ABSTRACT
Aims/Hypothesis
In diabetes haptoglobin (Hp) 2 vs Hp 1 allelic product is associated with cardiac and renal complications. Few studies report both Hp phenotype and Hp levels. In a Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial substudy we evaluated the Hp phenotype, Hp levels, and fenofibrate effects.
Materials and Methods
In 480 adults with type 2 diabetes (T2D) the Hp phenotype was assessed and the Hp level quantified (both using ELISAs assays) in plasma from baseline, after 6 weeks of fenofibrate, and (in n = 200) at 2 years post‐randomization to fenofibrate or placebo.
Results
The Hp phenotypes 1‐1, 2‐1, and 2‐2 frequencies were 15%, 49%, and 36%, respectively. Baseline Hp levels differed by phenotype (P < 0.0001) and decreased (median 21%) after 6 weeks fenofibrate in all phenotypes (adjusted mean (95% CI): −0.27 (−0.32, −0.23) mg/mL in Hp 1‐1, −0.29 (−0.31, −0.27) mg/mL in Hp 2‐1 and −0.05 (−0.07, −0.02) mg/mL in Hp 2‐2 (P = 0.005 and P = 0.055 vs Hp 1‐1 and Hp 2‐1, respectively)). At 2 years post‐randomization the Hp levels in the placebo group had returned to baseline, whilst the fenofibrate‐group levels remained similar to the 6 week levels.
Conclusions
In type 2 diabetes, Hp levels differ by Hp phenotype and are decreased by fenofibrate in all phenotypes, but the effect is diminished in Hp 2‐2.
Keywords: Fenofibrate, Haptoglobin, Type 2 diabetes
Haptoglobin (Hp) levels differed by Hp phenotype in adults with type 2 diabetes. Fenofibrate decreased Hp levels in all Hp phenotypes. A higher baseline Hp level and a smaller fenofibrate‐related decrease in Hp levels in Hp 2‐2 phenotype subjects might be indicative of a lower protective efficacy.
INTRODUCTION
Haptoglobin (Hp) is an abundant circulating α2‐glycoprotein, accounting for 0.9–3.6% (by mass) of plasma proteins 1 , 2 . This acute phase reactant irreversibly binds and neutralizes free (toxic) hemoglobin and exhibits potent antioxidant, anti‐inflammatory, and pro‐angiogenic effects 3 , 4 , 5 . Hp exists in plasma as a tetrameric protein with two α (allelic forms α1, α2) and two β subunits, hence three phenotypes 1‐1, 2‐1, 2‐2 which differ in structure: Hp 1‐1 is a monomer and Hp 2‐1 and 2‐2 are oligomers. In Caucasian individuals Hp 1‐1, Hp 2‐1, and Hp 2‐2 are found in 16%, 48%, and 36% of the population, respectively 6 . Hp is predominantly synthesized by hepatocytes, and is also expressed in kidney, heart, adipose tissue, spleen, and lung. The main clearance route is via the CD163 receptor and phagocytosis by monocytes/macrophages and exocytosis by hepatic Kupffer cells. CD163 expression is reduced in people with type 2 diabetes (T2D), prolonging the Hp half‐life, especially in those with the Hp 2‐2 phenotype 3 , 4 , 5 . A secondary clearance route for Hp and Hp‐hemoglobin complexes is via the renal proximal tubule cells. The circulating Hp half‐life is 3–5 days, but once bound to hemoglobin, Hp‐hemoglobin complexes are cleared within 20 min 3 . Circulating Hp reaches adult levels, 0.15–2.5 mg/mL, by approximately 4–6 months of age, and thereafter is stable in individuals in the absence of an acute phase stimulus 7 .
Compared with Hp 2, Hp 1 is smaller, has greater anti‐oxidant and anti‐inflammatory effects, is less angiogenic, binds free hemoglobin and CD163 receptors more avidly, and is more rapidly cleared from plasma 3 . Atherosclerotic plaques from Hp 1‐1 or 2‐1 mice and humans have lower lipid peroxides, iron, and macrophage accumulation than Hp 2‐2 counterparts 8 . Hp inhibition of lipoprotein oxidation is diminished if the lipoprotein is glycated, as occurs in diabetes according to the degree of hyperglycemia 9 . Oxidized LDL and HDL lipid peroxides are lower in people with Hp1‐1 vs Hp2‐1 or Hp2‐2, consistent with the greater anti‐oxidant activity of Hp1‐1 4 . Iron‐containing Hp‐hemoglobin complexes can bind to HDL, rendering HDL pro‐oxidant and pro‐inflammatory, and impairing (vasoprotective) reverse cholesterol transport 4 .
Many studies in people with type 2 diabetes report that the Hp 2‐2 phenotype is associated with a higher risk of cardiovascular disease (CVD) 10 , 11 . In 2000, Levy et al. 12 were one of the first to report an association between Hp phenotype and the development of vascular complications in 45 patients with type 2 diabetes; Hp 1‐1 was associated with reduced re‐stenosis after percutaneous transluminal coronary angioplasty vs Hp 2‐1/Hp 2‐2 12 . This small cohort was later included in a larger study (n = 98) showing a significant difference in re‐stenosis by Hp phenotype in patients with diabetes, and even in those without diabetes 13 . In a case–control sub‐study of the Strong Heart Study of Native Americans (206 CVD cases, 206 controls), subjects with type 2 diabetes with Hp 2‐2 were five and three times more likely to have a CVD event than those with Hp 1‐1 (P = 0.002) or Hp 2‐1 (P = 0.010), respectively 14 . In agreement, Hochberg et al. reported that in patients with diabetes and coronary artery disease, those with Hp 2‐2 /Hp 2‐1 had fewer collateral coronary arteries than Hp 1‐1, no such association was observed in people without diabetes 15 . The relative risk associated with Hp alleles was magnified in the presence of hyperglycemia 16 . The Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial results support that glycemia modulates the complication risk differentially by Hp phenotype 17 and that fenofibrate (vs placebo) reduces coronary artery disease (CAD) events only in those without the Hp 2‐2 phenotype (multivariable adjusted HR 0.74 (95% CI 0.60–0.90)) 18 ; however, the Hp levels were not measured. Proactive testing of Hp genotype or phenotype of people with type 2 diabetes may help to identify patients at particularly high complication risk and targeted treatment may improve clinical outcomes.
The Hp phenotype may determine the response to treatments, such as Vitamin E. In both the Israel Cardiovascular Events Reduction with vitamin E (ICARE) Study and the Nurses’ Health Study, people with Hp 2‐2 and HbA1c ≥6.5% had a >10‐fold increased risk of coronary heart disease compared with those with at least one Hp‐1 allele and HbA1c <6.5% 19 , 20 . Individual trials and a meta‐analysis show Vitamin E can decrease CVD death risk (OR 0.47, 95%CI 0.26–0.85, P = 0.013) in people with diabetes and Hp 2‐2 21 .
Most Hp‐related clinical studies only report Hp phenotype or levels, not both. In a Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) trial substudy we aimed to evaluate the frequency distribution of the Hp phenotype, the association with Hp levels, and the effects of fenofibrate on Hp levels.
MATERIALS AND METHODS
We determined the Hp phenotype and levels in (citrate) plasma samples of 480 randomly selected FIELD trial participants (Figure S1) from Australia and New Zealand (trial details 22 , 23 ). The Hp phenotype was assessed using baseline samples and the Hp levels were measured in samples from subjects at baseline, at the end of 6 week ‘run‐in’ (200 mg daily fenofibrate), and in 200 subjects 2 years post‐randomization, with all subject's samples for Hp levels analyzed in the same batch with the operator masked to sample order, subject identity, and treatment allocation. Among the analyzed subjects 463 (96.5%) were Caucasian (all ethnicity data were self‐reported) and of the remaining 3.5% the majority (n = 11, 64.7%) were Asian. There were 249 and 231 subjects selected at baseline and after 6 weeks fenofibrate ‘run‐in’ who were later randomized to placebo and fenofibrate arms, respectively. The proportions of women to men were the same in the selected subjects in placebo and fenofibrate arms: 49.0% and 49.4% women in placebo and fenofibrate arms, respectively. At the 2 years timepoint the number of subjects (n = 200) randomly selected from two treatment arms and women to men ratios were 1:1 (n = 100 placebo: n = 100 fenofibrate, n = 50 women to n = 50 men in both placebo and fenofibrate groups).
The Hp phenotype and levels were determined by ELISA (Savyon Diagnostics, Ashdod, Israel and R&D Systems, Inc., Minneapolis, MN, respectively) with intra‐ and inter‐assay CVs: Hp phenotype: 2% and 1% (for absorbance values); Hp level: 5% and 5%, respectively. Comparisons between Hp phenotypes were analyzed using anova (with post‐hoc Tukey test) or Kruskal‐Wallis anova (depending on distribution). Correlations were assessed using Spearman R coefficient. Categorical variables were analyzed using χ2 test. Data are presented as mean ± SD or median (LQ, UQ). Adjusted (least square) means are reported as mean (95% CI) or mean ± SEM. Comparisons were made with Bonferroni correction. Statistical significance was taken at P < 0.05.
RESULTS
Baseline subject characteristics are shown in Table 1, with no significant differences by Hp phenotype.
Table 1.
Baseline characteristics of study subjects, including plasma Hp levels for all subjects and by Hp phenotype. Data shown are mean ± SD or median (LQ, UQ)
| All | Hp 1‐1 | Hp 2‐1 | Hp 2‐2 | P * | |
|---|---|---|---|---|---|
| N (%) | 480 (100.0) | 74 (15.4) | 233 (48.5) | 173 (36.0) | – |
| Gender (M/F; % male) | 244/236 (51) | 36/38 (49) | 123/110 (53) | 85/88 (49) | 0.71 |
| Age (years) | 61 ± 6 | 60 ± 6 | 61 ± 6 | 61 ± 6 | 0.47 |
| Known type 2 diabetes duration (years) | 6 ± 6 | 7 ± 5 | 6 ± 7 | 6 ± 6 | 0.85 |
| HbA1c (%) | 6.9 ± 1.4 | 6.7 ± 1.5 | 7.1 ± 1.4 | 6.9 ± 1.2 | 0.08 |
| HbA1c (mmol/mol) | 52.4 ± 15.1 | 49.7 ± 16.4 | 53.9 ± 15.7 | 51.6 ± 13.3 | 0.08 |
| BMI (kg/m2) | 30.8 ± 5.6 | 30.0 ± 5.0 | 31.1 ± 5.6 | 30.6 ± 5.7 | 0.33 |
| TC (mmol/L) | 5.0 ± 0.7 | 4.9 ± 0.7 | 4.9 ± 0.7 | 5.0 ± 0.6 | 0.69 |
| TG (mmol/L) | 1.7 (1.3, 2.3) | 1.7 (1.4, 2.5) | 1.7 (1.3, 2.3) | 1.7 (1.3, 2.2) | 0.60 |
| HDL‐C (mmol/L) | 1.1 ± 0.3 | 1.1 ± 0.4 | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.64 |
| LDL‐C (mmol/L) | 3.0 ± 0.7 | 2.9 ± 0.7 | 3.0 ± 0.7 | 3.1 ± 0.6 | 0.15 |
| SBP (mmHg) | 138 ± 14 | 138 ± 15 | 138 ± 14 | 130 ± 12 | 0.30 |
| DBP (mmHg) | 81 ± 8 | 81 ± 7 | 82 ± 8 | 78 ± 8 | 0.09 |
| MAP (mmHg) | 100 ± 9 | 100 ± 9 | 100 ± 9 | 99 ± 9 | 0.10 |
| eGFR (CKD‐EPI) | 95 (83, 102) | 97 (82, 103) | 95 (83, 101) | 95 (83, 102) | 0.71 |
| Complications micro/macro (%) | 29 / 15 | 31 / 11 | 33 / 19 | 23 / 12 | 0.10/0.11 |
| Haptoglobin (mg/mL) | |||||
| Baseline | 1.00 ± 0.45 | 1.13 ± 0.46 | 1.10 ± 0.44 | 0.81 ± 0.41 | <0.0001 |
| End of run‐in | 0.80 ± 0.37 | 0.94 ± 0.35 | 0.87 ± 0.35 | 0.65 ± 0.36 | <0.0001 |
| 2 years** | 0.95 ± 0.42 | 1.18 ± 0.42 | 0.99 ± 0.37 | 0.76 ± 0.42 | <0.0001 |
Unadjusted Hp levels and difference are presented. Bolded P‐values with statistical significance.
Indicates P‐value: χ2‐test, anova or Kruskal‐Wallis anova between Hp phenotypes.
Indicates 2 years data in a subset of n = 200 subjects.
The Hp phenotype frequencies (Hp 1‐1, 2‐1, and 2‐2 constituting 15%, 49%, and 36%, respectively) in this FIELD trial substudy were similar to those published in the general Caucasian population 6 . Hp levels (mean ± SD) at baseline were lower in men than in women (0.89 ± 0.47 vs 1.11 ± 0.47 mg/mL respectively; P < 0.0001). Baseline plasma Hp levels were higher in Hp 1‐1 and Hp 2‐1 than in Hp 2‐2 subjects (Figure 1a), although differences were not sufficient to infer Hp phenotype from Hp level. After adjustment for sex, baseline mean arterial pressure (MAP), BMI, total cholesterol, HDL‐C and HbA1c Hp levels (mean (95% CI)) were higher in Hp 1‐1 and Hp 2‐1 vs Hp 2‐2 phenotype (1.15 (1.05, 1.24) mg/mL and 1.10 (1.04, 1.15) mg/mL vs 0.81 (0.74, 0.87) mg/mL respectively, both P < 0.0001).
Figure 1.
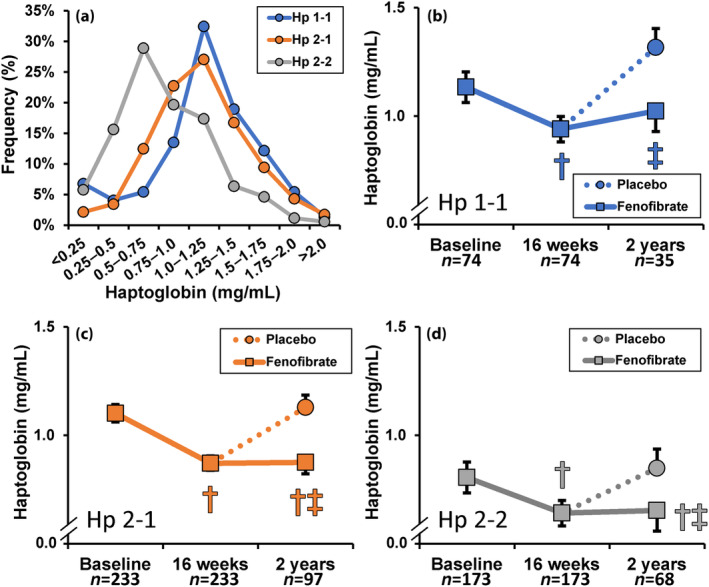
(a) Frequency of baseline Hp concentration by phenotype in 480 FIELD trial participants. (b–d) Effect of fenofibrate on plasma Hp levels according to phenotype in a subset of FIELD trial participants: all received treatment for the last 6 weeks of the 16 weeks ‘run‐in phase’; then they were randomized to fenofibrate or placebo. Fenofibrate (200 mg daily). significantly lowered Hp levels in all phenotypes after the 6 weeks active run‐in phase. Hp levels remained low at 2 years in those allocated to ongoing fenofibrate, whilst in those subsequently allocated to placebo at 2 years the levels were similar to those at baseline. Data (mean ± SEM) shown after adjustment for gender, baseline MAP, BMI, total cholesterol, HDL‐C, and HbA1c. † P < 0.0001 vs baseline, ‡ P < 0.04 vs placebo.
At baseline Hp levels correlated significantly with HbA1c, BMI, blood pressure, total‐ and HDL‐C differentially by Hp phenotype (Table 2). Comparison of clinical chemistry biomarkers at baseline and after 6 weeks of fenofibrate treatment (at the end of 16 weeks of FIELD trial run‐in phase) are shown in Table S1.
Table 2.
Correlations (Spearman R coefficient and P‐values) between baseline Hp levels and other biomarkers for all subjects and by Hp phenotype
| All | Hp 1‐1 | Hp 2‐1 | Hp 2‐2 | |
|---|---|---|---|---|
| Age (years) | R = −0.03, P = 0.58 | R = 0.01, P = 0.94 | R = −0.02, P = 0.78 | R = 0.01, P = 0.93 |
| Known type 2 diabetes duration (years) | R = 0.02, P = 0.72 | R = 0.06, P = 0.62 | R = −0.02, P = 0.72 | R = −0.01, P = 0.85 |
| HbA1c (%) | R = 0.10, P = 0.023 | R = 0.04, P = 0.76 | R = 0.09, P = 0.16 | R = 0.15, P = 0.048 |
| HbA1c (mmol/mol) | R = 0.10, P = 0.023 | R = 0.04, P = 0.76 | R = 0.09, P = 0.16 | R = 0.15, P = 0.048 |
| BMI (kg/m2) | R = 0.21, P < 0.0001 | R = 0.23, P = 0.048 | R = 0.25, P = 0.0001 | R = 0.17, P = 0.024 |
| TC (mmol/L) | R = 0.07, P = 0.12 | R = ‐0.04, P = 0.73 | R = 0.15, P = 0.021 | R = 0.08, P = 0.29 |
| TG (mmol/L) | R = 0.01, P = 0.85 | R = −0.13, P = 0.26 | R = 0.02, P = 0.78 | R = 0.01, P = 0.92 |
| HDL‐C (mmol/L) | R = 0.08, P = 0.085 | R = 0.004, P = 0.97 | R = 0.07, P = 0.32 | R = 0.18, P = 0.020 |
| LDL‐C (mmol/L) | R = 0.04, P = 0.43 | R = 0.09, P = 0.42 | R = 0.12, P = 0.056 | R = 0.02, P = 0.79 |
| SBP (mmHg) | R = 0.14, P = 0.003 | R = 0.07, P = 0.56 | R = 0.20, P = 0.002 | R = 0.03, P = 0.66 |
| DBP (mmHg) | R = 0.06, P = 0.16 | R = 0.04, P = 0.76 | R = 0.14, P = 0.03 | R = −0.09, P = 0.25 |
| MAP (mmHg) | R = 0.10, P = 0.025 | R = 0.05, P = 0.67 | R = 0.19, P = 0.004 | R = −0.05, P = 0.54 |
| eGFR (CKD‐EPI) | R = −0.01, P = 0.82 | R = 0.08, P = 0.51 | R = −0.01, P = 0.83 | R = −0.05, P = 0.50 |
Bolded P‐values with statistical significance.
As shown in Figure 1c,d, the Hp levels were significantly lowered by fenofibrate in all phenotypic groups vs baseline values. Adjusted for baseline Hp values, Hp levels decreased in all phenotypes after 6 weeks of fenofibrate (mean (95% CI)): −0.27 (−0.32, −0.23) mg/mL in Hp 1‐1, −0.29 (−0.31, −0.27) mg/mL in Hp 2‐1 and −0.05 (−0.07, −0.02) mg/mL in Hp 2–2 (change in Hp ‐22 P = 0.005 and P = 0.055 vs Hp 1‐1 and Hp 2‐1, respectively). After 2 years, in those randomized to placebo, the Hp concentrations reverted to baseline levels, whereas in those receiving fenofibrate post‐randomization, the reduction evident after 6 weeks of fenofibrate was sustained.
We were unable to detect any significant difference in the frequency of T2D complications (coronary event, non‐fatal MI, CVD, stroke, CABG, revascularization, amputation, laser treatment) within and between any Hp phenotype between subjects randomized to placebo or fenofibrate treatment (data not shown).
DISCUSSION
In our FIELD trial based substudy we found a similar Hp phenotype distribution to that reported in the literature 3 , 4 , 5 , 24 . We have observed differences in Hp levels by phenotype and phentoype differences in Hp levels reduction by fenofibrate. The Hp phenotype may be an important determinant of vascular risk, which can be modulated by glycemia 25 . As shown by the FIELD, ACCORD, and The Diabetes Atherosclerosis Intervention Study (DAIS) trials 26 , 27 , 28 fenofibrate can reduce the risk of many diabetes‐related chronic complications. The ACCORD trial reported that the fenofibrate benefit on CAD is observed only in those without the Hp 2‐2 phenotype 18 . The ACCORD trial did not report Hp levels. While there is much evidence linking the Hp 2‐2 phenotype with adverse CVD outcomes, not all studies concur. In people with diabetes in the Framingham Offspring Study, Hp 1‐1 was associated with more prevalent coronary heart disease (CHD) than Hp 2‐1 and Hp 2‐2 (although people with CHD were over‐represented in the Hp 1‐1 group) 29 . A recent study of Chinese living in Singapore also found an increased CVD risk in people with type 2 diabetes and Hp 1‐1 25 , emphasizing the need for studies in different ethnic groups. Our FIELD substudy is based in Australia and New Zealand. In the Bruneck study, no association of Hp 2‐2 with atherosclerotic events was evident in subjects with or without diabetes 30 , although this might be the result of low event numbers (only 123/9,863 cases/person‐years of follow‐up) and merging of cardiac and stroke events. The Bruneck study did, however, report differences in CVD risk factors by Hp phenotype: total cholesterol and LDL‐C were higher in Hp 2‐2 subjects than in Hp 2‐1/Hp 1‐1 subjects combined (P = 0.008 and P = 0.016, respectively). In our small FIELD substudy CVD risk factors did not differ significantly by Hp phenotype, but the Hp levels were correlated with HbA1c, blood pressure, and HDL‐C. Larger studies are of interest.
The Hp levels in urine have been related to diabetic renal damage. In the type 2 diabetes Veterans Affairs Diabetes Trial (VADT) 31 , urinary Hp levels were a predictor of diabetic nephropathy (DN), in a substudy of 204 subjects with type 2 diabetes and 3.8 years mean follow‐up. They had at baseline a urinary albumin:creatinine ratio (ACR) of <300 μg/mg and eGFR stage 2 or better. The urinary Hp:creatinine ratio (uHp:Cr) predicted early renal function decline (ERFD; ≥3.3% eGFR decline per annum). Compared with people in the lowest uHp:Cr tertile those in the highest tertile had a greater risk of ERFD: OR 2.70 (95% CI 1.15–6.32), P < 0.05, after adjustment for VADT treatment group allocation and ACEi use. Addition of uHp:Cr to ACR improved the end stage kidney disease prediction 32 . FIELD urine samples are not available to replicate this analysis.
The Hp levels have been linked to diabetes complications 31 , 32 . Circulating Hp levels differ by Hp phenotype 24 , but Hp phenotype cannot be inferred from the Hp level or vice‐versa, as confirmed by our FIELD study in which Hp levels in each phenotype overlap substantially. Studies in people with type 2 diabetes suggest that the Hp 2‐2 phenotype is associated with an increased risk of cardiac and kidney complications which is modulated by glycemia. It is desirable to measure both Hp phenotype and levels as we have done herein, and plan to extend to the majority of the FIELD trial cohort. The fenofibrate benefit may relate to reductions in circulating Hp levels, which we demonstrated with significant reductions in Hp levels in all phenotype groups, with the smallest reduction in Hp 2‐2 phenotype (after adjustment for baseline level). Whilst fenofibrate was protective against advanced diabetic retinopathy in the FIELD and ACCORD Lipid trials, currently there are no papers reporting associations between retinopathy or other microvascular complications, fenofibrate benefit and Hp levels. As there are many pleiotropic effects of fenofibrate 33 the macro‐ and micro‐vascular benefits of fenofibrate in people with type 2 diabetes may relate, at least partially, to reductions in Hp levels.
In this FIELD trial sub‐study baseline Hp levels differed by Hp phenotype in adults with type 2 diabetes, being lowest in Hp 2‐2 phenotype subjects. Fenofibrate decreased Hp levels in all Hp phenotypes, but the effect was diminished in the Hp 2‐2 group (after adjustment for baseline levels). Lower baseline Hp level and a lower fenofibrate‐related decrease in Hp levels in Hp 2‐2 phenotype subjects might be indicative of a lower protective efficacy against type 2 diabetes vascular complications. The study of most or all of the FIELD cohort (n = 9,795) may establish relationships between Hp phenotype, Hp levels, fenofibrate effects, and type 2 diabetes vascular complications.
DISCLOSURE
The authors declare no conflict of interest.
Approval of the research protocol: The project was approved by the Human Research Ethics Committee of The University of Sydney, Australia HREC no: 08–2007/10216.
Informed consent: All participants involved in the study were provided written, informed consent.
Approval date of registry and the registration no. of the study/trial: FIELD trial HREC approval date of registry: 19 October 2004, registration No ISRCTN 64783481.
Animal studies: N/A.
PRIOR PUBLICATIONS
Part of this work was presented at the American Diabetes Association 79th Scientific Sessions, San Francisco, CA 7–9 June 2019.
FUNDING
The FIELD study was supported by a grant from Laboratories Fournier SA, Dijon, France (now part of Abbott) and the National Health and Medical Research Council (NHMRC) of Australia, and was coordinated independently by the NHMRC Clinical Trials Centre, University of Sydney. FIELD was endorsed by the National Heart Foundation, Diabetes Australia, Diabetes New Zealand, and the Finnish Diabetes Association. AJJ and ACK were supported by NHMRC Program Grant (GNT1150467), AJJ was supported by NHMRC Practitioner Fellowship (GNT1121272). ACK was supported by NHMRC Senior Fellowship (GNT1137071) and Investigator Grant (GNT2018537).
Supporting information
Figure S1. Flow chart of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study (left, reproduced from Cardiovasc Diabetol 3(1), 9 (2004). https://doi.org/10.1186/1475‐2840‐3‐9) and the timepoints for sample selection for these analyses (right).
Table S1. Comparison of clinical chemistry results at baseline and at the end of trial run‐in (post 6 weeks of fenofibrate treatment).
Acknowledgment
Open access publishing facilitated by The University of Sydney, as part of the Wiley ‐ The University of Sydney agreement via the Council of Australian University Librarians.
REFERENCES
- 1. Longo LV, Nakayasu ES, Matsuo AL, et al. Identification of human plasma proteins associated with the cell wall of the pathogenic fungus Paracoccidioides brasiliensis. FEMS Microbiol Lett 2013; 341: 87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soejima M, Koda Y. Identification and diagnosis of complete haptoglobin gene deletion, one of the genes responsible for adverse posttransfusion reactions. Biomedicine 2024; 12: 790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Levy AP, Asleh R, Blum S, et al. Haptoglobin: Basic and clinical aspects. Antioxid Redox Signal 2010; 12: 293–304. [DOI] [PubMed] [Google Scholar]
- 4. Costacou T, Levy AP. Haptoglobin genotype and its role in diabetic cardiovascular disease. J Cardiovasc Transl Res 2012; 5: 423–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacKellar M, Vigerust DJ. Role of haptoglobin in health and disease: A focus on diabetes. Clin Diabetes 2016; 34: 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem 1996; 42: 1589–1600. [PubMed] [Google Scholar]
- 7. Hashim IA. Tutorials in Clinical Chemistry. Amsterdam: Elsevier Science, 2023. [Google Scholar]
- 8. Moreno PR, Purushothaman KR, Purushothaman M, et al. Haptoglobin genotype is a major determinant of the amount of iron in the human atherosclerotic plaque. J Am Coll Cardiol 2008; 52: 1049–1051. [DOI] [PubMed] [Google Scholar]
- 9. Lyons TJ, Baynes JW, Patrick JS, et al. Glycosylation of low density lipoprotein in patients with type 1 (insulin‐dependent) diabetes: Correlations with other parameters of glycaemic control. Diabetologia 1986; 29: 685–689. [DOI] [PubMed] [Google Scholar]
- 10. Somer S, Levy AP. The role of haptoglobin polymorphism in cardiovascular disease in the setting of diabetes. Int J Mol Sci 2020; 22: 287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalan R, Liew H, Goh LL, et al. The haptoglobin 2‐2 genotype is associated with inflammation and carotid artery intima‐media thickness. Diab Vasc Dis Res 2016; 13: 373–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Levy AP, Roguin A, Hochberg I, et al. Haptoglobin phenotype and vascular complications in patients with diabetes. N Engl J Med 2000; 343: 969–970. [DOI] [PubMed] [Google Scholar]
- 13. Roguin A, Hochberg I, Nikolsky E, et al. Haptoglobin phenotype as a predictor of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol 2001; 87: 330–332. [DOI] [PubMed] [Google Scholar]
- 14. Levy AP, Hochberg I, Jablonski K, et al. Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The strong heart study. J Am Coll Cardiol 2002; 40: 1984–1990. [DOI] [PubMed] [Google Scholar]
- 15. Hochberg I, Roguin A, Nikolsky E, et al. Haptoglobin phenotype and coronary artery collaterals in diabetic patients. Atherosclerosis 2002; 161: 441–446. [DOI] [PubMed] [Google Scholar]
- 16. Costacou T, Evans RW, Orchard TJ. Glycaemic control modifies the haptoglobin 2 allele‐conferred susceptibility to coronary artery disease in type 1 diabetes. Diabet Med 2016; 33: 1524–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carew AS, Levy AP, Ginsberg HN, et al. Haptoglobin phenotype modifies the influence of intensive glycemic control on cardiovascular outcomes. J Am Coll Cardiol 2020; 75: 512–521. [DOI] [PubMed] [Google Scholar]
- 18. Warren RA, Carew AS, Andreou P, et al. Haptoglobin phenotype modifies the effect of fenofibrate on risk of coronary event: ACCORD lipid trial. Diabetes Care 2022; 45: 241–250. [DOI] [PubMed] [Google Scholar]
- 19. Cahill LE, Levy AP, Chiuve SE, et al. Haptoglobin genotype is a consistent marker of coronary heart disease risk among individuals with elevated glycosylated hemoglobin. J Am Coll Cardiol 2013; 61: 728–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cahill LE, Jensen MK, Chiuve SE, et al. The risk of coronary heart disease associated with glycosylated hemoglobin of 6.5% or greater is pronounced in the haptoglobin 2‐2 genotype. J Am Coll Cardiol 2015; 66: 1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Asleh R, Briasoulis A, Berinstein EM, et al. Meta‐analysis of the association of the haptoglobin genotype with cardiovascular outcomes and the pharmacogenomic interactions with vitamin E supplementation. Pharmgenomics Pers Med 2018; 11: 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Keech A, Simes RJ, Barter P, et al. Effects of long‐term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005; 366: 1849–1861. [DOI] [PubMed] [Google Scholar]
- 23. Davis TM, Ting R, Best JD, et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: The fenofibrate intervention and event lowering in diabetes (FIELD) study. Diabetologia 2011; 54: 280–290. [DOI] [PubMed] [Google Scholar]
- 24. Van Vlierberghe H, Langlois M, Delanghe J. Haptoglobin polymorphisms and iron homeostasis in health and in disease. Clin Chim Acta 2004; 345: 35–42. [DOI] [PubMed] [Google Scholar]
- 25. Gurung RL, Yiamunaa M, Liu S, et al. Association of haptoglobin phenotype with incident acute myocardial infarction in Chinese patients with type 2 diabetes. Cardiovasc Diabetol 2019; 18: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsunoda F, Asztalos IB, Horvath KV, et al. Fenofibrate, HDL, and cardiovascular disease in type‐2 diabetes: The DAIS trial. Atherosclerosis 2016; 247: 35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mottl AK, Buse JB, Ismail‐Beigi F, et al. Long‐term effects of intensive glycemic and blood pressure control and fenofibrate use on kidney outcomes. Clin J Am Soc Nephrol 2018; 13: 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart S, Lois N. Fenofibrate for diabetic retinopathy. Asia Pac J Ophthalmol 2018; 7: 422–426. [DOI] [PubMed] [Google Scholar]
- 29. Levy AP, Larson MG, Corey D, et al. Haptoglobin phenotype and prevalent coronary heart disease in the Framingham offspring cohort. Atherosclerosis 2004; 172: 361–365. [DOI] [PubMed] [Google Scholar]
- 30. Pechlaner R, Kiechl S, Willeit P, et al. Haptoglobin 2‐2 genotype is not associated with cardiovascular risk in subjects with elevated glycohemoglobin‐results from the Bruneck study. J Am Heart Assoc 2014; 3: e000732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jenkins AJ, Fu D, Azar M, et al. Clinical correlates of serum pigment epithelium‐derived factor in type 2 diabetes patients. J Diabetes Complications 2014; 28: 353–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhensdadia NM, Hunt KJ, Lopes‐Virella MF, et al. Urine haptoglobin levels predict early renal functional decline in patients with type 2 diabetes. Kidney Int 2013; 83: 1136–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsimihodimos V, Liberopoulos E, Elisaf M. Pleiotropic effects of fenofibrate. Curr Pharm Des 2009; 15: 517–528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study (left, reproduced from Cardiovasc Diabetol 3(1), 9 (2004). https://doi.org/10.1186/1475‐2840‐3‐9) and the timepoints for sample selection for these analyses (right).
Table S1. Comparison of clinical chemistry results at baseline and at the end of trial run‐in (post 6 weeks of fenofibrate treatment).


