67/M | 30/M |
| Total duration of illness | 2 months | 1 week | 2 months | 15 days |
| Presenting symptoms | Fever | Altered sensorium and focal neurological deficit | Fever and shortness of breath | Fever, shortness of breath |
| Comorbidity | Systematic lupus erythematosus | None | Chronic kidney disease | Chronic kidney disease |
| Type of NTM diseases | Bone marrow involvement, pancytopenia | Brain abscess, meningoencephalitis, hydrocephalus and arachnoiditis | Left pleural effusion | Lymphadenitis |
| NTM species | Slow growing NTM (species not identified) | Mycobacterium abscessus | Mycobacterium fortuitum | Slow growing NTM (species not identified) |
| Treatment | Rifampicin, ethambutol, clarithromycin | Imipenem, amikacin, linezolid and clarithromycin | Levofloxacin, imipenem and clarithromycin | Complete resolution with surgical excision, no antibiotics required |
| Outcome and follow-up | Survived, resolution of pancytopenia at 3 months follow-up | Survived, resolution of CNS symptoms at 3 months follow-up | Deceased on day 6 of hospitalization | No relapse at 3 months of follow-up |
CNS – central nervous system; NTM – non-tuberculous mycobacteria.
Discussion
Non-tuberculous mycobacteria are facultative organisms which are ubiquitously found in the environment (surface water, tap water, soil, animal products).8 Mycobacteria are clinically classified based on rate of growth and pigment production.9 NTM are differentiated into slow-growing (requiring >7 days to produce mature colonies on solid media) and rapidly growing bacteria (producing mature colonies ≥7 days on solid media) with a majority of them belonging to a rapidly growing group.10 Lungs are the most frequently involved organ in NTM infections (65-90%).11 Lung involvement can lead to cough, fever, shortness of breath and weight loss, with most of the clinical findings being non-specific, occurring in the setting of pre-existing lung disease and may mimic tuberculosis or malignancy. The radiographic picture varies according to the immune status of the host. In immunocompetent patients, nodules, bronchiectasis, and fibrocavitary lesions are usual findings on radiographic imaging. In contrast, the X-ray may be normal or show only mediastinal or hilar lymphadenopathy in immunocompromised hosts.12 Lymph nodes, skin and soft tissues, bones and joints are the other extrapulmonary organs affected by NTM disease. Disseminated infections are more common in immunocompromised hosts.
Lymph node involvement mainly results in unilateral cervical lymphadenopathy. There is a recent shift in the etiological agent from Mycobacterium scrofulaceum to Mycobacterium avium complex (MAC).12 NTM lymphadenitis usually presents in the pediatric population and may need to be differentiated from pyogenic abscess, histoplasmosis, toxoplasmosis, infectious mononucleosis, and lymphoma.12 CNS disease in NTM infections is rare and mostly associated with HIV and MAC infections and manifests as a spectrum of disseminated disease.13-14 However, rapidly growing NTM are now increasingly recognized as etiology of CNS disease, particularly in immunocompetent hosts.15 Our patient had primary CNS meningitis due to Mycobacterium abscessus, without overt immunodeficiency. There is a significant delay in the diagnosis of CNS NTM disease, resulting in the use of conventional anti-tubercular therapy in these patients, which contributes to the inadequate response.
Bone marrow involvement is seen with disseminated infections and almost exclusively with immunosuppression. Isolation of NTM from bone marrow is rarely reported in the literature. Bourlon et al. reported a case of bone marrow suppression in an HIV-positive 40-year-old male, caused by Mycobacterium genavense.16 Yazisiz et al. described a case of a 36-year-old female with systemic lupus erythematosus who had primary bone marrow disease due to MAC.17 In contrast to the aforementioned reports, our patient was immunocompetent with no signs of disseminated infection yet had NTM-related bone marrow suppression.
Diagnosis of NTM disease is based on clinical suspicion as well as laboratory identification. ZN staining cannot differentiate Mycobacterium tuberculosis and NTM; therefore, a nucleic acid amplification test is required for further differentiation. Culture is the gold standard for confirmation, which also helps in genotyping and drug susceptibility testing. In all of our cases, the diagnosis was confirmed by isolation of NTM in culture. Gene sequencing is now emerging as an effective tool for rapid diagnosis.18,19 NTM disease should be differentiated from colonization. NTM can form biofilms on a wide range of organic and inorganic materials due to the hydrophobic nature of their cell wall. Pseudoinfections may be a result of contamination of specimens or during laboratory testing. Treatment of NTM infections is determined by the primary site of involvement and drug susceptibility pattern. Surgical excision is more effective than antimicrobial therapy in cases with NTM lymphadenitis.12,20 Our patient recovered with surgical resection alone without antibiotics (case 4). The management of disseminated infections is challenging. Since NTM isolation is difficult and delayed, most patients are often misdiagnosed and receive anti-tubercular treatment, which was the case in our patient. In disseminated disease, prolonged treatment is required (6-12 months), which includes the combination of at least 2-3 drugs (amikacin, imipenem, macrolides, linezolid). In CNS disease, a combination of 3-4 drugs is recommended (preferably with good CNS penetration).13
In this article, we discussed four cases of extrapulmonary NTM infection, which mimic tuberculosis. Clinically, it is difficult to differentiate NTM, especially in immunocompetent patients. Skin and soft tissue represent the most common site affected in NTM compared to tuberculosis, where lymphadenopathy is a more common manifestation. ATT is usually initiated based on positive AFB reports; however, it is prudent to follow the culture reports, which later on might indicate the growth of NTM. In addition, cases with positive AFB and negative GeneXpert should be carefully evaluated for NTM disease. MPT-64 antigen is specific for M. tuberculosis cell wall, which is negative in NTM growth and can help in further differentiation from Mycobacterium tuberculosis. A limitation of this study was the small number of patient data, the relatively short duration of follow-up and the lack of drug adverse effects reporting. A large prospective study is required to delineate the risk factors and outcomes of extrapulmonary NTM infections.
Conclusions
NTM infections are an underreported disease and are often misdiagnosed as tubercular infections, especially in developing countries where M. tuberculosis is prevalent. The absence of an immunodeficiency state should not preclude the possibility of NTM infections.
Footnotes
Authors’ contributions statement: PY, NK, DSM, NJ and DK conceived the study; DSM, DK, GKB, SK and ST designed the study protocol; DSM, GKB, VN and DK drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
Consent: Written informed consent for the publication of this article has been provided by the patients and by the next of kin (in case of the deceased).
References
- 1.Gupta N, Mittal A, Niyas VKM, et al. Nontuberculous mycobacteria: a report of eighteen cases from a tertiary care center in India. Lung India. 2020;37:495–500. doi: 10.4103/lungindia.lungindia_365_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed I, Tiberi S, Farooqi J, et al. Non-tuberculous mycobacterial infections-a neglected and emerging problem. Int J Infect Dis. 2020;92S:S46–50. doi: 10.1016/j.ijid.2020.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Sharma SK, Sharma R, Singh BK, et al. A prospective study of non-tuberculous mycobacterial disease among tuberculosis suspects at a tertiary care centre in north India. Indian J Med Res. 2019;150:458–67. doi: 10.4103/ijmr.IJMR_194_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wani SR, Wattal C, Raveendran R. Epidemiology and risk factors associated with NTM pulmonary and extrapulmonary infections in a high tuberculosis endemic region. Indian J Med Microbiol. 2020;38:169–75. doi: 10.4103/ijmm.IJMM_20_274. [DOI] [PubMed] [Google Scholar]
- 5.Umrao J, Singh D, Zia A, et al. Prevalence and species spectrum of both pulmonary and extrapulmonary nontuberculous mycobacteria isolates at a tertiary care center. Int J Mycobacteriol. 2016;5:288–93. doi: 10.1016/j.ijmyco.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Mohajeri P, Norozi B, Atashi S, Farahani A. Anti tuberculosis drug resistance in west of Iran. J Glob Infect Dis. 2014;6:114–7. doi: 10.4103/0974-777X.138506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohajeri P, Moradi S, Atashi S, Farahani A. Mycobacterium tuberculosis Beijing genotype in western Iran: distribution and drug resistance. J Clin Diagn Res. 2016;10:DC05–7. doi: 10.7860/JCDR/2016/20893.8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porvaznik I, Solovič I, Mokrý J. Non-tuberculous mycobacteria: classification, diagnostics, and therapy. Adv Exp Med Biol. 2017;944:19–25. doi: 10.1007/5584_2016_45. [DOI] [PubMed] [Google Scholar]
- 9.Johansen MD, Herrmann JL, Kremer L. Non-tuberculous mycobacteria and the rise of Mycobacterium abscessus. Nat Rev Microbiol. 2020;18:392–407. doi: 10.1038/s41579-020-0331-1. [DOI] [PubMed] [Google Scholar]
- 10.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 11.Stout JE, Koh WJ, Yew WW. Update on pulmonary disease due to non-tuberculous mycobacteria. Int J Infect Dis. 2016;45:123–34. doi: 10.1016/j.ijid.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Mandell DL, Wald ER, Michaels MG, Dohar JE. Management of nontuberculous mycobacterial cervical lymphadenitis. Arch Otolaryngol Head Neck Surg. 2003;129:341–4. doi: 10.1001/archotol.129.3.341. [DOI] [PubMed] [Google Scholar]
- 13.Meena DS, Kumar D, Meena V, Bohra GK, Tak V, Garg MK. Epidemiology, clinical presentation, and predictors of outcome in nontuberculous mycobacterial central nervous system infection: a systematic review. Trop Med Health. 2023;51:54. doi: 10.1186/s41182-023-00546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flor A, Capdevila JA, Martin N, Gavaldà J, Pahissa A. Nontuberculous mycobacterial meningitis: report of two cases and review. Clin Infect Dis. 1996;23:1266–73. doi: 10.1093/clinids/23.6.1266. [DOI] [PubMed] [Google Scholar]
- 15.Talati NJ, Rouphael N, Kuppalli K, Franco-Paredes C. Spectrum of CNS disease caused by rapidly growing mycobacteria. Lancet Infect Dis. 2008;8:390–8. doi: 10.1016/S1473-3099(08)70127-0. [DOI] [PubMed] [Google Scholar]
- 16.Bourlon C, Vargas-Serafín C, López-Karpovitch X. Mycobacterium genavense invading the bone marrow in a HIV-positive patient. Clin Case Rep. 2017;5:1043–45. doi: 10.1002/ccr3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yazisiz V, Erbasan F, Inan D, et al. Bone marrow infection caused by Mycobacterium avium complex in a patient with systemic lupus erythematosus. Lupus. 2010;19:323–6. doi: 10.1177/0961203309346904. [DOI] [PubMed] [Google Scholar]
- 18.Mohajeri P, Yazdani L, Shahraki AH, et al. Verification of frequency in species of nontuberculous mycobacteria in Kermanshah drinking water supplies using the PCR-sequencing method. Microb Drug Resist. 2017;23:359–64. doi: 10.1089/mdr.2016.0064. [DOI] [PubMed] [Google Scholar]
- 19.Khosravi AD, Mirsaeidi M, Farahani A, et al. Prevalence of nontuberculous mycobacteria and high efficacy of d-cycloserine and its synergistic effect with clarithromycin against Mycobacterium fortuitum and Mycobacterium abscessus. Infect Drug Resist. 2018;11:2521–32. doi: 10.2147/IDR.S187554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindeboom JA, Kuijper EJ, Bruijnesteijn van Coppenraet ES, Lindeboom R, Prins JM. Surgical excision versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children: a multicenter, randomized, controlled trial. Clin Infect Dis. 2007;44:1057–64. doi: 10.1086/512675. [DOI] [PubMed] [Google Scholar]