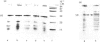Abstract
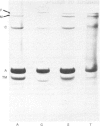
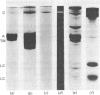
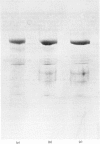
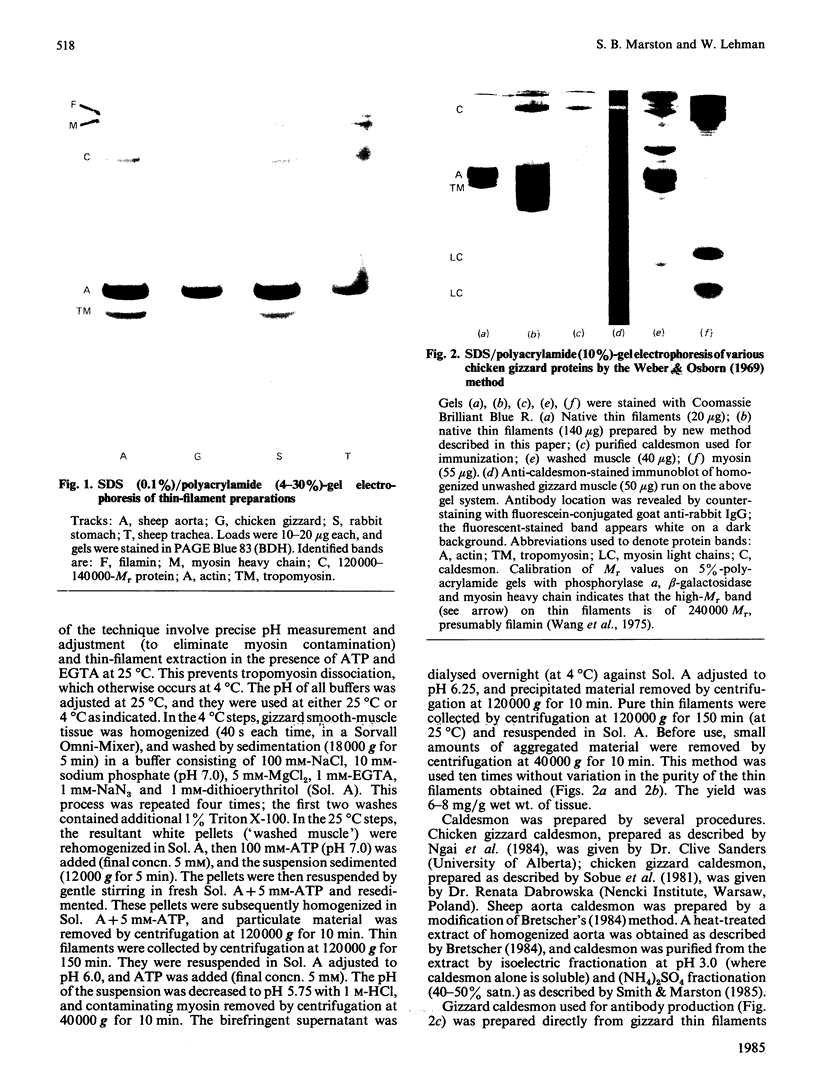
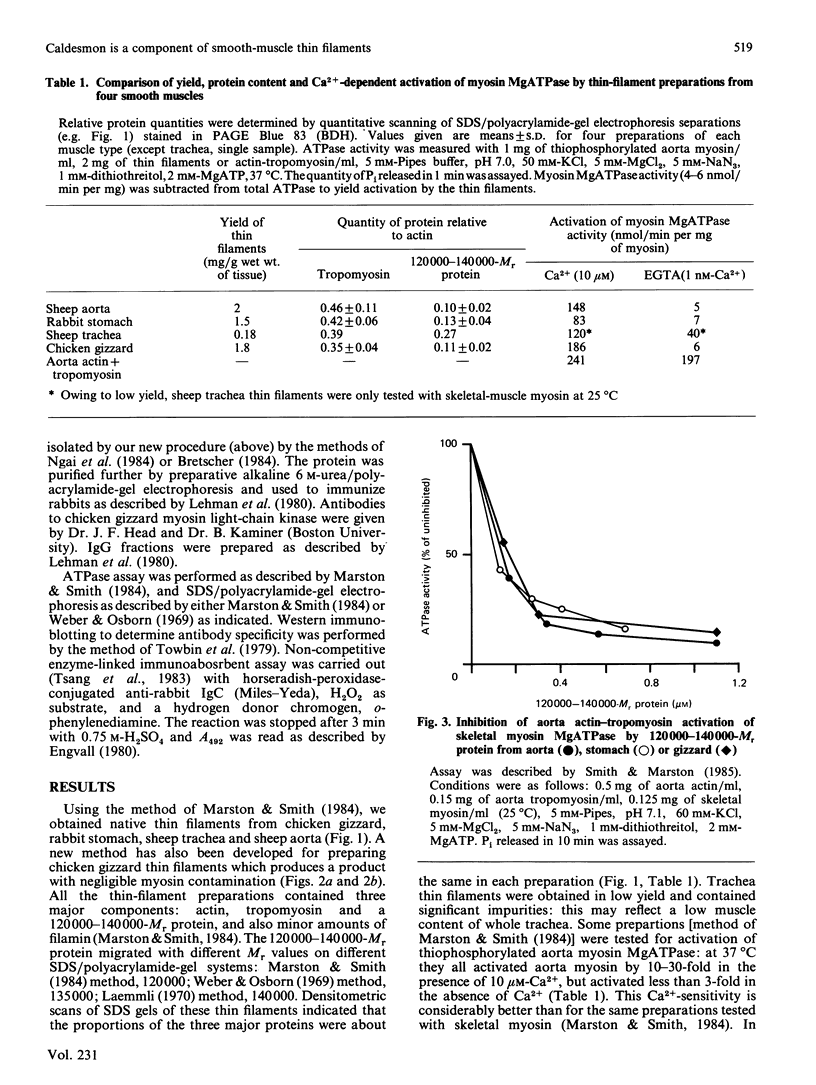
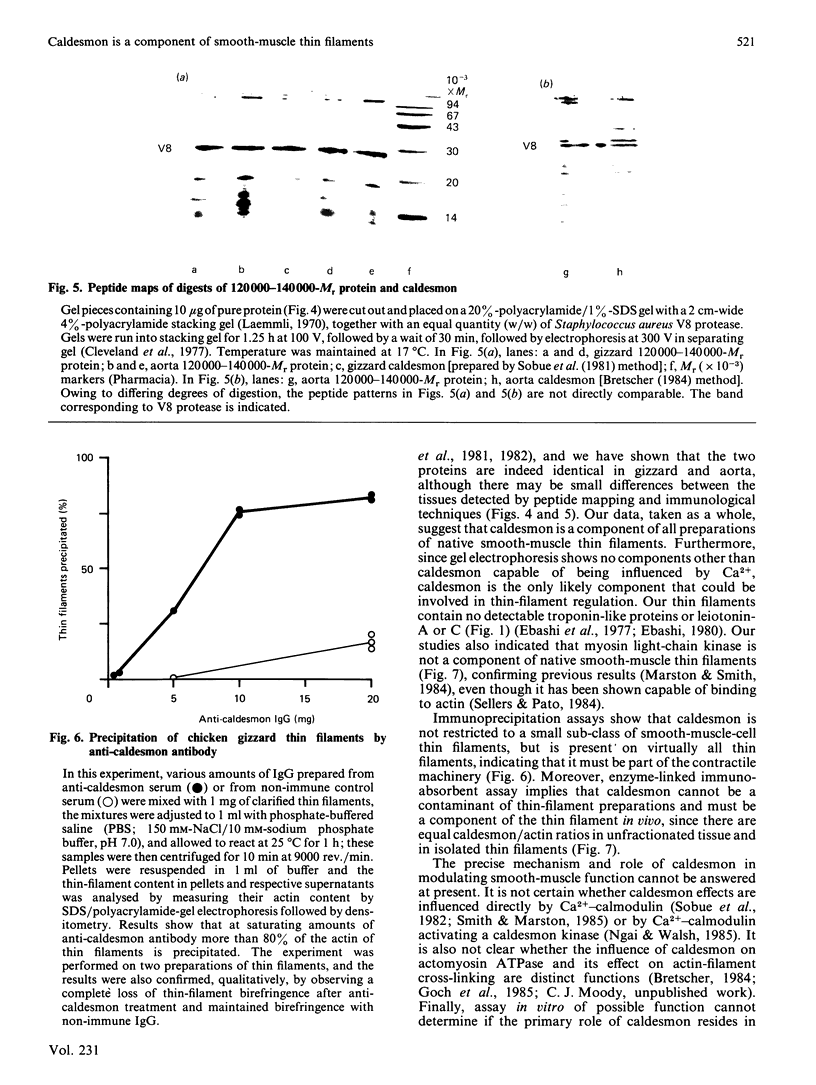
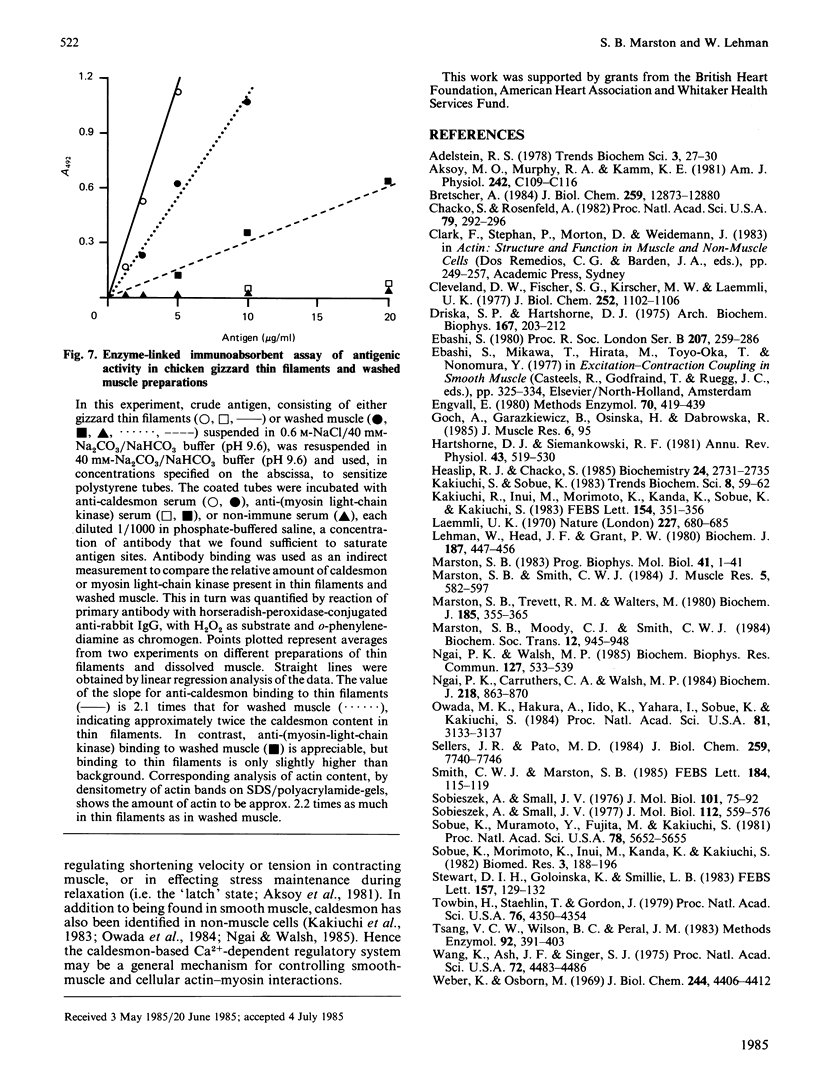
Thin-filament preparations from four smooth muscle types (gizzard, stomach, trachea, aorta) all activate myosin MgATPase activity, are regulated by Ca2+, and contain actin, tropomyosin and a 120000-140000-Mr protein in the molar proportions 1:1/7:1/26. The 120000-140000-Mr protein from all sources is a potent inhibitor of actomyosin ATPase activity. Peptide-mapping and immunological evidence is presented showing that it is identical with caldesmon. Quantitative immunological data suggest that caldesmon is a component of all the thin filaments and that the thin-filament-bound caldesmon accounts for all the caldesmon in intact tissue. The myosin light-chain kinase content of thin-filament preparations was found to be negligible. We propose that caldesmon-based thin-filament Ca2+ regulation is a physiological mechanism in all smooth muscles.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bretscher A. Smooth muscle caldesmon. Rapid purification and F-actin cross-linking properties. J Biol Chem. 1984 Oct 25;259(20):12873–12880. [PubMed] [Google Scholar]
- Chacko S., Rosenfeld A. Regulation of actin-activated ATP hydrolysis by arterial myosin. Proc Natl Acad Sci U S A. 1982 Jan;79(2):292–296. doi: 10.1073/pnas.79.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Driska S., Hartshorne D. J. The contractile proteins of smooth muscle. Properties and components of a Ca2+-sensitive actomyosin from chicken gizzard. Arch Biochem Biophys. 1975 Mar;167(1):203–212. doi: 10.1016/0003-9861(75)90457-9. [DOI] [PubMed] [Google Scholar]
- Ebashi S. The Croonian lecture, 1979: Regulation of muscle contraction. Proc R Soc Lond B Biol Sci. 1980 Mar 21;207(1168):259–286. doi: 10.1098/rspb.1980.0024. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Siemankowski R. F. Regulation of smooth muscle actomyosin. Annu Rev Physiol. 1981;43:519–530. doi: 10.1146/annurev.ph.43.030181.002511. [DOI] [PubMed] [Google Scholar]
- Heaslip R. J., Chacko S. Effects of Ca2+ and Mg2+ on the actomyosin adenosine-5'-triphosphatase of stably phosphorylated gizzard myosin. Biochemistry. 1985 May 21;24(11):2731–2736. doi: 10.1021/bi00332a020. [DOI] [PubMed] [Google Scholar]
- Kakiuchi R., Inui M., Morimoto K., Kanda K., Sobue K., Kakiuchi S. Caldesmon, a calmodulin-binding, F actin-interacting protein, is present in aorta, uterus and platelets. FEBS Lett. 1983 Apr 18;154(2):351–356. doi: 10.1016/0014-5793(83)80181-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehman W., Head J. F., Grant P. W. The stoichiometry and location of troponin I- and troponin C-like proteins in the myofibril of the bay scallop, Aequipecten irradians. Biochem J. 1980 May 1;187(2):447–456. doi: 10.1042/bj1870447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston S. B., Moody C., Smith C. Mechanisms of the regulation of myofibrillar function in vascular smooth muscle. Biochem Soc Trans. 1984 Dec;12(6):945–948. doi: 10.1042/bst0120945. [DOI] [PubMed] [Google Scholar]
- Marston S. B. The regulation of smooth muscle contractile proteins. Prog Biophys Mol Biol. 1983;41(1):1–41. doi: 10.1016/0079-6107(83)90024-x. [DOI] [PubMed] [Google Scholar]
- Marston S. B., Trevett R. M., Walters M. Calcium ion-regulated thin filaments from vascular smooth muscle. Biochem J. 1980 Feb 1;185(2):355–365. doi: 10.1042/bj1850355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai P. K., Carruthers C. A., Walsh M. P. Isolation of the native form of chicken gizzard myosin light-chain kinase. Biochem J. 1984 Mar 15;218(3):863–870. doi: 10.1042/bj2180863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai P. K., Walsh M. P. Detection of caldesmon in muscle and non-muscle tissues of the chicken using polyclonal antibodies. Biochem Biophys Res Commun. 1985 Mar 15;127(2):533–539. doi: 10.1016/s0006-291x(85)80192-3. [DOI] [PubMed] [Google Scholar]
- Owada M. K., Hakura A., Iida K., Yahara I., Sobue K., Kakiuchi S. Occurrence of caldesmon (a calmodulin-binding protein) in cultured cells: comparison of normal and transformed cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3133–3137. doi: 10.1073/pnas.81.10.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers J. R., Pato M. D. The binding of smooth muscle myosin light chain kinase and phosphatases to actin and myosin. J Biol Chem. 1984 Jun 25;259(12):7740–7746. [PubMed] [Google Scholar]
- Smith C. W., Marston S. B. Disassembly and reconstitution of the Ca2+-sensitive thin filaments of vascular smooth muscle. FEBS Lett. 1985 May 6;184(1):115–119. doi: 10.1016/0014-5793(85)80665-7. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Myosin-linked calcium regulation in vertebrate smooth muscle. J Mol Biol. 1976 Mar 25;102(1):75–92. doi: 10.1016/0022-2836(76)90074-7. [DOI] [PubMed] [Google Scholar]
- Sobieszek A., Small J. V. Regulation of the actin-myosin interaction in vertebrate smooth muscle: activation via a myosin light-chain kinase and the effect of tropomyosin. J Mol Biol. 1977 Jun 5;112(4):559–576. doi: 10.1016/s0022-2836(77)80164-2. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Purification of a calmodulin-binding protein from chicken gizzard that interacts with F-actin. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5652–5655. doi: 10.1073/pnas.78.9.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart D. I., Golosinska K., Smillie L. B. Identification of a troponin-I like protein in platelet preparations as histone H2B. FEBS Lett. 1983 Jun 27;157(1):129–132. doi: 10.1016/0014-5793(83)81130-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Wilson B. C., Peralta J. M. Quantitative, single-tube, kinetic-dependent enzyme-linked immunosorbent assay (k-ELISA). Methods Enzymol. 1983;92:391–403. doi: 10.1016/0076-6879(83)92033-5. [DOI] [PubMed] [Google Scholar]
- Wang K., Ash J. F., Singer S. J. Filamin, a new high-molecular-weight protein found in smooth muscle and non-muscle cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4483–4486. doi: 10.1073/pnas.72.11.4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]