Abstract
Tricholoma matsutake is an edible ectomycorrhizal mushroom that forms a symbiotic association with Pinaceae trees by constructing a large extraradical mycelial area (called a shiro) in the soil. The detection of this fungal mycelium in the soil is crucial for estimating the success of outplanted mycorrhizal seedlings inoculated with T. matsutake under experimental conditions. Although several T. matsutake-specific DNA markers have been reported for efficient detection in the field, no comparative study has been conducted to assess their effectiveness. In the present study, we targeted the nuclear ribosomal DNA intergenic spacer 2 (IGS2) region for the detection of T. matsutake. The newly designed TmSP-I-2F/TmSP-I-2R primer pair, which targets a partial IGS2 sequence (543 bp), effectively detected T. matsutake from pine root and soil samples via PCR assay, outperforming other T. matsutake-specific primers. In combination with a PCR system targeting LTR DNA markers that were previously developed, a PCR system with the TmSP-I-2F/TmSP-I-2R primer pair set can expedite investigations of the dynamics of T. matsutake genets in mycorrhizas and shiro.
Keywords: DNA maker, Ectomycorrhizal mushroom, Environmental microbiology, Molecular traceability, Non-timber forest resources
1. Introduction
Tricholoma matsutake (S. Ito & S. Imai) Singer is a well-studied wild edible ectomycorrhizal mushroom that associates with Pinaceae trees in Asia and Europe (Aoki et al., 2022; Murata et al., 2023; Vaario et al., 2017; Yamanaka et al., 2020). The fungus forms a large extraradical mycelial area, called a shiro, which expands from the ectomycorrhizal root system. Although the shiro can be visible to the naked eye during the mycelial growing seasons (Yamada, 2022), it is often challenging to determine the boundary between the front edge of the developing shiro mycelium and the outer region of the soil (Supplementary Fig. S1). Additionally, it is often unclear whether the older mycelium present in the area is living biomass or dead residue, i.e., necromass, despite the whitish color of those regions (Yamaguchi et al., 2016).
Multiple techniques are available to detect and quantify fungal biomass in environmental samples, including dilution plating, selection medium cultivation, and the measurement of chemical compounds such as chitin, phospholipid fatty acids, ergosterol, and DNA (Baldrian et al., 2013). Kikuchi et al. (2000) developed a specific PCR primer pair, TmF/TmR, that amplified the nuclear ribosomal DNA (nuc rDNA) internal transcribed spacer (ITS) region of T. matsutake and was successfully used to detect the fungus in soil samples. Similarly, Yamaguchi et al. (2016) reported specific and quantitative PCR amplification of T. matsutake DNA by targeting a 202-bp-long single-copy region with the primer pair M201f/M101r. Other DNA markers that target genotypes of T. matsutake can be applied for single-nucleotide polymorphism (SNP) analysis (Amend et al., 2009; Xu et al., 2007), simple sequence repeat analysis (Lian et al., 2003), and long terminal repeat (LTR) retroelement analysis (Murata et al., 2005a, 2005b), all of which can also be used for the specific detection of T. matsutake DNA in environmental samples. The copy number of the rDNA operon in T. matsutake and its related species is estimated to be approximately 100 (Murata et al., 2013; Yamaguchi et al., 2016), which makes ITS-based markers advantageous because they can detect T. matsutake in a limited amount of DNA template due to the high number of ITS copies present. However, the accuracy of such markers can be low due to the likely detection of phylogenetically closely related species. Because environmental samples may be accompanied by unknown factors that affect the detection of target fungal taxa in these samples by PCR-based methods, several sets of DNA markers are expected to be valid for the detection of specific taxa. However, in T. matsutake, only two DNA markers are available for its species-specific detection from soil samples as described above. Therefore, it is desirable to find another DNA marker that can be specifically and easily amplified from T. matsutake by PCR.
In the present study, we alternatively analyzed the intergenic spacer 2 (IGS2) region of the rDNA operon in T. matsutake to determine its accuracy for the detection of T. matsutake. The IGS2 region is estimated to be 4~5 kbp in length based on draft genome data but is otherwise poorly studied. Therefore, we first analyzed the basic structure of the IGS2 region to determine its suitability as a DNA marker for the genetic typing and specific detection of T. matsutake. After finding a promising partial sequence of the IGS2 region, we developed specific primers and tested them on T. matsutake samples collected in the field.
2. Material and methods
2.1. Tricholoma matsutake cultures and DNA extraction
We tested three dikaryotic sibling T. matsutake strains (#52, #84, and #99) obtained from a single basidioma using the spore isolation technique (Horimai et al., 2020; Yamada et al., 2019), which were on MNC agar plates (Yamada & Katsuya, 1995). We extracted DNA from the cultured mycelia following the CTAB method (Gardes & Bruns, 1993; Horimai et al., 2020; Supplementary Table S1). For each strain, a 5 mm × 5 mm mycelial block grown on MNC agar plates was used for the DNA extraction, and the extracted DNA was dissolved in 30 µL of 0.1× TE buffer.
We also extracted DNA from four phylogenetically related and potentially sympatric pine-associated Tricholoma species: dried basidioma specimens of T. robustum (Alb. & Schwein.) Ricken (AY-2211102-002), T. auratum sensu Hongo (Imazeki & Hongo, 1987) (AY-2221104-005), and T. kakishimeji W. Aoki & A. Yamada (TUA-164); and cultured mycelium of T. sejunctum (Sowerby) Quél. (Strain WA-Tpor; WA-201007-002). DNA was extracted as described above.
2.2. PCR amplification of the nuc rDNA IGS2 region
We developed and tested multiple PCR primer pairs to fully sequence the IGS2 region. We first attempted to amplify the full-length IGS2 region (Supplementary Fig. S2) using the primer pair 5SA' (F)/TmI-4R (Supplementary Table S2) with the following cycle parameters: initial denaturation at 95 °C for 3 min; 40 cycles of denaturation at 95 °C for 30 s, annealing for 30 s at the optimum temperature for each primer pair, and extension at 72 °C for 4 min; and final extension at 72 °C for 7 min. The revNS1 (R) primer, which partly overlapped with the TmI-4R primer, did not work well. Next, the IGS2 region was partially amplified by each of the primer pair (Supplementary Fig. S2, Supplementary Table S1) with the following cycle parameters: initial denaturation at 95 °C for 3 min; 40 cycles of denaturation at 95 °C for 30 s, annealing for 30 s at the optimum temperature for each primer pair, and extension at 72 °C for 1 min; and final extension at 72 °C for 7 min. PCR was conducted using the ProFlexTM PCR System (Applied Biosystems, Waltham, MA, USA). For PCR, 25 µL reaction mixtures were assembled as follows: 2.5 µL 10× DreamTaq Buffer (Thermo Scientific, Waltham, MA, USA), 2.5 µL 2 mM dNTP mix, 2.5 µL 5 µM forward primer, 2.5 µL 5 µM reverse primer, 0.125 µL DreamTaq DNA Polymerase, 0.5 µL template DNA, and 14.375 µL autoclaved distilled water. The size of PCR amplicons was determined using electrophoresis as described in Horimai et al. (2020).
2.3. Sequencing of the nuc rDNA IGS2 region
The amplified full-length IGS2 region (~4.5 kbp) was first sequenced directly. The purified PCR amplicon (single band) was extracted from the electrophoresis gel and purified using the QIAquick Gel Extraction Kit (QIAGEN, Hilden, Germany) following the manufacturer's instructions. The purified PCR amplicons were processed directly using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Waltham, MA, USA). We prepared 10-µL reaction mixtures for cycle sequencing as follows: 1.5 µL 5× Sequencing Buffer, 1.5 µL 1.6 µM primers (5SA'(F) or TmI-4R), 1 µL BigDye Terminator v3.1 Ready Reaction Mix, 4 µL purified DNA amplicon, and 2.5 µL autoclaved distilled water. PCRs were conducted using the ProFlexTM PCR System (Applied Biosystems) with the following cycle parameters: initial denaturation at 96 °C for 1 min; 25 cycles of denaturation at 96 °C for 10 s, annealing for 5 s using the optimum temperature for each primer pair, and extension at 60 °C for 4 min.
Since the inner region of the IGS2 contained sequence variation, cloning was conducted for the analysis. The PCR amplicon from the primer pair TmI-8F/TmI-5R (3.3-3.4 kbp) was cloned using the Mighty TA-cloning Kit (TaKaRa Bio, Kusatsu, Shiga, Japan), followed by colony PCR using the same primer pair and cycle sequencing using each primer individually. The PCR amplicons were sequenced using the Applied Biosystems 3130 Genetic Analyzer (Applied Biosystems). We designed new primers on the determined sequence and repeated PCR and sequencing to obtain the full complementary sequence in the IGS2 region. The sequences were aligned using ClustalW ver. 2.0 (Larkin et al., 2007) and MEGA7 (Kumar et al., 2016), and the consensus sequence of the full-length IGS2 region was assembled and deposited into the DNA Data Bank of Japan (DDBJ; LC761959-LC761964). Based on Basic Local Alignment Search Tool (BLAST) searches, the 543-bp-long amplicon generated using the primer pair TmSP-I-2F/TmSP-I-2R was unique to T. matsutake; therefore, we further tested the primer pair on its specificity for T. matsutake in environmental samples.
We used endonucleases to determine how the IGS2 region retains sequence variation between and within T. matsutake strains. We reacted AfaI, AluI, AsuI, HaeIII, HinfI, MspI, TaqI, MaeIII, CviRI, and XapI (TaKaRa Bio) with PCR amplicons according to the manufacturers' recommendations.
2.4. Preparation on environmental samples of T. matsutake and DNA extraction
Two types of T. matsutake samples (ectomycorrhizae and soil mycelium) were collected at an experimental plot in Matsumoto, Nagano Prefecture, Japan, where T. matsutake grows in a Pinus densiflora forest (Fig. 1; Furukawa et al., 2021). In previous studies, T. matsutake-colonized P. densiflora seedlings were generated in vitro (Horimai et al., 2021), and non-mycorrhizal P. densiflora plants were air-layered (Kawai, 1997); these had been outplanted in the plot in May and Oct 2016, respectively. Air layering is a technique for propagating cloned plants from the branches of a tree under field conditions (Kawai, 2019; Lerner & Dana, 2001). The outplanting procedures of the ectomycorrhizal seedlings and air-layered non-mycorrhizal pine plants followed Kobayashi et al. (2015) and Kawai (2019), respectively. In Oct 2017, during the fruiting season of T. matsutake, five to ten pine ectomycorrhizal root tips were collected from the root systems of outplanted pine seedlings and air-layered pine plants, along with rhizosphere soil samples (2~3 mL/sample) for DNA analyses. Additionally, eight 2-y-old juvenile pine seedlings naturally grown on the front zone of the shiro area of T. matsutake were sampled in Jun 2016 for DNA analyses. The sampled materials were stored at −20 °C in the laboratory until processing.
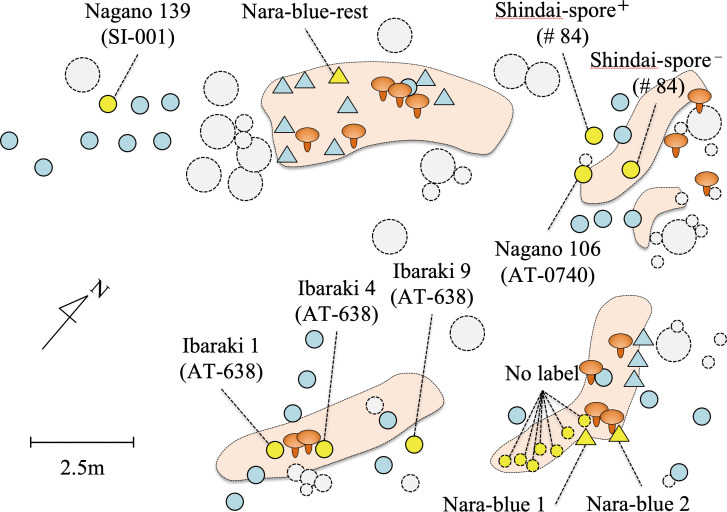
Fig. 1 - Map showing the sampling points in the experimental plot. Dotted large circle: mature pine tree, dotted intermediate circle: pine tree ca. 1-2 m in height, dotted small circle: pine seedlings ca. 2-5-y-old, closed lined circle: outplanted pine ectomycorrhizal seedling, triangle: outplanted air-layered pine tree. The plants analyzed in terms of their root system are highlighted in yellow, and their sample names are provided (fungal strain number is in parentheses in the case of mycorrhizal seedlings). In the brown area enclosed with the dotted black line, there was whitish soil in the soil B-layer (observed on May 24, 2016), suggesting the shiro area of Tricholoma matsutake. The fruit body icon shows where a basidioma of T. matsutake occurred in the autumn in 2015 or 2016.
For each sample, a few ectomycorrhizal root tips or 0.2 mL of soil were crushed using a bead cell disrupter (MS-100; TOMY, Tokyo, Japan), following the manufacturer's instructions with minor modifications. Specifically, 1 mg of sample, a small amount of 1-mm-diam zirconia beads, and 600 µL of CTAB buffer (Supplementary Table S1) were placed in a 1.5-mL microtube. The microtube was then placed in the bead cell disrupter and centrifuged at 5,000 rpm for 3 min. The subsequent DNA extraction steps followed Horimai et al. (2020).
2.5. Accuracy of the TmSP-I-2F/TmSP-I-2R primer pairs for environmental samples of T. matsutake
We compared the accuracy of the TmSP-I-2F/TmSP-I-2R primer pair targeting the nuc rDNA IGS2 region with that of other PCR primers that target different loci in the T. matsutake genome, namely the nuc rDNA ITS region (primer pairs TmF/TmR and ITS1/ITS4B), the nuc rDNA IGS1 region (primer pair CNL12 (F)/5SA (R)), the LTR retroelement marY1 (pS1 primer, primer pair pS48/pL281), and a T. matsutake-specific genomic DNA region (primer pair MY201f/MY101r) (Supplementary Table S3). Of these targeted regions, the primer pairs ITS1/ITS4B (targeting the ITS region) and CNL12 (F)/5SA (R) (targeting the IGS1 region) are supposed to be universal for at least basidiomycetous fungi, whereas the others are specific to T. matsutake. The amplification efficiency of nuc rDNA regions in T. matsutake is approximately 100 tandem repeats of the operon, but the MY201f/MY101r region is a single copy (Murata et al., 2013; Yamaguchi et al., 2016).
PCR amplification was performed using EmeraldAmp® MAX PCR Master Mix (TaKaRa Bio) for each DNA region (Supplementary Table S3). The PCR cycle parameters were as follows: initial denaturation at 96 °C for 2 min; 40 cycles of denaturation at 96 °C for 30 s, annealing at 61 °C for 30 s, and extension at 72 °C for 1 min; and final extension at 72 °C for 5 min. The 20-µL reaction mixture for PCR included the following components: 5.5 µL autoclaved distilled water, 2 µL 5 µM forward primer, 2 µL 5 µM reverse primer, 10 µL 2× EmeraldAmp® Premix, and 0.5 µL template DNA solution. If no amplicon was detected in a DNA extraction sample, a portion of the stored residual environmental sample was subjected to DNA extraction again. If PCR amplification was unsuccessful twice, the DNA extraction procedure was repeated three times. To test the effect of bovine serum albumin (BSA) on PCR, each DNA extraction sample was tested with or without BSA in the reaction mixture. In samples containing BSA, 1 µL of 0.1% BSA solution replaced an equal volume of distilled water.
PCR amplification was performed on other DNA regions using DNA extraction samples that showed an approximately 550-bp-long band with the TmSP-12F/TmSP12R primer pair. The composition of the PCR reaction mixtures was the same as that used for the IGS2 region. The cycle parameters for PCR were almost the same as those used for the IGS2 region, except for the extension step, which was increased to 1.5 min for the LTRs. The ITS1/ITS4B primer pair was used to amplify the IGS1 and ITS regions, and restriction fragment length polymorphism (RFLP) analysis was performed to confirm the specific pattern of T. matsutake (Horimai et al., 2020, 2021). After the RFLP analyses, all ITS and IGS1 amplicons were sequenced, and the fungal taxa were identified using BLAST. For other PCR primers, the presence or absence of DNA amplicons was confirmed using electrophoresis (Horimai et al., 2020, 2021).
3. Results
3.1. Structure of the full-length IGS2 region
The PCR amplicons using the 5SA' (F)/TmI-4R primer pair showed bands around 5 kbp (Supplementary Fig. S3). Tricholoma matsutake strains #52 and #84 showed almost identical band size. We sequenced the full-length IGS2 region directly from both sides, i.e., approximately 400 bp from the 5SA' (F) primer and approximately 900 bp from the TmI-4R primer. We then designed and tested the primers TmI-3F, TmI-8F, TmI-8R, and TmI-5R. The primer pair TmI-8F/TmI-5R provided a single PCR amplicon with an expected fragment size of approximately 3.3 kbp, which showed no diverse heterogeneity (Supplementary Fig. S4). Finally, we sequenced the full-length IGS2 regions in strains #52 and #84. The full length of the IGS region between TmI-3F and TmI-4R was 4.5 kb in strain #52 (DDBJ accession numbers: LC761959 and LC761960) and 4.6 kbp in strain #84 (LC761961-761964; Supplementary Fig. S7). The sequence from base positions 2424 to 2521 in strain #84 was not present in strain #52. The other regions were approximately 99% identical between the two strains. In total, 50 SNPs were detected between the strains, most of which were base substitutions. Half of these polymorphic sites showed polymorphism between cloned sequences in each strain. For #99, we sequenced part of it, i.e., the first 380 bp and the last 1.76 kbp (Supplementary Fig. S7).
3.2. PCR amplification of the partial IGS2 region of T. matsutake using the primer pair TmSP-I-2F/TmSP-I-2R
The primer pair TmSP-I-2F/TmSP-I-2R amplified a region of 543 bp in #52 and #84 or 544 bp in #99 (Supplementary Figs. S5-S7) that showed low homology with other fungal species. The nearest sequence identified via a BLAST search was Hypsizygus marmoreus, a basidiomycetous agaric fungus phylogenetically related to Tricholomataceae, the family to which T. matsutake belongs (Matheny et al., 2006). The query cover was only 29%, and the percent identity was 82.5% (GenBank accession number: KC832881). Therefore, we selected the primer pair TmSP-I-2F/TmSP-I-2R as a potential specific PCR primer for T. matsutake in environmental samples. We conducted additional comparative analysis using four taxonomically related Tricholoma species potentially present in T. matsutake habitats: T. robustum, T. auratum, T. portentosum, and T. kakishimeji. PCR with the TmSP-I-2F/TmSP-I-2R primer pair showed distinct amplicons of the IGS2 region in T. robustum and T. auratum but no amplicon in T. portentosum and T. kakishimeji (Supplementary Fig. S9). The amplified bands of T. robustum and T. auratum were different in size compared to that of T. matsutake.
3.3. Detection of T. matsutake in environmental samples using the primer pair TmSP-I-2F/TmSP-I-2R
Out of seven pine mycorrhizal seedlings that had been inoculated with T. matsutake strains and outplanted to the experimental forest site 17 months prior to DNA analysis, we detected the IGS2 region of T. matsutake in root samples from four seedlings (Table 1). In addition, we detected the IGS2 region of T. matsutake in soil samples (extraradical mycelium) from two pine mycorrhizal seedlings (Nagano 106, Ibaraki 1). Among the air-layered pine plants, we detected the IGS2 region of T. matsutake in the ectomycorrhizal root tips of two out of three individuals. Furthermore, we detected the IGS2 region of T. matsutake in the ectomycorrhizal root tips of one naturally grown juvenile pine seedling.
Table 1. PCR amplification of the partial IGS2 region of Tricholoma matsutake using the TmSP-I-2F/TmSP-I-2R primer pair.
| Sample group of pine plants | Name of pine plant | Sample name3 | N | Number of replicated samples detected T. matsutake | ||
| −BSA | +BSA | Total | ||||
| Outplanted mycorrhizal seedling1 | Nagano 106 (AT-0740) | EMRT | 23 | 0/23 | 1/23 | 1 |
| ERM in soil | 2 | 0/2 | 0/2 | 0 | ||
| ERM in soil | 5 | 0/5 | 2/5 | 2 | ||
| EMRT (not T. matsutake) | 1 | 0/1 | 0/1 | 0 | ||
| Nagano 139 (SI-001) | EMRT | 26 | 1/26 | 9/25 | 10 | |
| EMRT (not T. matsutake) | 1 | 0/1 | 0/1 | 0 | ||
| Ibaraki 1 (AT-638) | EMRT | 3 | 0/3 | 0/3 | 0 | |
| ERM in soil* | 9 | 1/9 | 0/8 | 1 | ||
| ERM in soil | 1 | 1/1 | ND | 1 | ||
| EMRT (not T. matsutake) | 1 | 0/1 | 0/1 | 0 | ||
| Ibaraki 4 (AT-638) | EMRT | 5 | 0/5 | 0/5 | 0 | |
| ERM in soil | 3 | 0/3 | 0/3 | 0 | ||
| EMRT (not T. matsutake) | 1 | 0/1 | 0/1 | 0 | ||
| Ibaraki 9 (AT-638) | EM root tips | 4 | 0/4 | 0/4 | 0 | |
| Shindai-spore+ (# 84) | EMRT | 3 | 1/3 | 0/2 | 1 | |
| EMRT (not T. matsutake) | 1 | 0/1 | 0/1 | 0 | ||
| Shindai-spore− (# 84) | EMRT | 2 | 0/2 | 1/2 | 1 | |
| EMRT (not T. matsutake) | 2 | 0/2 | 0/2 | 0 | ||
| Outplanted non-mycorrhizal air-layered plant1 | Nara-blue 1 | EMRT | 3 | 1/3 | 0/2 | 1 |
| Nara-blue 2 | EMRT** | 3 | 3/3 | ND | 3 | |
| EMRT (not T. matsutake) | 1 | 0/1 | 0/1 | 0 | ||
| EMRT on oak tree | 1 | 0/1 | 0/1 | 0 | ||
| Nara-blue-rest | EMRT | 10 | 0/10 | 0/10 | 0 | |
| EMRT (not T. matsutake) | 2 | 0/2 | 0/2 | 0 | ||
| Growing wild seedling2 | No label | EMRT | 8 | 1/8 | 0/7 | 1 |
| Total | 121 | 9 | 13 | 22 | ||
1 These mycorrhizal seedlings and air-layered non-mycorrhizal plants were planted in May 2016 and Oct 2016, respectively, at a point 10-15 cm from the front zone of the shiro area of naturally colonized T. matsutake in the forest site.
2 The pine seedlings were 2-y-old when collected in Jun 2016, and grew naturally in the front zone of the shiro area of naturally colonized T. matsutake in the forest site.
3 EMRT: ectomycorrhizal root tips on the transplanted plant; ERM: extraradical mycelium around the root system of the transplanted plant.
* Adult pine ectomycorrhizal root tips were included in each sample.
** A fruiting body of T. matsutake occurred during sampling (Supplementary Fig. S2), and the shiro mycelium was associated with the ectomycorrhizal root tip samples.
The addition of BSA to the reaction mixture for PCR increased the amplification frequency of the IGS2 region. The IGS2 region of T. matsutake was not detected in ectomycorrhizal root tips that had distinct rhizomorphs exhibiting suilloid-type colonization. In total, 22 out of 121 samples were positive for the IGS2 region of T. matsutake.
3.4. Detection of T. matsutake by commonly used PCR primer pairs that targeted DNA loci other than IGS2
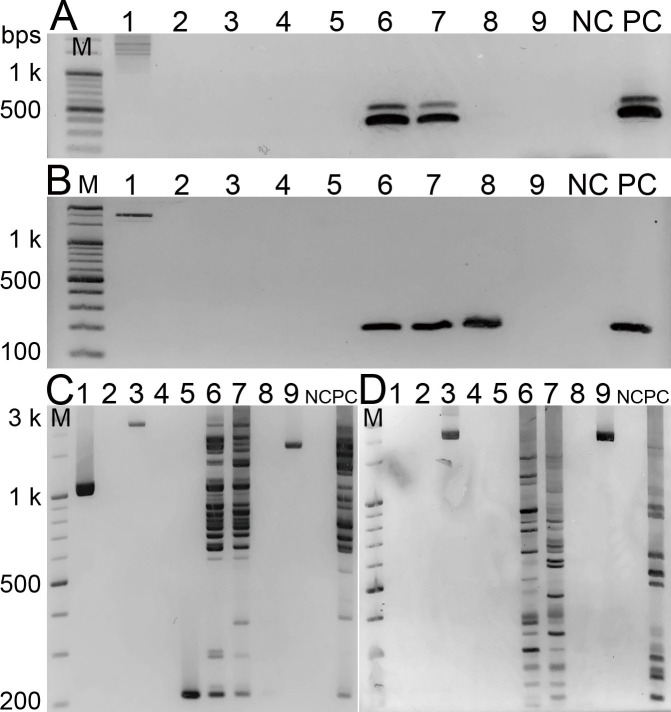
To evaluate the accuracy of the primer pair TmSP-I-2F/TmSP-I-2R for the PCR-based detection of T. matsutake, we tested five other primer pairs and a single primer on nine samples in which we detected the IGS2 region of T. matsutake using the primer pair TmSP-I-2F/TmSP-I-2R (Table 1). As shown in Table 2 and Fig. 2, four specific primers, namely TmF/TmR (targeting the ITS region), MY201f/MY101r (targeting the LTR-related region), and pS1 and pS48/pL281 (targeting the LTR retroelement marY1), yielded significantly lower detection frequencies. The universal primer ITS1/ITS4B (targeting the ITS region) yielded a lower detection frequency, whereas CNL12 (F)/5SA (R) (targeting the IGS1 region) had approximately the same detection frequency as TmSP-I-2F/TmSP-I-2R. For pS1 and pS48/pL281, two samples showed a single unknown band (Fig. 2). All of the tested primers detected T. matsutake in two mycorrhizal samples collected from the root system of an air-layered pine (Nara-blue-2). These samples were associated with actively growing shiro mycelium, which was also associated with a fruiting body of T. matsutake when the root samples were collected.
Table 2. PCR detection of Tricholoma matsutake DNA by the known specific and universal primers from 9 samples in which the fungal DNA was detected by the TmSP-I-2F/TmSP-I-2R primer pair as shown in Table 1.
| Plant name | Samples1 | Detection of T. matsutake using the following specific PCR primers2 | Homology of the sequence obtained using the following universal PCR primer pair with T. matsutake #84 sequence3 | ||||
| TmF/TmR | MY201f/MY101r | pS1 | pS48/pL281 | ITS1/ITS4B | CNL12 (F)/5SA (R) | ||
| Nagano 139 (seedling) | EMRT | ND | ND | ND | − | ND | 411/412 (99.8 %) |
| Ibaraki 1 (seedling) | ERM | − | − | − | − | ND | 410/412 (99.5 %) |
| ERM* | − | − | ND | ND | 635/664 (95.6 %)** | 420/422 (99.5 %) | |
| Shindai-spore+ (seedling) | EMRT | − | − | − | − | ND | ND |
| Nara-blue 1 (air-layered tree) | EMRT | − | − | ND | − | 791/792 (99.9 %) | 455/456 (99.8 %) |
| Nara-blue 2 (air-layered tree) | EMRT | + | + | + | + | 787/789 (99.7 %) | 422/423 (99.8 %) |
| EMRT | + | + | + | + | 746/748 (99.7 %) | 422/423 (99.8 %) | |
| EMRT | − | + | ND | ND | 741/743 (99.7 %) | 423/424 (99.8 %) | |
| Growing wild seedling | EMRT | − | − | − | − | 746/748 (99.7 %) | 413/420 (96.0 %) |
1 Abbreviation is as in Table 1.
2 +: detection of T. matsutake, −: no detection of T. matsutake, ND: not determined due to unknown amplification pattern.
3 All sequences obtained were highly identical to T. matsutake sequences by BLAST search.
* Ectomycorrhizal root tips from adult pines were included in each sample.
** The sequence was also highly matched to the T. matsutake sequence by BLAST search.
Fig. 2 - Electrophoresis bands of PCR amplicons using different primers. A: primer pair TmF/TmR (targeting the ITS region), B: primer pair MY201f/MY101r (targeting the marY1 region), C and D: primer pS1 and primer pair pS48/pL281 (targeting the LTR region), respecitvely. Lane 1: EMRT of Nagano 139, Lane 2: ERM of Ibaraki 1, Lane 3: ERM (including ectomycorrhizae of adult pine tree) of Ibaraki 1, Lane 4: EMRT of Shindai-spore+, Lane 5: EMRT of Nara-blue 1, Lanes 6-8: EMRT of Nara-blue 2, Lane 9: EMRT of a seedling (no label), NC: negative control (no template DNA); PC: positive control (extracted DNA from T. matsutake strain #84). The abbreviations are the same as in Table 1.
For the 112 IGS2-negative samples using the TmSP-I-2F/TmSP-I-2R primer pair without BSA for PCR (Table 1), the TmF/TmR primer pair was used for confirmation PCR. No samples showed amplification (data not shown).
4. Discussion
We showed that our newly designed primer pair TmSP-I-2F/TmSP-I-2R, which targets a partial, 543-bp-long sequence of the nuc rDNA IGS2 region of T. matsutake, effectively detected T. matsutake in field samples. The primer pair has a better combination of amplification efficiency and specificity compared to any other known specific PCR primers for this fungus. Therefore, we recommend targeting the IGS2 region for the detection of T. matsutake in soil from forests with various conditions, as well as in pot cultivation experiments.
The nuc rDNA IGS1 region and the ITS region are established DNA barcoding regions for the identification of fungi at the species level (Aoki et al., 2022; Guerin-Laguette et al., 2002; Henrion et al., 1992; Horimai et al., 2020; Matsushita et al., 2005). However, due to the length of the full-length IGS2 region of approximately 5 kbp and the heterogeneity of sequences among tandem repeat copies in the nuc rDNA operon, limited data on the full-length IGS2 region in T. matsutake are available, even though next-generation sequencing techniques have recently covered this region. The full length of the 4.5-4.6 kbp IGS2 region can only be sequenced without cloning at both ends, i.e., approximately the first 0.4 kbp and last 0.9 kbp, but the inner region requires cloning. Therefore, some regions showed a low level of query cover percentage in BLAST results of T. matsutake based on short-read next-generation sequencing (Ichida et al., 2023; Kurokochi et al., 2023). We also found that the length of the IGS2 region differed by 100 bp between strains #52 and #84. In addition, the IGS2 region was approximately 500 bp longer in strain #99 than in strains #52 and #84. These intraspecific variations might be valuable for specifying strains and individuals or for detecting chromosomal recombination events during reproduction.
The nuc rDNA IGS2 region was amplified using the primer pair TmSP-I-2F/TmSP-I-2R, which targets the last region (Supplementary Fig. S2). This sequence showed only a few base substitutions between strains (#52, #84, and #99) and between clones within the strain. Therefore, we selected this site as a simple DNA marker for detecting T. matsutake using electrophoresis of PCR amplicons. Additionally, the site can be digested by the restriction enzymes CviRI (Supplemetntary Fig. S5) and XpoI (Supplementary Fig. S6), and probably also by ApoI, AfaI, and HincII, making RFLP analysis available for species-level discrimination between Tricholoma matsutake and related species in the genus.
The use of the TmSP-I-2F/TmSP-I-2R primer pair has advantages in detecting T. matsutake from environmental samples with low fungal biomass, i.e., low template DNA levels compared with the primer pairs TmF/TmR and MY201f/MY101r. The TmF/TmR primer pair (Kikuchi et al., 2000), targeting the ITS region, showed less amplification efficiency than the TmSP-I-2F/TmSP-I-2R primer pair. Meanwhile, the primer pair MY201f/MY101r targets a single-copy region in the nuclear genome of T. matsutake (Yamaguchi et al., 2016), explaining its lower amplification efficiency. These detection efficiencies were demonstrated in two mycorrhizal samples from an air-layered pine plant (Nara-blue-2), in which all tested primers detected T. matsutake. As these samples were associated with developed T. matsutake mycelium occurring in a fruiting body, the mycelial biomass at the ectomycorrhizal root tips was likely higher than in other samples. Therefore, to validate the detection of T. matsutake from the environment, it might be recommendable to use both TmSP-I-2F/TmSP-I-2R and MY201f/MY101r primer pairs because environmental samples can potentially include unexpected and unknown factors. Especially in the DNA-degraded environmental samples, MY201f/MY101r might be preferred for its ability to detect T. matsutake DNA because its PCR amplicon length is less than half (approximately 200 bp) that of TmSP-I-2F/TmSP-I-2R.
Based on the detection data of T. matsutake, we present new findings on fungal ecophysiology. The outplanted pine mycorrhizal seedlings showed different detection patterns of T. matsutake (Table 1). The Ibaraki-1 seedling, in which we detected T. matsutake only from the rhizosphere soil, suggests that the detected fungus was a native genet, not the inoculated strain in the mycorrhization experiment in vitro. However, it was unclear whether we detected native or introduced T. matsutake in the other four seedlings (Nagano 106, Nagano 139, Shindai-spore+, and Shindai-spore−). Outplanted seedlings showed limited growth of T. matsutake-associated ectomycorrhizal root tips on their root system 1.5 y after outplanting. Kobayashi et al. (2015) reported that outplanted pine seedlings previously inoculated with T. matsutake in vitro sustained the fungal colonization status for 2 y, but the newly developed root systems were colonized with suilloid fungi. Therefore, it is necessary to monitor outplanted seedlings for a longer period to determine whether introduced T. matsutake genets adapt and grow on the forest site, as has been reported in the transplantation of T. matsutake-colonized pine seedings established under in situ experimental conditions (Ka et al., 2006, 2010; Kareki & Kawakami, 1985).
By contrast, two out of three outplanted non-mycorrhizal air-layered pine plants were colonized by native T. matsutake genets within a year. In particular, we detected T. matsutake in all three tested mycorrhizal samples from one seedling (Nara-blue-2) that were connected with densely developed whitish mycelium, resulting in a fruiting body. This suggests that if a pine root system encounters infective T. matsutake mycelium at a specific time, ectomycorrhization can progress quickly in situ. In vitro experiments suggest that only 2 wk are necessary for T. matsutake mycelium to establish ectomycorrhizal colonization (Vaario et al., 2000). Additionally, a naturally grown 2-y-old juvenile pine seedling in the shiro area was colonized by T. matsutake at the experimental site. This suggests that row thinning of pine trees might serve as forest management for T. matsutake cultivation in relation to tree age because open sites in the pine forest provide the next generation of offspring by maintaining the shiro mycelium among tree generations.
In the present study, our first aim was to compare the detection efficiency of T. matsutake in soil using different PCR primers. While LTR retroelement marY1 target primers, namely pS1 and pS48/pL281, showed a detection frequency of T. matsutake similar to that of the primer pair MY201f/MY101r (Table 2), both pS1 and pS48/pL281 detected different T. matsutake genets in the root system of an air-layered pine plant (Nara-blue-2) (Supplementary Fig. S9C, D). This demonstrates how different mycelial genets of T. matsutake colonize a small root system in situ. It remains to be tested whether both genets compete on a host root system or share the root system to improve survival and growth. Another advantage of being able to distinguish T. matsutake genets in soil is that it can be determined whether an introduced T. matsutake genet has been successfully established in an outplanted forest site. In the past, reports of successful outplantation of pine seedlings that had previously been colonized by T. matsutake and subsequent fruiting from the outplanted host pines were conducted in forest sites where native T. matsutake genets were already present (Ka et al., 2018; Kareki & Kawakami, 1985). The analysis of LTR DNA markers can help to determine whether an introduced T. matsutake genet has taken root among outplanted hosts, whether genet alternation has occurred, and whether introduced and native genets are mutually associated with each other.
Disclosure
The authors of this study have no conflicts of interest to declare. All experiments in this study were performed in compliance with the current laws of Japan.
Supplementary Materials
Supplementary online materials (Supplementary Figures, Supplementary Tables) are available at https://doi.org/10.47371/mycosci.2024.05.001
Acknowledgements
This study was supported by a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan, “Technology development for the optimal use of forest resources”.
References
- Amend, A., Keeley, S., & Garbelotto, M. (2009).Forest age correlates with fine-scale spatial structure of Matsutake mycorrhizas. Mycological Research, 113, 541-551. https://doi.org/10.1016/j.mycres.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Anderson, J. B., & Stasovski, E. (1992) Molecular phylogeny of northern hemisphere species of Armillaria. Mycologia, 84, 505-516. https://doi.org/10.1080/00275514.1992.12026170. [Google Scholar]
- Aoki, W., Bergius, N., Kozlan, S., Fukuzawa, F., Okuda, H., Murata, H., Ishida, T. A., Vaario, L. M., Kobayashi, H., Kalmiş, E., Fukiharu, T., Gisusi, S., Matsushima, K., Terashima, Y., Narimatsu, M., Matsushita, N., Ka, K. H., Yu, F., Yamanaka, T., Fukuda, M., & Yamada, A. (2022). New findings on the fungal species Tricholoma matsutake from Ukraine, and revision of its taxonomy and biogeography based on multilocus phylogenetic analyses. Mycoscience, 63, 197-214. https://doi.org/10.47371/mycosci.2022.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldrian, P., Větrovský, T., Cajthaml, T., Dobiášová, P., Petránková, M., Šnajdr, J., & Eichlerová, I. (2013). Estimation of fungal biomass in forest litter and soil. Fungal Ecology, 6, 1-11. https://doi.org/10.1016/j.funeco.2012.10.002. [Google Scholar]
- Furukawa, H., Katagiri, K., Masuno, K., Yamada, A., Kawai, M., Kobayashi, H., & Yamanaka, T. (2021). Development of technique to establish Tricholoma matsutake in forest areas using seedlings previously infected with the fungus. Annual Report of Nagano Prefecture Forestry Research Center, 35, 69-82. (In Japanese). https://www.pref.nagano.lg.jp/ringyosogo/seika/kenkyu/shido/documents/35-07.pdf. [Google Scholar]
- Gardes, M., & Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes - application to the identification of mycorrhizae and rusts. Molecular Ecology, 2, 113-118. https://doi.org/10.1111/j.1365-294X.1993.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Guerin-Laguette, A., Matsushita, N., Kikuchi, K., Iwase, K., Lapeyrie, F., & Suzuki, K. (2002). Identification of a prevalent Tricholoma matsutake ribotype in Japan by rDNA IGS1 spacer characterization. Mycological Research, 106, 435-443. https://doi.org/10.1017/S0953756202005725. [Google Scholar]
- Henrion, B., Le Tacon, F., & Martin, F. (1992). Rapid identification of genetic variation of ectomycorrhizal fungi by amplification of ribosomal RNA genes. New Phytologist, 112, 289-298. https://doi.org/10.1111/j.1469-8137.1992.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Horimai, Y., Misawa, H., Suzuki, K., Fukuda, M., Furukawa, H., Masuno, K., Yamanaka, T., & Yamada, A. (2020). Sibling spore isolates of Tricholoma matsutake vary significantly in their ectomycorrhizal colonization abilities on pine hosts in vitro and form multiple intimate associations in single ectomycorrhizal roots. Fungal Ecology, 43, 100874. https://doi.org/10.1016/j.funeco.2019.100874. [Google Scholar]
- Horimai, Y., Misawa, H., Suzuki, K., Tateishi, Y., Furukawa, H., Yamanaka, T., Yamashita, S., Takayama, T., Fukuda, M., & Yamada, A. (2021). Spore germination and ectomycorrhizae formation of Tricholoma matsutake on pine root systems with previously established ectomycorrhizae from a dikaryotic mycelial isolate of T. matsutake. Mycorrhiza, 31, 335-347. https://doi.org/10.1007/s00572-021-01028-3. [DOI] [PubMed] [Google Scholar]
- Ichida, H., Murata, H., Hatakeyama, S., Yamada, A., & Ohta, A. (2023). Complete de novo assembly of Tricholoma bakamatsutake chromosomes revealed the structural divergence and differentiation of Tricholoma genomes. bioRxiv, preprint. https://doi.org/10.1101/2023.02.12.528224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazeki, R., & Hongo, T. (1987). Colored illustrations of mushrooms of Japan I (in Japanese). Hoikusha. [Google Scholar]
- Ka, K. H., Hur, T. C., Park, H., Kim, H. S., Bak, W. C., & Yoon, K. H. (2006). Production and transplanting of ectomycorrhizal pine seedlings using the old fairy ring of Tricholoma matsutake. Journal of Korean Forest Society, 95, 636-642. (In Korean). https://koreascience.kr/article/JAKO200610103457570.pdf. [Google Scholar]
- Ka, K. H., Hur, T. C., Park, H., Kim, H. S., & Bak, W. C. (2010). Mycelial growth and fairy-ring formation of Tricholoma matsutake from matsutake-infected pine trees. Korean Journal of Mycology, 38, 16-20. (In Korean). https://doi.org/10.4489/KJM.2010.38.1.016. [Google Scholar]
- Ka, K. H., Kim, H. S., Hur, T. C., Park, H., Jeon, S. M., Ryoo, R., & Jang, Y. (2018). Analysis of environment and production of Tricholoma matsutake in matsutake-infected pine trees. Korean Journal of Mycology, 46, 34-42. (In Korean). https://doi.org/10.4489/KJM.20180005. [Google Scholar]
- Kareki, K., & Kawakami, Y. (1985). Artificial formation of Shiro (fungus colony) by planting the pine saplings infected with Tricholoma matsutake (Ito et Imai) Sing. Bulletin of the Hiroshima Prefecture Forestry Experimental Station, 20: 13-23. (In Japanese). https://agriknowledge.affrc.go.jp/RN/2010340592.pdf. [Google Scholar]
- Kawai, M. (1997). Artificial ectomycorrhiza formation on roots of air-layered Pinus densiflora saplings by inoculation with Lyophyllum shimeji. Mycologia, 89, 228-232. https://doi.org/10.1080/00275514.1997.12026774. [Google Scholar]
- Kawai, M. (2019). Studies on the cultivation of mycorrhizal mushrooms in Nara Prefecture - on the cultivation of Honshimeji and Bakamatsutake mushrooms. Shinrin-kagaku, 86, 30-34. (In Japanese). https://doi.org/10.11519/jjsk.86.0_30. [Google Scholar]
- Kikuchi, K., Matsushita, M., Guerin-Laguette, A., Ohta, A., & Suzuki, K. (2000). Detection of Tricholoma matsutake by specific ITS primers. Mycological Research, 104, 1427-1430. https://doi.org/10.1017/S0953756200002653 [Google Scholar]
- Kobayashi, H., Terasaki, M., & Yamada, A. (2015). Two-year survival of Tricholoma matsutake ectomycorrhizas on Pinus densiflora seedlings after outplanting to a pine forest. Mushroom Science and Biotechnology, 23, 108-113. (In Japanese). https://doi.org/10.24465/msb.23.3_108. [Google Scholar]
- Kumar, S., Stecher, G., & Tamura, K. (2016). MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870-1874. https://doi.org/10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokochi, H., Tajima, N., Sato, M. P., Yoshitake, K., Asakawa, S., Isobe, S., & Shirasawa, K. (2023). Telomere-to-telomere genome assembly of matsutake (Tricholoma matsutake). DNA Research, 30, 1-6. https://doi.org/10.1093/dnares/dsad006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGettigan, P. A., McWilliam, H., Valentin, F., Wallace, I. M., Wilm, A., Lopez, R., Thompson, J. D., Gibson, T. J., & Higgins, D. G. (2007). Clustal W and Clustal X version 2.0. Bioinformatics, 23, 2947-2948. https://doi.org/10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Lerner, B. R., & Dana, M. N. (2001). New plants from layering. Purdue University Cooperative Extension Service, General Horticulture, HO-1-W. https://web.archive.org/web/20010614031022/http://www.hort.purdue.edu/ext/ho-1.pdf. [Google Scholar]
- Lian, C., Hogetsu, T., Matsushita, N., Guerin-Laguette, A., Suzuki, K., & Yamada, A. (2003). Development of microsatellite markers from an ectomycorrhizal fungus, Tricholoma matsutake, by an ISSR-suppression-PCR method. Mycorrhiza, 13, 27-31. https://doi.org/10.1007/s00572-002-0193-6. [DOI] [PubMed] [Google Scholar]
- Matheny, P. B., Curtis, J. M., Hofstetter, V., Aime, M. C., Moncalvo, J. M., Ge, Z. W., Slot, J. C., Ammirati, J. F., Baroni, T. J., Bougher, N. L., Hughes, K. W., Lodge, D. J., Kerrigan, R. W., Seidl, M. T., Aanen, D. K., DeNitis, M., Daniele, G. M., Desjardin, D. E., Kropp, B. R., Norvell, L. L., Parker, A., Vellinga, E. C., Vilgalys, R., & Hibbett, D. S. (2006). Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia, 98, 982-995. https://doi.org/10.3852/mycologia.98.6.982. [DOI] [PubMed] [Google Scholar]
- Matsushita, N., Kikuchi, K., Sasaki, Y., Guerin-Laguette, A., Vaario, L.-M., Suzuki, K., Lapeyrie, F., & Intini, M. (2005). Genetic relationship of Tricholoma matsutake and T. nauseosum from the Northern Hemisphere based on analyses of ribosomal DNA spacer regions. Mycoscience, 46, 90-96. https://doi.org/10.1007/S10267-004-0220-X. [Google Scholar]
- Murata, H., Babasaki, K., & Yamada, A. (2005. a). Highly polymorphic DNA markers to specify strains of the ectomycorrhizal basidiomycete Tricholoma matsutake based on σmarY1, the long terminal repeat of gypsy-type retroelement marY1. Mycorrhiza, 15, 179-186. https://doi.org/10.1007/s00572-004-0319-0. [DOI] [PubMed] [Google Scholar]
- Murata, H., Ohta, A., Yamada, A., Narimatsu, M., & Futamura, N. (2005. b). Genetic mosaics in the massive persisting rhizosphere colony “shiro” of the ectomycorrhizal basidiomycete Tricholoma matsutake. Mycorrhiza, 15, 505-512. https://doi.org/10.1007/s00572-005-0358-1. [DOI] [PubMed] [Google Scholar]
- Murata, H., Ota, Y., Yamaguchi, M., Yamada, A., Katahata, S., Otsuka, Y., Babasaki, K., & Neda, H. (2013). Mobile DNA distributions refine the phylogeny of “matsutake” mushrooms, Tricholoma sect. Caligata. Mycorrhiza, 23, 447-461. https://doi.org/10.1007/s00572-013-0487-x. [DOI] [PubMed] [Google Scholar]
- Murata, H., Yamada, A., Ichida, H., Nakamura, N., & Neda, H. (2023). Biodiversity of Tricholoma matsutake (syn. T. nauseosum) and its related species based on repetitive DNA and genomics. Botany, 101, 138-154. https://doi.org/10.1139/cjb-2022-0122. [Google Scholar]
- Vaario, L. M., Guerin-Laguette, A., Gill, W. M., Lapeyrie, F., & Suzuki, K. (2000). Only two weeks are required for Tricholoma matsutake to differentiate ectomycorrhizal Hartig net structures in roots of Pinus densiflora seedlings cultivated on artificial substrate. Journal of Forest Research, 5, 293-297. https://doi.org/10.1007/BF02767125. [Google Scholar]
- Vaario, L.-M., Yang, X., & Yamada, A. (2017). Biogeography of the Japanese gourmet fungus, Tricholoma matsutake: a review of the distribution and functional ecology of Matsutake. In: Tedersoo, L. (Ed.), Biogeography of Mycorrhizal Symbiosis. Ecological Studies (Analysis and Synthesis), vol. 230. (pp. 319-344). Springer; .https://doi.org/10.1007/978e3-319e56363e3_15. [Google Scholar]
- White, T. J., Bruns, T., Lee, S., & Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M. A., Gelfand, D. H., Sninsky, J. J., & White, T. J. (Eds), PCR protocols: A guide to methods and applications (pp.315-322). Academic Press [Google Scholar]
- Xu, J., Guo, H., & Yang, Z. L. (2007). Single nucleotide polymorphisms in the ectomycorrhizal mushroom Tricholoma matsutake. Microbiology, 153, 2002-2012. https://doi.org/10.1099/mic.0.2006/005686-0. [DOI] [PubMed] [Google Scholar]
- Yamada, A. (2022). Cultivation studies of edible ectomycorrhizal mushrooms: successful establishment of ectomycorrhizal associations in vitro and efficient production of fruiting bodies. Mycoscience, 63. 235-246. https://doi.org/10.47371/mycosci.2022.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, A., Hayakawa, N., Saito, C., Horimai, Y., Misawa, H., Yamanaka, T., & Fukuda, M. (2019). Physiological variation among Tricholoma matsutake isolates generated from basidiospores obtained from one basidioma. Mycoscience, 60, 102-109. https://doi.org/10.1016/j.myc.2018.12.001 [Google Scholar]
- Yamada, A., & Katsuya, K. (1995). Mycorrhizal association of isolates from sporocarps and ectomycorrhizas with Pinus densiflora seedlings. Mycoscience, 36, 315-323. https://doi.org/10.1007/BF02268607. [Google Scholar]
- Yamaguchi, M., Narimatsu, M., Fujita, T., Kawai, M., Kobayashi, H., Ohta, A., Yamada, A., Matsushita, N., Neda, H., Shimokawa, T., & Murata, H. (2016). A qPCR assay that specifically quantifies Tricholoma matsutake biomass in natural soil. Mycorrhiza, 26, 847-861. https://doi.org/10.1007/s00572-016-0718-z. [DOI] [PubMed] [Google Scholar]
- Yamanaka, T., Yamada, A., & Furukawa, H. (2020). Advances in the cultivation of the highly-prized ectomycorrhizal mushroom Tricholoma matsutake. Mycoscience, 61, 49-57. https://doi.org/10.1016/j.myc.2020.01.001. [Google Scholar]