Abstract
Cancer neuroscience is an emerging field that investigates the intricate relationship between the nervous system and cancer, gaining increasing recognition for its importance. The central nervous system governs the development of the nervous system and directly affects brain tumors, and the peripheral nervous system (PNS) shapes the tumor microenvironment (TME) of peripheral tumors. Both systems are crucial in cancer initiation and progression, with recent studies revealing a more intricate role of the PNS within the TME. Tumors not only invade nerves but also persuade them through remodeling to further promote malignancy, creating a bidirectional interaction between nerves and cancers. Notably, immune cells also contribute to this communication, forming a triangular relationship that influences protumor inflammation and the effectiveness of immunotherapy. This review delves into the intricate mechanisms connecting the PNS and tumors, focusing on how various immune cell types influence nerve‒tumor interactions, emphasizing the clinical relevance of nerve‒tumor and nerve‒immune dynamics. By deepening our understanding of the interplay between nerves, cancer, and immune cells, this review has the potential to reshape tumor biology insights, inspire innovative therapies, and improve clinical outcomes for cancer patients.
Keywords: cancer neuroscience, cancer therapy, neuroimmune crosstalk, peripheral nervous system, tumor microenvironment, tumor‒nerve interactions
The figure depicts the intricate interactions between cancer cells, the immune system, and nerves within the tumor microenvironment. At the center, the diagram highlights the tripartite relationship among cancer, immune, and nerve cells, illustrating the dynamic and reciprocal influences they exert on one another. Surrounding this core, key biological processes are illustrated, including proliferation and apoptosis, cell cycle arrest and DNA damage, invasion and metastasis, angiogenesis and lymphangiogenesis, and immune modulation. The diagram also underscores the feedback loop driving cancer progression, where nerves facilitate cancer initiation and immune evasion, while cancer cells activate neural pathways, further enhancing their invasive potential.

1. INTRODUCTION
The nervous system is crucial for organ development, tissue homeostasis, and cancer regulation, as recent research extensively explores the connection between nerves and cancer, thereby establishing a solid foundation for cancer neuroscience. 1 , 2 , 3 The central nervous system (CNS) is directly involved in the formation of brain tumors, with its development closely correlated with tumor progression. 4 Alterations in different brain regions or neural pathways are intertwined, and abnormal signals originating from brain regions can also modulate peripheral tumor development through the PNS. 5 Numerous studies have demonstrated that the PNS significantly contributes to the pathogenesis of extracerebral cancers.
The PNS consists of afferent (sensory) and efferent (motor and autonomic) nerves, which branch throughout the body alongside microvessels, connecting the CNS to organs and tissues to maintain local homeostasis. 6 Over a century ago, pathologists recognized the relationship between peripheral nerves and malignant cells, termed perineural infiltration (PNI), which is associated with increased cancer aggressiveness and poor prognosis. 7 , 8 Additionally, researchers have observed a notable increase in nerve density as precancerous lesions progress to cancer. 9 , 10 Tumors not only invade nerves but also accelerate cancer progression by secreting molecules that promote nerve infiltration. 11 For example, neurotrophic factors such as nerve growth factor (NGF) stimulate nerve growth in the tumor microenvironment (TME), leading to sensory and sympathetic nerve infiltration. 12 , 13 Cancer cells also release axon guidance molecules, such as semaphorins, 14 , 15 that reprogram sensory nerves into sympathetic ones, further driving tumor invasion and metastasis. This interplay underscores the critical role of nerves in promoting tumor aggressiveness. Emerging evidence reveals the intricate interactions between immune cells, cancer cells, and nerves within the TME. 16 Besides tumor cells, immune cells—such as T cells, myeloid‐derived suppressor cells (MDSCs), and tumor‐associated macrophages (TAMs)—express neural signaling receptors, including adrenergic and cholinergic receptors. These neural signals regulate the movement, proliferation, activation, and function of immune cells, influencing tumor inflammation, anti‐tumor immunity, and immunosuppression. 17 , 18 Thus, the peripheral neural network is now recognized as a key component of the TME, contributing to cancer progression, proliferation, invasion, and metastasis through via neural signaling pathways. 19 , 20 , 21 , 22
The interplay between the nervous system and tumors presents promising therapeutic opportunities, such as inhibiting tumor growth, 23 , 24 , 25 overcoming treatment resistance, 26 , 27 , 28 enhancing immune responses, 29 , 30 and preventing metastasis. 31 Modulating adrenergic and cholinergic signaling can influence these aspects of cancer biology. β‐adrenergic receptor (β‐AR) blockers and parasympathomimetics show potential, especially when combined with chemotherapy or immunotherapy, to enhance treatment efficacy and reduce resistance. 32 , 33 , 34 , 35 , 36 , 37 Notably, clinical trials have demonstrated the effectiveness of combining β‐AR blockers with immune checkpoint inhibitors (ICIs) in treating melanoma. 38 , 39 , 40 Nonetheless, due to the variability in response among different cancer types to therapies targeting nerve‒tumor‒immune interactions, more preclinical trials are necessary for further validation. Targeting neural regeneration pathways, especially those involving NGF, may inhibit tumor progression, as cancers such as pancreatic and breast cancer promote nerve growth to facilitate metastasis. 41 , 42 , 43 Consequently, disrupting these neurogenic processes could offer a strategic approach to limiting metastasis. In conclusion, elucidating the role of the nervous system in cancer progression and its impact on the immune microenvironment is essential for advancing therapeutic strategies and enhancing patient outcomes.
In this review, we outline the interactions between peripheral nerves and cancer, emphasizing the key nerve types and signaling pathways that promote tumor progression. We highlight the bidirectional relationship in which nerves not only regulate tumor initiation and progression but are also hijacked and manipulated by the tumor, creating a self‐sustaining cycle that accelerates tumor development. Additionally, we discuss the role of immune cell phenotypes in nerve‒tumor crosstalk, highlighting their impact on both nerve and tumor dynamics, as well as the molecular mechanisms involved. The review further assesses current clinical treatment strategies and proposes future research directions based on these insights. To our knowledge, it is the first comprehensive review of peripheral nerve‒tumor‒immune crosstalk and its clinical significance.
2. NEURAL REGULATION OF THE TME
Research has shown that the autonomic nervous system (ANS), 44 , 45 , 46 , 47 , 48 sensory nerves, 49 , 50 and the enteric nervous system (ENS) 51 , 52 play significant roles in the initiation and progression of peripheral tumors (Figure 1A). Denervation studies have demonstrated the direct impact of nerves on tumor growth. 53 , 54 , 55 Neural signals influence by acting on nerve‐related receptors on various cell types within the TME, such as tumor cells and immune cells (Figure 1B). Interestingly, while the connection between physical activity and cancer is well established, 56 , 57 the direct involvement of motor nerves in tumor development remains unexplored, warranting further investigation in future research.
FIGURE 1.
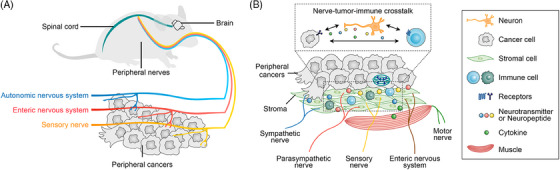
Schematic diagram of peripheral nerves intertwined with peripheral cancers. (A) The sympathetic, parasympathetic, and sensory nerves play a regulatory role in peripheral cancers. (B) This diagram shows how nerves, tumor cells, immune cells, and other components interact within the tumor microenvironment (TME) of peripheral cancers. Various types of nerves—sympathetic, parasympathetic, sensory, motor, and enteric—enter the tissue around tumor cells. The diagram highlights how these nerves communicate with cancer cells by releasing neurotransmitters and neuropeptides, which in turn influence tumor growth and the immune response.
2.1. Principal nerves involved in peripheral tumors
2.1.1. Autonomic nervous system
The ANS, consisting of the sympathetic and parasympathetic nerves, regulates homeostasis throughout the body. 58 , 59 , 60 The sympathetic (adrenergic) nerves control the “fight or flight” response, 61 , 62 while the parasympathetic (cholinergic) nerves manage “rest and digest” functions. 63 , 64 These systems maintain physiological balance by exerting opposing effects through the release of neurotransmitters such as norepinephrine and acetylcholine (ACh). Beyond homeostasis, these neurotransmitters are crucial in tumor development by interacting with receptors on tumor and/or immune cells in the TME, directly influencing tumor growth and metastasis. 65
Adrenergic receptors (ARs) are primarily classified into α and β types, 66 , 67 while cholinergic receptors include muscarinic (M) receptors and nicotinic (N) receptors, 68 both widely distributed on peripheral tumor and immune cells. 69 , 70 , 71 , 72 , 73 The α‐ARs have two subtypes, α1 and α2, while β‐ARs consist of β1, β2, and β3. Among these, β2‐AR is the most studied in tumors, present in most cell types, including tumor cells and immune cells. 74 , 75 , 76 Numerous studies have shown that ARs primarily promote tumor growth, and ARs blockers have been used as therapeutic agents for cancers such as prostate, 77 pancreatic, 44 gastric, 78 , 79 oral, 80 ovarian, 81 melanoma, 82 hepatocellular carcinoma, 83 high‐grade serous carcinoma, 84 and breast cancers. 85 , 86 Muscarinic receptors, which are G‐protein‐coupled, have five subtypes (M1‒M5), 87 while nicotinic receptors are ion channel receptors composed of 17 different subunits. 88 Compared to ARs, cholinergic receptors are less studied in tumors. Activation of M1 and M4 muscarinic receptors can promote the growth and metastasis of prostate cancer cells and contribute to chemotherapy resistance. 89 , 90 The M3 muscarinic receptor is highly expressed in colorectal cancer (CRC), with its high expression associated with increased survival rates. 91 Inhibitors of the M3 receptor can reduce lung cancer growth and immune suppression by inhibiting the AKT and ERK pathways, thereby enhancing anti‐cancer immune responses. 36 In pancreatic ductal adenocarcinoma (PDAC), the M1 muscarinic receptor signaling pathway suppresses tumor formation, inhibits proliferation, and prolongs survival. 44 Nicotinic receptors, crucial for rapid excitatory signaling in synaptic transmission, are strongly associated with lung cancer, where they promote proliferation, epithelial‒mesenchymal transition (EMT), angiogenesis, and anti‐apoptotic effects. 92 , 93 , 94 Additionally, tumors can induce the growth of autonomic nerves, creating a feedback loop that increases tumor aggressiveness and supports tumor progression. Both adrenergic and cholinergic receptors are involved in the recruitment, activation, and function of immune cells, such as T cells and TAMs, influencing anti‐tumor immunity and immune exhaustion, thereby affecting tumor progression. 95 , 96 , 97 The complex interactions between the ANS, tumor cells, and immune cells within the TME underscore the significant role of the ANS in cancer biology.
2.1.2. Sensory nerves
Sensory nerves are distributed throughout tissues and organs, including the skin, mucous membranes, muscles, and internal organs, where they detect external stimuli and relay information to the CNS, aiding in homeostasis and responses to environmental changes. 98 Neuropeptides released by sensory nerves are crucial in pain transmission and inflammatory responses. For example, substance P (SP) amplifies pain signals and induces local inflammation by activating specific receptors, promoting vasodilation, particularly in the trigeminal nervous system. 99 , 100 In contrast, somatostatin reduces neuroinflammation and protects nerve function by inhibiting hormone secretion, and the neuropeptide galanin modulates pain and promotes nerve repair. 101 Together, these neuropeptides act synergistically to regulate pain and inflammation. SP and calcitonin gene‐related peptide (CGRP) are the most extensively studied neuropeptides in the TME, where they interact with tumor cells to stimulate angiogenesis, enhance cancer cell proliferation, and promote metastasis. 102 , 103 , 104 , 105 , 106 , 107 , 108 The roles of other neuropeptides, such as somatostatin and galanin, in tumors remain largely unexplored, presenting an interesting direction for future exploration in cancer neuroscience. Additionally, sensory nerves influence the TME by affecting immune cells, such as macrophages and lymphocytes, thereby altering inflammatory responses and immune surveillance. 109 , 110 Furthermore, interactions between sensory nerves and tumor cells can trigger tumor‐associated pain, 111 , 112 , 113 which reduces the patient's quality of life and may also promote tumor growth and progression.
2.1.3. Enteric nervous system
The ENS consists of enteric neurons and glial cells, often referred to as the “second brain” that functions independently of the ANS. 114 , 115 The ENS contains both autonomic and sensory components, expressing various neurotransmitters and neuropeptides that together regulate gastrointestinal function. 116 Molecules, released by the ENS, can promote cancer cell proliferation, invasion, and resistance to chemotherapy, playing a critical role in the progression of gastrointestinal tumors, particularly colorectal cancer (CRC). 117 , 118 , 119 Studies have demonstrated that increased neural innervation and the severity of neural invasion in CRC, pancreatic cancer, and esophagogastric junction cancer are correlated with reduced overall survival (OS) rates. 120 Studies have demonstrated that increased neural innervation and the severity of neural invasion in CRC, 10 pancreatic cancer, 120 , 121 and esophagogastric junction cancer are associated with reduced OS rates. In the invasive front of CRC, myenteric plexus neurons are often surrounded by abundant extracellular matrix, accompanied by numerous myelin‐like structures, and significant infiltration of mast cells and plasma cells. 122 In heterotypic three‐dimensional (3D) culture models, CRC and pancreatic cancer cells are chemotactically attracted to ENS neurons through neurotrophic factors such as NGF and glial cell line‐derived neurotrophic factor (GDNF), migrating along axons via adhesion molecules such as L1 cell adhesion molecule (L1CAM) and N‐cadherin. 123 , 124 , 125 In gastric cancer, doublecortin‐like kinase 1 (DCLK1)+ tuft cells and nerves have been identified as the main source of ACh in the mouse gastric mucosa. 21 These cells promote tumor proliferation and local nerve growth by releasing ACh and upregulating NGF, creating a self‐perpetuating cycle. Complex interactions between gastrointestinal cancers—such as pancreatic and CRCs—and both extrinsic innervation via the vagus nerve and intrinsic ENS innervation contribute to tumor growth. 120 , 126 , 127 Neural inputs enhance tumor growth by upregulating neurotrophic factors, expanding the cancer stem cell population, and modulating tumor‐associated inflammation.95 The reciprocal relationship between the ENS and gastrointestinal tumors supports mutual development, with nerves serving as pathways for cancer invasion and neuropeptides from extrinsic nerve terminals promoting further tumor proliferation and invasion. 128
2.1.4. Motor nerves
Currently, there is a lack of research demonstrating that motor nerves directly influence tumor progression. However, the benefits of exercise for cancer patients are widely recognized. 56 Regular physical activity has been shown to reduce the risk of developing cancers such as colorectal, breast, and prostate cancer, with relative risk reductions ranging from 10% to 27%. 129 Exercise has a significant regulatory effect on the TME, particularly in metabolic processes, including specific metabolites such as lactate, tumor signaling pathways, and central carbon metabolism such as the tricarboxylic acid cycle, thereby exhibiting anti‐tumor activity. 130 Furthermore, exercise modulates the infiltration and function of immune cells within the TME. 131 , 132 , 133 For example, exercise has been shown to inhibit tumor growth in mouse models of breast cancer, induce vascular normalization, promote CD8+ T‐cell infiltration, enhance effector functions, and improve the effectiveness of immunotherapy for breast cancer. 134 Overall, current research suggests that exercise exerts anti‐tumor effects primarily by altering the metabolic and immune characteristics of tumors. Additionally, cytokines or neurotrophic factors produced by motor nerves or skeletal muscles during exercise may have unexpected effects on body cells, warranting further investigation. 135 , 136 , 137 For instance, although interleukin‐6 (IL‐6) is generally recognized for its tumor‐promoting effects, IL‐6 released from skeletal muscle during exercise enhances insulin sensitivity, stimulates the production of anti‐inflammatory cytokines, and reduces proliferation and DNA damage in cancer cells. 129 GDNF, initially isolated from rat glial cell lines, is closely associated with the PNI in tumor tissues, with motor nerves and skeletal muscles serving as significant sources, and exercise shown to increase its protein levels in tissues. 138 , 139 This finding contrasts with the observation that high GDNF levels promote PNI, leading to poorer outcomes for cancer patients. Therefore, exploring the regulatory role of motor nerves in the TME presents an intriguing and promising research area.
2.2. Denervation
A common approach to investigate the effect of nerves on tumors is to observe tumorigenesis following denervation (Figure 2). Sympathectomy can be achieved through surgical operations or chemical means, such as with 6‐hydroxydopamine (6OHDA). 140 In both the autochthonous transgenic prostate cancer mouse model and the prostate cancer xenograft mouse model, sympathectomy of bilateral hypogastric nerves inhibited tumor growth and induced tumor regression, respectively. 141 Sympathectomy of bilateral superior cervical ganglia suppressed tumor growth in 4‐nitroquinoline‐n‐oxide (4‐NQO) induced and orthotopic tongue cancer xenograft mouse models. 142 , 143 In addition, sympathectomy can inhibit the growth of breast cancer and melanoma. 144 , 145 Sympathetic nerves are also associated with tumor metastasis, and in a mouse model of melanoma, sympathectomy eliminated peak neural activity that is highly correlated with metastasis. 146 The vagus nerves are mixed nerves that contain both parasympathetic and sensory components and are among the major parasympathetic nerves. The vagus nerve distributed in the gastrointestinal tract is primarily parasympathetic. 147 Gastric vagotomy attenuated tumor development in three separate gastric cancer mouse models but only in vagotomized tissue. 53 Similarly, the vagus nerve innervates the pancreas through a parasympathetic component, and unlike its procancer effects in gastric cancer, it has anti‐tumor effects in pancreatic cancer. 126 In a mouse model of PDAC, vagotomy caused malignant epithelial cell proliferation and elevated pancreatic tumor incidence. 44 Similar to parasympathetic nerves, sensory nerves may play opposite roles in different tumors. In breast cancer, sensory nerves act as parasympathetic nerves, and disruption of sensory neurons by capsaicin increases cancer metastasis. 148 The development of PDAC is strongly related to preexisting pancreatitis. Enhanced expression of TRPV1 and TRPA1 channels in sensory neurons might drive inflammation and accelerate precancerous pancreatic cancer through a neurogenic mechanism in a PDAC mouse model. 149 , 150 , 151 Ablating sensory neurons in a mouse model of pancreatitis eliminated inflammation and reduced progression to cancer. 152 Nociceptors are the nerve endings of sensory neurons that can be involved in regulating immune and inflammatory responses. 153 Oral squamous cell carcinoma (OSCC) is densely innervated by nociceptive nerves originating from the trigeminal ganglion, and denervation of sensory nerves can inhibit tumor growth. 54 , 154 Nociceptive stimuli such as peripheral nerve‐derived neuropeptides, including CGRP and SP, and increased infiltration of αCGRP+ sensory nerve fibers were found in OSCC histologic samples. 155 , 156 These findings highlight the varying roles of specific types of nerves in different cancer types.
FIGURE 2.
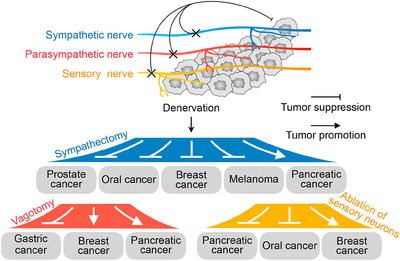
The impact of denervation on peripheral cancers. This diagram illustrates how denervation of different nerve types—sympathetic, parasympathetic, and sensory—affects tumor growth in different peripheral cancers, highlighting the diverse effects based on nerve type and cancer type.
Denervation in PDAC is a multifaceted process shaped by the immunosuppressive TME and the intricate interplay between the nervous system and tumor biology, with the TME being defined by limited effector T‐cell infiltration and a dominance of M2 macrophages and regulatory T (Treg) cells that release anti‐inflammatory factors, thereby suppressing anti‐tumor immune responses and enabling tumor evasion and growth. 157 , 158 , 159 Denervation, particularly the removal of sympathetic nerves, can disrupt neural signals that facilitate tumor progression but also triggers intricate immune and cellular responses. For example, sympathetic nerve removal has been associated with an increase in M2 macrophages, which contribute to immunosuppression and tumor growth by secreting cytokines such as IL‐10 that inhibit T‐cell‐mediated immunity. 160 , 161 The interaction between the nervous and immune systems shapes immune activity within the TME and influences tumor behavior, with denervation potentially disrupting these pathways, impairing the function of immune cells, particularly macrophages, while also directly altering the tumor's immune landscape by reducing the anti‐tumor activity of T cells, thereby weakening immune surveillance and promoting tumor growth. 96 , 162 Sympathetic nerves also play a critical role in tumor angiogenesis, and their removal may indirectly affect tumor growth and invasion. In summary, denervation in PDAC poses a complex therapeutic challenge, as it may inhibit tumor growth by disrupting neural‐tumor signaling but could also inadvertently promote tumor progression through immune dysregulation and altered angiogenesis, underscoring the need for cautious evaluation of denervation as a treatment strategy to prevent unintended consequences.
2.3. Neural signals and channels
Ion channels are membrane proteins that enable ion movement between cellular compartments, a process critical for electrical signaling and cell motility. 163 These channels help maintain normal tissue homeostasis, and dysregulation of their expression or function can contribute to the transformation of normal cells into cancer cells, characterized by unchecked growth and spread. 164 Neural signals modulate ion channels on cancer cells, influencing growth, cell death, and migration. 165 , 166 , 167 These signals primarily affect the electrical activity and cell cycle of cancer cells by regulating voltage‐gated potassium and sodium channels. 168 , 169 Neurotransmitters such as ACh and norepinephrine can bind to receptors on cancer cells, leading to abnormal ion channel activation; for instance, norepinephrine released by the sympathetic nervous system activates β‐ARs, altering ion channel activity and increasing calcium influx, which promotes cancer cell survival and growth. 92 , 170 Additionally, neuropeptides and other signaling molecules from nerve cells can regulate ion channel expression and function in cancer cells. Neuropeptides such as SP and CGRP can influence ion channel activity, impacting cancer cell behavior and contributing to tumor progression. 108 , 171 In conclusion, neural signals regulate ion channels on cancer cells by altering the local electrical and chemical environment, thereby influencing tumor growth, invasion, and treatment resistance.
3. INTERACTIONS BETWEEN PERIPHERAL TUMORS AND NERVES
Clinical samples consistently show that tumor tissues have a higher density of nerve fibers compared to surrounding normal tissues, indicating that cancer cells may promote nerve fiber growth, thereby increasing the presence of neural structures and terminals within the TME. 172 , 173 , 174 Emerging evidence suggests that tumor growth depends not only on blood vessels formed through angiogenesis but also on nerves generated through neurogenesis. This section explores the composition of nerves within tumors, neural signals, and the complex tumor‐nerve interactions, including the role of neural signals in tumor progression, as well as the invasion and remodeling of nerves by tumors.
3.1. How the PNS interferes with cancer
Research indicates that neural signals in the TME influence every stage of peripheral tumor development, from initiation to invasiveness, while the peripheral nervous system (PNS) impacts cancer progression by affecting DNA damage repair, cell cycle regulation, proliferation, apoptosis resistance, migration, invasion, EMT, angiogenesis, and lymphangiogenesis (Figure 3). 65 , 175
FIGURE 3.
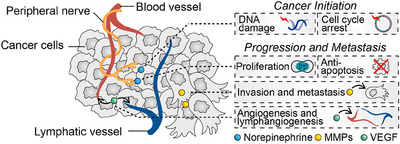
Neural regulation of tumor initiation and progression. Neural signals regulate tumorigenesis, including their effects on cellular DNA damage and the cell cycle, as well as tumor progression, including cell proliferation, anti‐apoptosis, invasion, metastasis, angiogenesis, and lymphangiogenesis.
3.1.1. Initiation
Research indicates that the intrinsic risks of tumor development include DNA damage responses, cell cycle checkpoint regulation, oxidative stress responses, chromatin structure alterations, and transcription/translation networks, all of which can confer a selective advantage to cells and drive malignant growth. 176 , 177 , 178 Additionally, some tumors are associated with specific environmental factors such as smoking (lung cancer) and sun exposure (skin cancer), as well as endogenous factors such as immune status, metabolism, and hormone levels, collectively defined as non‐intrinsic risk factors. 177
Neural signals in tumors are influenced by both intrinsic and non‐intrinsic factors. Among the non‐intrinsic factors, environmental influences such as smoking and sun exposure are closely linked to neural signaling pathways. For example, during smoking, nicotine binds to nicotinic ACh receptors, opening ion channels to allow sodium and potassium ion flow, thereby altering neuronal membrane potential and triggering the release of neurotransmitters such as dopamine (DA). 179 , 180 , 181 Similarly, sun exposure has a complex relationship with stress, as the sympathetic nervous system, by releasing catecholamines such as adrenaline and noradrenaline, triggers various physiological responses. This exposure influences the release of stress hormones, which affect hormone levels, metabolism, and immunity, thereby contributing to intrinsic cancer risks. 182 , 183 Stress is closely linked to cell cycle regulation and DNA damage, with the sympathetic nervous system releasing noradrenaline, which influences DNA damage/repair and cell cycle progression, potentially promoting cancer initiation. 184 , 185 , 186 In vitro studies have shown that human ovarian cancer cells exhibit accelerated DNA damage when exposed to stress. 186 Similarly, stress hormones can cause DNA damage in breast cancer cells and induce cell cycle arrest at the G1 phase. 187 Furthermore, noradrenaline activation of β‐ARs in human osteosarcoma cells leads to DNA damage accumulation and a decrease in tumor suppressor gene p53 levels. 188 , 189 In vivo studies have shown that stressed mice treated with noradrenaline exhibit increased DNA damage and reduced DNA repair. 190 Additionally, cholinergic activation results in the inhibition of p‐ERK1/2 and p‐p38 MAPK in human pancreatic cancer cells, reducing cell viability and invasion both in vitro and in vivo, and inducing cell cycle arrest. 191 Knockdown of the cholinergic receptor nicotinic α5 (CHRNA5) in breast cancer cells also promotes cell cycle arrest and inhibits DNA synthesis. 192 It is important to note that tumor development is not solely caused by DNA damage but also requires the accumulation and maintenance of these damages during cell division, along with increased proliferation and anti‐apoptotic capabilities. Moreover, malignant cells must overcome multiple barriers to successfully form tumors, such as evading immune attack and transforming the surrounding stroma into a tumor‐supportive TME.
3.1.2. Progression and metastasis
Neural signals play a critical role in multiple aspects of tumor development, including proliferation, apoptosis, invasion, metastasis, angiogenesis, and lymphangiogenesis. For instance, activation of β2‐adrenergic signaling enhances proliferation in gastric cancer cells, 78 , 79 whereas inhibition of this signaling induces apoptosis. 193 Similarly, in breast cancer cells, β2‐adrenergic signaling drives proliferation by activating the tyrosine kinase effector GSK3. 194 In ovarian cancer, adrenergic signaling enhances anti‐apoptotic mechanisms by increasing focal adhesion kinase levels. 195 In pancreatic cancer cells, blocking β2‐ARs leads to G1/S phase cell cycle arrest and induces cell death. 196 Likewise, selective inhibition of β2‐adrenergic signaling in colorectal cancer cells causes G1 phase cell cycle arrest and triggers apoptosis. 197 In liver cancer, β2‐adrenergic signaling supports tumor proliferation and survival by downregulating autophagy and stabilizing hypoxia‐inducible factor‐1α. 198 In gastric epithelium, cholinergic stimulation induces NGF production, promoting tumor formation and growth via the M3 receptor and Yes‐associated protein pathways. 199 Human gastric cancer cell lines also show overexpression of choline acetyltransferase, and autocrine and paracrine ACh activation of M3 cholinergic receptors, along with downstream epidermal growth factor receptor signaling, stimulates proliferation in these cells. 200
Neural signals also play a significant role in promoting cancer stemness, invasion, and metastasis. In depressed gastric cancer patients, increased plasma catecholamine levels activate β2‐ARs, leading to the upregulation of MACC1 expression, which enhances cell migration and invasion. 201 In colorectal cancer, stress‐induced norepinephrine activates β2‐ARs via the CEBPB/TRIM2/P53 axis, promoting EMT. 202 In a mouse model of prostate cancer, norepinephrine increases lymph node metastasis, an effect that can be blocked by propranolol. 77 In ovarian cancer cells, norepinephrine boosts prostaglandin E2 synthesis through the NF‐κB‒PTGS2 (nuclear factor kappa‐B and prostaglandin‐endoperoxide synthase 2) pathway, promoting tumor growth and metastasis. 81 High norepinephrine levels in ovarian cancer are associated with increased Src phosphorylation, mediating the β‐adrenergic/protein kinase A (PKA) axis, which enhances tumor cell migration, invasion, and growth. 203 Norepinephrine also increases STAT3 phosphorylation, leading to elevated matrix metalloproteinases (MMP‐2 and MMP‐9), further driving ovarian cancer cell invasiveness. Similarly, in nasopharyngeal carcinoma, norepinephrine treatment elevates MMP‐2 and MMP‐9 levels, enhancing the invasiveness of the cancer cells. 204 Adrenergic signaling activation is linked to EMT and increased invasiveness in several cancers, including ovarian, 205 hepatocellular, 206 breast, 207 pancreatic, 83 , 208 and lung cancer cells. 209 These findings highlight the critical role of neural signals in facilitating cancer progression through multiple mechanisms. Norepinephrine significantly increases levels of vascular endothelial growth factor (VEGF) in ovarian cancer, 210 , 211 melanoma, 212 pancreatic cancer, 213 oral cancer, 214 and nasopharyngeal carcinoma. 215 It can also stimulate angiogenesis in primary breast tissues by promoting VEGF signaling in TAMs. 85 Similarly, norepinephrine signaling in lung cancer promotes angiogenesis indirectly by stimulating VEGF secretion from M2 TAMs. 216 In prostate stroma, norepinephrine/β‐adrenergic signaling activates angiogenic switches, facilitating exponential tumor growth. 89 Knockdown of Adrb2 (encoding the β2‐AR) reduces vascular density and inhibits prostate cancer progression in mice. Tumor‐associated lymphatic vessel density correlates closely with patient prognosis, and the sympathetic nervous system can influence lymphatic systems. 217 In breast cancer mouse models, activation of β2‐ARs promotes VEGFC expression in cancer and stromal cells, increasing lymphatic vessel density and inducing stable expansion of lymphatic vessels draining the primary tumor. Conversely, blocking sympathetic nerves significantly (∼80%) inhibits lymphatic flow. 218
3.2. Perineural infiltration by tumors
PNI is a histopathological finding first identified in 1985, where malignant cells surround, invade, or reside within nerves, highlighting a key aspect of tumor‒nerve interactions (Figure 4A,B). 219 , 220 Clinically, PNI is an important factor in cancer management, often indicating the need for adjuvant radiotherapy and playing a role in the staging and risk stratification of various malignancies, including penile, head and neck, esophageal, and thyroid cancers. The incidence of PNI varies by cancer type, with rates reported as high as 98% in pancreatic cancer, 80% in head and neck squamous cell carcinoma, and 75% in prostate cancer, while colorectal cancer shows a lower incidence at around 33%. 221 PNI can act as a conduit for tumor spread, allowing cancer cells to migrate along nerve fibers to distant sites, which contributes to tumor recurrence and metastasis. For instance, PNI is correlated with advanced clinical stage and metastasis in breast cancer. 15 , 222 In prostate and pancreatic cancers, PNI involving larger nerve diameters is associated with poorer prognosis. 223 , 224 Similarly, the depth of nerve invasion in colorectal cancer serves as an independent predictor of OS and disease‐free survival.7 As a result, PNI is recognized as an important prognostic factor in various cancers. The interaction between tumor cells and nerves during PNI is bidirectional and intricate, often involving the modulation of peripheral sensory nerve paracrine signals. This can lead to hypersensitivity, nerve sprouting, and further perineural invasion, highlighting the complex dynamics of tumor‒nerve crosstalk in cancer progression. 225 , 226 , 227
FIGURE 4.
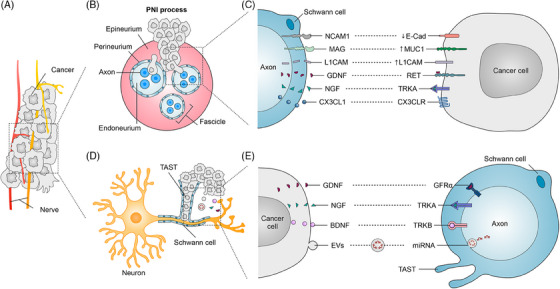
Perineural infiltration (PNI) and molecular interactions between Schwann cells and cancer cells. (A) Diagram shows how nerves interact with peripheral cancers. (B) A cross‐section of a nerve shows its protective layers—epineurium, perineurium, and endoneurium—surrounding the axons within a fascicle. The diagram illustrates how cancer cells infiltrate these layers during PNI. (C) Molecular signaling between Schwann cells and cancer cells. This section highlights the key molecular interactions driving communication between Schwann cells and cancer cells. Molecules such as neural cell adhesion molecule 1 (NCAM1), myelin‐associated glycoprotein (MAG), L1 cell adhesion molecule (L1CAM), glial cell line‐derived neurotrophic factor (GDNF), nerve growth factor (NGF), and fractalkine (CX3CL1) on Schwann cells interact with cancer cell receptors or ligands, including E‐cadherin (E‐Cad), Mucin 1 (MUC1), L1CAM, rearranged during transfection (RET), TRKA, and C‒X3‒C motif chemokine receptor 1 (CX3CR1). (D) The figure shows how cancer cells interact with Schwann cells and axons, highlighting the formation of the tumor‐activated Schwann cell tracks (TAST) by Schwann cells. (E) The figure illustrates the molecular crosstalk between cancer cells, Schwann cells, and axons. It highlights key signaling pathways through which cancer cells communicate with neurons. Cancer cells secrete growth factors such as GDNF, NGF, and brain‐derived neurotrophic factor (BDNF), which bind to their respective receptors (GFRα, TRKA, and TRKB) on Schwann cells and axons, promoting neuronal growth and cancer invasion. Additionally, cancer cells release extracellular vehicles (EVs) containing microRNAs (miRNAs), which contribute to neural reprogramming. The figure also showcases the TAST formed by Schwann cells, aiding in cancer progression.
Schwann cells, key glial cells in the PNS, surround nerve fibers and form myelin sheaths, playing a significant role in the TME (Figure 4C). These cells can interact directly with tumor cells via neural cell adhesion molecule 1, facilitating tumor cell invasion into nerves. 125 , 228 Schwann cells also release neurotrophic factors, such as GDNF, through paracrine signaling, which binds to the rearranged during transfection (RET) receptor on PDAC cells, promoting tumor cell migration toward neurons. 229 , 230 Additionally, Schwann cells secrete cell adhesion molecules and chemotactic factors, attracting tumor cells to nerves. 231 , 232 Schwann cells also play a role in modulating the immune response within tumors. For instance, they recruit TAMs to perineural invasion sites via C‒C motif chemokine ligand 2 (CCL2), which exacerbates nerve damage and tumor cell invasion. 233 Recent research explores Schwann cells as potential therapeutic targets. By disrupting the signaling pathways between Schwann cells and tumor cells—such as blocking tumor‐promoting factors secreted by Schwann cells or interfering with their interactions with cancer cells—it may be possible to hinder tumor progression. 234 , 235 In summary, Schwann cells are increasingly recognized for their role in tumor progression, and ongoing research may reveal their potential as therapeutic targets, offering new avenues for anti‐cancer treatment strategies. 18 , 236
3.3. Tumor‐induced nerve remodeling
Tumor neurogenesis, or innervation, is a process where tumors recruit new axons and respond to nerve regeneration (Figure 4A,D), a hallmark of aggressive tumor behavior associated with poor prognosis. 10 , 162 , 237 This phenomenon has been observed in various cancer types, including prostate and breast cancers. 12 , 238 Research shows that cancer cells express neurotrophic markers such as NGF, GDNF, and brain‐derived neurotrophic factor (BDNF), releasing axon‐guiding molecules that promote axonogenesis and attract nerves to the tumor site (Figure 4E). For instance, OSCC cells facilitate TME innervation by secreting NGF, which prompts nociceptive nerves to secrete CGRP and promote cancer growth. 239 Similarly, nociceptive neurons can interact with melanoma cells, promoting synaptic growth and increasing responsiveness to harmful ligands and neuropeptides. 108 Elevated levels of NGF precursors are strongly associated with neurogenesis in prostate cancer, where tumors can reprogram recruited nerves to develop into an adrenergic infiltrating phenotype that supports tumor growth and invasion. 240 In OSCC, the loss of the tumor suppressor gene p53 has been shown to drive nerve reprogramming, reconnecting established nerves to an adrenergic phenotype through exosomes carrying microRNAs. These reprogrammed nerves provide growth‐promoting signals to the tumor and facilitate cancer cell invasion. 54 Notably, PDAC cells reprogram proximal Schwann cells to become tumor‐activated cells, forming tumor‐activated Schwann cell tracks (TASTs), which create pathways for cancer cells to invade nerves. 241 Peripheral tumors can also interact remotely with the brain by attracting neural progenitor cells, as seen in prostate and breast cancers. These progenitor cells cross the blood‒brain barrier, infiltrate the tumor, initiate neurogenesis, and differentiate into adrenergic neurons, further supporting tumor progression and metastasis. 242 In summary, nerve reprogramming by tumors is a complex and critical process that drives cancer growth, invasion, and metastasis, representing an important area of research in tumor neuroscience.
4. NEUROIMMUNE CROSSTALK IN PERIPHERAL TUMORS
Neural signals modulate immune cell via adrenergic and cholinergic receptors, with norepinephrine binding to ARs on immune cells to influence cytokine production, cell migration, and immune function. This neural‒immune interaction is especially critical in the TME, where stress‐induced neural signals contribute to an immunosuppressive state, promoting tumor growth and metastasis. In turn, immune cells can influence neural structures by releasing cytokines and signaling molecules that affect nerve growth and function.
4.1. Stress and tumor immunology
Stress is a physiological response aimed at restoring homeostasis, primarily through the activation of the sympathetic nervous system and the hypothalamic‒pituitary‒adrenal (HPA) axis. 243 This activation triggers the release of adrenergic factors, such as norepinephrine and epinephrine, from sympathetic nerve endings and the adrenal medulla, along with glucocorticoids such as cortisol from the adrenal cortex. 244 , 245 , 246 These hormones work together to coordinate the body's adaptive response to stress in tumor patients (Figure 5).
FIGURE 5.
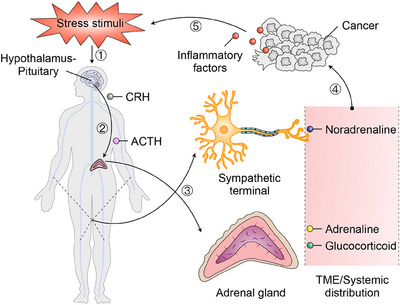
The stress response and its influence on the tumor microenvironment (TME). This schematic illustrates the biological pathway by which stress stimuli influence tumor progression via the hypothalamic‒pituitary‒adrenal (HPA) axis and the sympathetic nervous system. Upon exposure to stress, the hypothalamus releases corticotropin‐releasing hormone (CRH), which stimulates the anterior pituitary gland to secrete adrenocorticotropic hormone (ACTH) into the bloodstream. ACTH then prompts the adrenal glands to release adrenaline and glucocorticoids, which are distributed systemically, including to the TME. Simultaneously, the sympathetic nervous system is activated, releasing noradrenaline from sympathetic nerve terminals that innervate the tumor environment. These stress hormones, together with inflammatory factors, promote cancer cell survival, proliferation, and tumor progression by modulating the inflammatory conditions within the TME.
Cancer patients often experience peak stress during diagnosis, treatment, and relapse, which heightens the risk of anxiety and depression, potentially creating a synergistic interaction with the cancer itself. 247 , 248 , 249 For instance, in breast cancer patients, elevated anxiety, stress, depressive symptoms, or increased nocturnal cortisol levels are linked to immune suppression mediated by tumor cells. 250 , 251 Tumors can elevate norepinephrine release within the TME, disrupt CNS and HPA axis activity, and intensify local and systemic inflammation, contributing to depression, sleep disturbances, and fatigue, thereby perpetuating a harmful cycle between stress and cancer. 252 , 253 Although the precise role of stress in cancer development is not fully understood, research suggests that stress promotes cancer progression by altering tumor characteristics, with animal studies and clinical observations confirming that adrenergic stress responses and inflammation play a significant role in this process. 86 , 182 , 254 Recent animal studies provide strong evidence that stress accelerates cancer growth and metastasis by enhancing cancer markers, promoting immune evasion, and increasing inflammation, primarily through interactions with β‐adrenergic, prostaglandin, and glucocorticoid receptors. 255 , 256 , 257 , 258 Elevated stress in cancer patients has been shown to inhibit natural killer cell activity, reduce cytotoxicity, and promote tumor growth by inducing T helper (Th) cell differentiation and increasing the proportion of suppressive immune cells, such as MDSCs and Treg cells. 182 , 259 , 260 Additionally, stress‐induced β‐adrenergic signaling increases cyclooxygenase‐2 (COX‐2) expression, prostaglandin secretion, and the release of pro‐inflammatory cytokines such as IL‐6, while also enhancing macrophage recruitment and M2 polarization in tumors. 261 Social isolation is linked to increased M2 polarization in breast tumors, while higher depression levels are associated with increased norepinephrine in ovarian tumors. 262 Both preclinical and clinical research have demonstrated that stress can reduce the efficacy of adjuvant and neoadjuvant cancer treatments, including chemotherapy, radiation therapy, and immunotherapy, through mechanisms mediated by glucocorticoids and/or catecholamines. 263 , 264 , 265 , 266 For instance, in mouse models of melanoma and lymphoma, social disruption stress or activation of β‐ARs has impaired CD8+ T‐cell responses and diminished the effectiveness of several immunotherapies. 267 , 268 Similarly, in models of breast, pancreatic, melanoma, colon, and lung cancers, stress‐induced β‐adrenergic signaling has been shown to negatively impact the success of programmed cell death‐1 (PD1)‐targeted immunotherapies. 269 , 270 Furthermore, in mouse models of gastric and breast cancer, β‐adrenergic activation has been shown to induce resistance to HER2‐targeted therapy with trastuzumab, with clinical observations supporting these findings by linking tumor expression of β‐ARs to reduced efficacy of trastuzumab in breast cancer patients. 271 , 272 These studies highlight the critical role of stress in undermining cancer treatment outcomes and the importance of managing stress as part of comprehensive cancer care.
4.2. Characterization of immune cells regulated by neural signals
Crosstalk between immune cells, cancer cells, and nerves can impact cancer progression (Figure 6A). For instance, neurotransmitters can have immunomodulatory effects that influence protumor inflammation, anti‐tumor immunity, and immunosuppression. Immune cells present in the TME, including T cells, MDSCs, TAMs, B cells, neutrophils, and dendritic cells, participate in neural signaling regulation.
FIGURE 6.
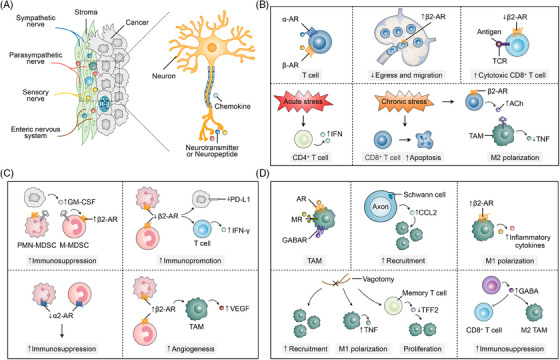
Neuro‐immune interactions in the tumor microenvironment (TME). (A) The diagram on the left shows sympathetic, parasympathetic, sensory, and enteric nerves infiltrating the tumor stroma. These neurons release neurotransmitters and neuropeptides, which interact with cancer cells and immune cells via chemokine signaling, influencing both tumor progression and immune responses. (B) T cells express both α‐ and β‐adrenergic receptors (β‐ARs), and their activation triggers distinct immune responses. Increased β2‐AR signaling impairs T‐cell egress and migration from lymphoid organs, limiting their ability to reach tumor sites. Conversely, the downregulation of β2‐AR on CD8+ T cells enhances their cytotoxic functions and improves antigen recognition through interactions with T‐cell receptor (TCR). Acute stress can boost immune responses by promoting interferon (IFN) production in CD4+ T cells, while chronic stress has the opposite effect, inducing apoptosis in CD8+ T cells and weakening immune surveillance, thereby promoting tumor progression. Moreover, chronic stress and β2‐AR activation increase acetylcholine (ACh) production, which drives M2 polarization of tumor‐associated macrophages (TAMs) and suppresses tumor necrosis factor (TNF) production, creating an immunosuppressive tumor microenvironment. (C) β2‐AR signaling in myeloid‐derived suppressor cells (MDSCs) enhances their immunosuppressive activity by increasing granulocyte‒macrophage colony‐stimulating factor (GM‐CSF) production. Conversely, reduced β2‐AR signaling lowers programmed death‐ligand 1 (PD‐L1) expression and boosts IFN‐γ production in T cells, leading to enhanced immune activation. Additionally, α2‐AR signaling in MDSCs further strengthens their immunosuppressive effects, promoting tumor immune evasion. In TAMs, β2‐AR activation supports angiogenesis by increasing vascular endothelial growth factor (VEGF) secretion, which facilitates tumor growth. (D) AR, MR, and GABAR on TAMs regulate their activation and function in the tumor microenvironment. Schwann cells, in conjunction with axons, release CCL2 to recruit TAMs to the tumor site, increasing immune cell infiltration. β2‐AR signaling in TAMs elevates the production of inflammatory cytokines, promoting M1 polarization and a pro‐inflammatory response. Vagotomy enhances TAM recruitment and M1 polarization by boosting TNF production, thereby supporting an anti‐tumor immune response. It also reduces TFF2 expression in memory T cells, which boosts their proliferation. Conversely, GABA production by CD8+ T cells promotes TAM polarization towards the M2 phenotype, leading to immunosuppression in the TME.
4.2.1. T cells
The functional stages of T cells—antigen recognition, activation, clonal expansion, effector functions, and memory formation—enable them to identify and eliminate pathogens while maintaining immune balance. 273 , 274 , 275 In the TME, T cells express β‐ARs (primarily β2‐AR), responding to neural signals, with β‐adrenergic signaling playing a key role in regulating anti‐tumor immune responses (Figure 6B). 276 , 277 The sympathetic nervous system influences the lymphatic system, responsible for transporting lymphocytes, by β2‐AR signaling in lymph nodes, which inhibits T‐cell outflow and reduces lymphocyte infiltration in tissues. 278 Conversely, β‐AR agonists rapidly suppress CD8+ T‐cell movement in tissues and temporarily inhibit CD4+ T‐cell movement, suggesting that sympathetic nervous system stimulation can impair leukocyte mobility and potentially weaken immune responses. 279 6OHDA‐mediated sympathectomy enhanced CD8+ T‐cell responses to antigens in mice, discovering that this enhanced response was mediated by β2‐AR, rather than β1‐AR or α‐AR. 280 Exhausted CD8+ T cells express β1‐AR in response to stress‐associated catecholamines, inhibiting their function and accumulating around sympathetic nerves, but blocking β1‐adrenergic signaling can slow T‐cell exhaustion, enhance anti‐tumor function, and synergistically promote effector T‐cell responses with ICIs therapy. 29 Acute stress activates ARs on CD4+ T cells and B cells, increasing IgG1 and interferon (IFN) production, while chronic stress promotes apoptosis of these cells. 281 Similarly, β2‐AR signaling knockdown increased the proportion of effector CD8+ T cells while decreasing the percentage of regulatory CD4+ T cells in the TME. 269 Daher et al. reported that β2‐AR signaling strongly inhibited the initial activation of effector CD8+ T‐cell responses, suggesting it suppresses the activation of naïve CD8+ T cells. 267 In the spleen, T cells expressing β2‐AR produce ACh when stimulated by sympathetic innervation. 282 Under stress conditions, Ach produced by T cells inhibits tumor necrosis factor (TNF) production in TAMs that express nicotinic ACh receptors. Additionally, autonomic innervation has a direct impact on the recruitment and fate of immune cells within the TME. 283 , 284
4.2.2. MDSCs
MDSCs, a class of immature immune cells including monocytic (M‐MDSCs) and granulocytic (PMN‐MDSCs) subsets, originate from bone marrow precursors and play a crucial role in the TME (Figure 6C). 285 , 286 Upon recruitment to the TME, MDSCs secrete various immunosuppressive factors, such as nitric oxide, reactive oxygen species, transforming growth factor‐beta, and IL‐10, which collectively inhibit T‐cell activity and function. 287 , 288 , 289 MDSCs also express β‐ARs, and the secretion of granulocyte‒macrophage colony‐stimulating factor by cancer cells leads to increased expression of β2‐AR on MDSCs, further promoting tumor‐induced immunosuppression. 290 , 291 , 292 Activation of β2‐AR on MDSCs downregulates glycolysis and enhances fatty acid oxidation, thereby augmenting the immunosuppressive function of MDSCs. 293 Research indicates that knocking down β2‐AR in MDSCs reverses their immunosuppressive function, enhancing T‐cell proliferation and IFN‐γ production, while knocking down Adrb2 slows tumor growth, reduces programmed death‐ligand 1 (PD‐L1) expression, and lowers serum levels of immunosuppressive cytokines. 292 β2‐AR positively regulates adenylate cyclase activity and cAMP levels, which is related to the mechanism of the anti‐tumor immune response. In contrast, α2‐AR exerts opposite regulatory effects on cAMP and downstream signals to those of β‐AR. Disruption of α2‐AR signaling leads to the accumulation of MDSCs, which suppress anti‐tumor immunity and promote tumor growth. 294 , 295 MDSCs also influence the maturation and function of dendritic cells and promote the generation of TAMs, affecting antigen presentation and immune responses. 296 Additionally, MDSCs can directly secrete VEGF to support tumor vascularization. 297 , 298 β2‐AR signaling has been found to increase the accumulation of MDSCs in the TME and promote tumor angiogenesis. 216 In lung cancer, adrenergic signaling indirectly stimulates angiogenesis by promoting VEGF secretion from M2 macrophages. 216
4.2.3. Macrophages
TAMs originate from peripheral blood monocytes and differentiate into macrophages upon entering the TME, where their surface is equipped with receptors that respond to neural signals, including adrenergic, cholinergic, and GABA receptors (Figure 6D). 299 , 300 , 301 Under stress conditions, increased adrenergic signaling and β2‐AR agonist administration lead to an increase in TAMs in breast cancer, while β2‐AR antagonists inhibit their recruitment and metastasis. 302 In prostate and pancreatic cancers, TAMs increase as the cancer progresses, and this increase is nerve dependent. Additionally, vagotomy may accelerate the onset of pancreatic cancer and promote tumor growth by recruiting TAMs and mediating inflammation. 303 , 304 Vagus nerve stimulation activates postsynaptic adrenergic nerves in the celiac ganglion and inhibits TNF release from TAMs, while vagotomy removes this immunosuppression, resulting in increased TNF levels. 126 , 127 , 305 , 306 Bilateral vagotomy reduces the expression of trefoil factor 2 (TFF2) in the spleen of mice, suggesting that cholinergic signaling enhances TFF2 release, which, in turn, inhibits the expansion of MDSCs and suppresses inflammation and colon cancer development. 307 GABA signaling can also produce anti‐inflammatory and immunosuppressive effects by directly inhibiting macrophage function. 308 GABA derived from cancer cells can activate GABA‐BR, suppressing the expression of CCL4 and CCL5, thus inhibiting the tumor infiltration of T cells and dendritic cells. 309 , 310 Additionally, in colon cancer, B cells can produce GABA, which limits the anti‐tumor response by inhibiting CD8+ T‐cell cytotoxicity and promoting the survival of M2‐type TAMs. 311
4.2.4. Interactions between immune cells and nerves
Immune cells, including T cells, MDSCs, and TAMs, express neural receptors on their surfaces, enabling them to receive neural signals. Interestingly, these immune cells can also affect neural structures, a dynamic area of research. For instance, in Alzheimer's disease, conditioned media from T cells treated with photobiomodulation therapy has been found to promote neural stem cell differentiation. 312 Macrophages have a complex role in neurogenesis. Brain macrophages produce essential factors for the migration of new neurons, yet inflammation mediated by macrophages can be detrimental to neurogenesis and the survival of new neurons. 313 However, similar studies exploring this interaction within peripheral TME remain limited.
4.3. Neuroinflammatory mediators and their roles
In peripheral tumor neuroscience, the interplay between neuroinflammation and tumor progression is crucial, as tumor‐associated inflammation significantly influences the development, growth, and treatment response of peripheral tumors, with various key mediators and cells playing essential roles in this process. M2‐TAMs are central to the inflammatory response in peripheral tumors, secreting cytokines, growth factors, and enzymes that promote tumor growth, angiogenesis, and metastasis, while also contributing to a tumor‐supportive microenvironment by remodeling the extracellular matrix and suppressing anti‐tumor immune responses. Moreover, while innate immune cells such as neutrophils and B cells have garnered significant attention in cancer research, their roles in tumor‒nerve interactions remain less understood. In the TME, tumor‐associated neutrophils are recruited by signals primarily from macrophages, as well as tumor and stromal cells, and are generally classified into two types: anti‐tumor (N1) and pro‐tumor (N2). 314 , 315 , 316 Neutrophils are closely linked to stress and inflammation as key mediators of the body's rapid immune response. 317 Neutrophils are also recruited into the TME by neuropeptides and in response to DA, which induces apoptosis and inhibits their functions. 318 , 319 Additionally, tumor cells can promote neutrophil infiltration into the perihippocampal meninges via the CCR2‒CCL2 axis, contributing to cognitive decline and anorexia. 320 In high‐grade gliomas, increased neuronal infiltration and neutrophil extracellular trap (NET) formation, identified as an oncogenic marker, promote glioma cell proliferation, migration, and invasion. 321 HMGB1 binds to NF3 and activates the NF‐κB pathway, leading to IL‐10 (IL‐8) secretion, which recruits neutrophils and induces further NET formation via the PI3K/AKT/ROS axis, while targeting NET formation or IL‐8 secretion inhibits glioma progression. 321 These findings suggest that neutrophils play a crucial role in tumor‒nerve interactions; however, while current research has primarily focused on brain tumors, their higher enrichment in peripheral tissues warrants further investigation. Moreover, B cells can express nerve‐related receptors, such as ACh receptors, and secrete neurotropic factors such as GABA, while also interacting with other immune cells, including T cells and macrophages, to shape the tumor's immune response. 311 , 322 , 323 These complex interactions between neutrophils, B cells, other immune cells, and neural cells complicate tumor‒nerve crosstalk, highlighting the need for further investigation into the roles of neutrophils and B cells in cancer.
In summary, neuroinflammatory mediators and inflammation‐associated cells play complex and diverse roles in peripheral tumors. Macrophages, neutrophils, cytokines, chemokines, and prostaglandins significantly influence the TME, affecting tumor growth, metastasis, and treatment outcomes. A deeper understanding of these interactions is essential for developing advanced therapeutic strategies in peripheral tumor neuroscience.
5. THERAPEUTIC IMPLICATIONS AND STRATEGIES
The primary goal of translating cancer neuroscience research into clinical practice is to develop more effective treatments, with the complex interactions between cancer‒nerve‒immune driving several clinical trials aimed at improving therapeutic outcomes (see Table 1). Clinically, neuromodulatory drugs, such as β‐adrenergic agents and DA and glutamate receptor modulators, have shown anti‐cancer potential, with β‐blockers, in particular, being associated with improved tumor prognosis. Although these strategies have been tested in small prospective trials, large‐scale randomized controlled trials are still needed to verify their clinical efficacy. Drugs targeting neural paracrine signaling are less commonly used, although targeting neural activators in these pathways may prove effectiveness; however, genetic alterations in many cancers complicate this approach. Repurposing existing drugs offers a faster pathway to cancer treatment due to their known safety profiles, but the lack of tumor specificity and potential side effects remain significant challenges for neuromodulatory therapies.
TABLE 1.
Clinical trials investigating inhibitors of nerve‒cancer interactions in peripheral cancers.
| Cancer type | Therapeutic targets | Intervention | Trials no. |
|---|---|---|---|
| Prostate cancer | β‐AR | Carvedilol | NCT02944201 |
| Advanced melanoma | β‐AR, opioid receptor, and immune checkpoints | Propranolol, naltrexone, and ICIs | NCT05968690 |
| Hepatocellular carcinoma | β‐AR | Propranolol and carvedilol | NCT06233708 |
| Malignant soft tissue sarcoma | β‐AR and chemotherapy | Propranolol and doxorubicin | NCT03108300 |
| Pancreatic, hepatocellular, and biliary tract cancer | β‐AR, immune checkpoints, and chemotherapy | Propranolol, ICIs, and paclitaxel | NCT05451043 |
| PDAC | β‐AR | Propranolol | NCT06145074 |
| Breast cancer | β‐AR | Carvedilol | |
| Breast cancer and melanoma | β‐AR | Propranolol | NCT02013492 |
| Prostate cancer | β‐AR | Propranolol | NCT03152786 |
| Cancer bone metastases | NGF signaling | Tanezumab | NCT02609828 |
| PDAC | Acetylcholine receptor | Bethanechol | NCT03572283 |
| Prostate cancer | Denervation | Botulinum toxin | NCT01520441 |
Abbreviations: β‐AR, β‐adrenergic receptor; ICI, immune checkpoint inhibitor; NGF, nerve growth factor; PDAC, pancreatic ductal adenocarcinoma.
Source: https://clinicaltrials.gov/.
5.1. Targeting tumor‒nerve interactions
Given the diverse mechanisms of tumor‒nerve crosstalk and their clinical and pathological significance, this knowledge can be translated into several key intervention strategies: inhibiting tumor growth, overcoming therapeutic resistance and tumor adaptation to stress, enhancing anti‐tumor immunity, and preventing metastasis. 324
Neural signaling plays a significant role in tumor biology by modulating both adrenergic and cholinergic pathways. Pharmacological interventions using selective and non‐selective β‐blockers (e.g., propranolol, metoprolol) and parasympathomimetics (e.g., bethanechol) have demonstrated potential as adjuvant cancer therapies. 325 Notably, differences in cholinergic responses have been observed between various cancers, such as gastric and pancreatic cancers. Retrospective studies suggest improved outcomes in cancer patients who use β‐blockers. 326 Clinical trials such as NCT02944201, NCT0383802, NCT03572283, and NCT04245644 are investigating these drugs in both non‐metastatic and metastatic cancers. β‐AR antagonists have been found to enhance the therapeutic effects of chemotherapy and immunotherapy. Adrenergic signaling has been implicated in chemotherapy resistance, with evidence suggesting that the addition of β‐adrenergic antagonists can improve chemotherapy outcomes. For instance, norepinephrine‐induced overexpression of DUSP1 in ovarian cancer cells increases apoptosis, indicating that adrenergic signaling may reduce chemotherapy sensitivity. 26 In a mouse model of pancreatic cancer, cold stress was shown to activate sympathetic nerve responses and β‐AR, leading to increased resistance to cisplatin and paclitaxel. 27 Similarly, in triple‐negative breast cancer, β‐AR antagonists combined with anthracycline chemotherapy reduced metastasis and improved chemotherapy efficacy. β1‐AR antagonists, such as nebivolol, have also been shown to inhibit the growth of colon and breast cancers by suppressing oxidative phosphorylation in cancer cells and preventing endothelial cell proliferation, thereby reducing tumor angiogenesis. 31 , 327 β‐ARs signaling plays a crucial role in chemoresistance in cancer, and blockade of ARs can act as a chemosensitizer in combination with treatment for breast cancer, 23 lung cancer, 28 , 34 colorectal cancer, 328 , 329 , 330 cervical cancer, 331 uveal melanoma, 332 and multiple myeloma. 24 In addition, a β1‐AR blocker (nebivolol) specifically impeded oxidative phosphorylation in cancer cells, preventing tumor angiogenesis by blocking endothelial cell proliferation and limiting colon and breast cancer growth. 25 Overall, β‐ARs appear to hold promise in improving cancer‐related biomarkers, and large‐scale randomized controlled trials are currently underway to assess the long‐term efficacy of these drugs for managing cancer‐related stress responses.
5.2. Modulating neuroimmune crosstalk
Immune checkpoint blockade is now a standard cancer treatment, with studies showing that combining neuroactive drugs with ICIs can modulate the TME. Research has demonstrated that β‐AR blockade enhances immunotherapy efficacy by regulating immune responses, and preclinical studies across various cancers suggest that inhibiting adrenergic signaling can improve anti‐tumor immune effects. Propranolol co‐administered with an anti‐CTLA‐4 (cytotoxic T‐lymphocyte associated protein 4) antibody improved ICB efficacy in patients with soft tissue sarcoma. 30 Nine patients with metastatic melanoma treated with a combination of propranolol and pembrolizumab, an anti‐PD1 checkpoint inhibitor, achieved an objective response rate of 78%, with elevated IFN‐γ and reduced IL‐6. 38 C‒X‒C motif chemokine receptor 4 (CXCR4) is a promising target for anti‐cancer therapy, and it has been found that β2‐AR can bind to CXCR4 and that CXCR4‒β2AR heteromers are present in human cancer cells, which might help to develop targeted therapeutics for the treatment of CXCR4‐positive cancer drugs. 333 ICIs show limited efficacy in immunologically cold tumors, partly due to the absence of CD8+ T cells near malignant cells. 334 , 335 Combining radiation therapy with β‐ARs blockade has the potential to enhance the response in tumors that are resistant to immunotherapy alone, such as pancreatic and prostate cancers. Immune dysfunction is linked to prolonged antigen exposure, impairing the effector function of CD8+ T cells. Research indicates that β1‐ARs, rather than β2, play a significant role in immune failure. Inhibiting β1‐AR activity enhances CD8+ T‐cell function in melanoma and helps prevent T‐cell exhaustion. 29 Advances in multi‐omics technology and artificial intelligence (AI) have enabled multi‐scale mapping of nerve‒cancer‒immune interactions, offering insights into the specific role of nerves in tumor immune regulation. Future research should prioritize exploring the regulatory effects of neural signals on immune cell phenotypes within the TME. These findings could enhance current immunotherapies and support the development of novel treatment strategies.
5.3. Emerging therapies
Strategies to inhibit neuroregeneration focus on the complex interplay between tumors and the nervous system, as tumor cells exploit regenerative mechanisms to support their growth and metastasis. 336 Under normal conditions, the nervous system's regenerative capacity can facilitate tumor expansion, particularly in cancers such as pancreatic and breast cancer, which stimulate nerve growth to create a favorable microenvironment. 241 , 337 Inhibiting neuroregeneration typically targets growth factors, such as NGF, and key signaling pathways such as MAPK and PI3K/Akt. 338 Developing drugs or immunotherapies that target these elements can help prevent nerve regeneration, thereby restricting tumor growth and spread. Unlike surgical or chemical neurolysis, targeting neurotrophic factors involved in tumor axonogenesis may offer a more effective approach, as it preserves the essential neural support within organs without damaging existing structures. While neurotrophic factors such as NGF are crucial during peripheral nerve development, they play a minimal role in maintaining neuronal function in adults. Instead, NGF primarily mediates pain by activating the tropomyosin receptor kinase A (TRKA) receptor in sensory neurons. 339 , 340 Studies have shown that systemic administration of anti‐NGF antibodies does not significantly affect neuronal or cognitive functions, making NGF an attractive therapeutic target for preventing nerve growth within the TME. 341 , 342 Anti‐NGF antibodies, TRKA inhibitors, and NGF‐targeted siRNA delivered via nanoparticles have shown promise in reducing cancer growth and metastasis in animal models, and these approaches are advancing to clinical trials. Beyond inhibiting tumor axonogenesis, anti‐NGF antibodies also offer the benefit of alleviating cancer‐related pain, demonstrating their dual potential as effective cancer therapies. 343
6. CHALLENGES AND FUTURE DIRECTIONS
Research in peripheral tumor neuroscience is uncovering the intricate connections between the nervous system and tumor progression. Sensory and sympathetic nerves within the PNS interact with tumor and immune cells through the release of specific signaling molecules, influencing tumor growth and metastasis. These peripheral nerves also play a role in critical tumor‐related processes such as angiogenesis, immune evasion, and metabolic regulation. As this field evolves, researchers are exploring novel treatment strategies, such as blocking nerve‒tumor cell signaling or employing neuroregulation techniques to inhibit tumor growth. Additionally, applying neuroscience tools to study the interactions between the nervous system and tumors is revealing new therapeutic targets. This growing understanding holds the potential for more personalized and effective cancer treatments, as novel interventions may be tailored to disrupt the neural mechanisms driving tumor development.
6.1. Research gaps and limitations
Surgical denervation has demonstrated tumor‐suppressing effects in mouse models of prostate and gastric cancer, but translating this approach to clinical practice presents significant challenges. Complete denervation may lead to severe side effects, limiting its broad application. The complexity of the TME and the intricate nerve innervation of different organs complicate the implementation of this strategy. Additionally, complete denervation can result in postoperative discomfort and potentially cause organ dysfunction. 344 While denervation has shown efficacy in experimental settings, its potential side effects must be carefully considered in clinical practice. Future research should prioritize optimizing denervation techniques to develop safer and more effective methods. Minimally invasive surgery, focusing on local nerve resection, could offer a viable option, particularly in cases where direct tumor resection poses high risks. This approach may help reduce surgical complications while inhibiting cancer progression. Alternatively, local injection of chemical agents or drugs targeting nerves involved in tumor growth offers a less invasive solution. For example, botulinum toxin (BoNT‐A) has been used to induce autonomic denervation, with clinical trials showing that it can trigger apoptosis in prostate cancer cells. This method holds promise for reducing the side effects of traditional surgery and could become a valuable tool in cancer treatment. 345
Mouse cancer models fail to fully capture the complexity of human diseases, particularly in cancer neuroscience. Future research must prioritize the development of animal models that better simulate the neural invasion characteristics seen in human cancers. Genetically modified and humanized patient‐derived xenograft models can induce neural invasion in mice that more closely resembles human pathology. 346 , 347 , 348 Additionally, using species with anatomical and physiological similarities to humans, such as pigs, may provide a more accurate platform for studying human pathophysiology. While various neural signaling inhibitors have shown promise in preclinical studies, these pathways are not only involved in tumor growth but are also critical for normal neural and bodily functions. This makes it challenging to develop therapies that target nerve‒cancer interactions without compromising normal neural function. Furthermore, the effectiveness of cancer treatments depends on disease stage and patient‐specific responses to neural signaling inhibitors. Identifying predictive biomarkers for tailored therapies will enhance treatment selection and advance personalized neuro‐oncology care. 325
6.2. Personalized medicine approaches
Research demonstrates that a patient's psychological state significantly influences the effectiveness of tumor treatments. Stress hormones (e.g., norepinephrine, epinephrine, glucocorticoids) may negatively impact both adjuvant and neoadjuvant therapies, although this issue has been insufficiently addressed in clinical studies. Synthetic glucocorticoids, such as dexamethasone, are commonly used in cancer treatment to alleviate chemotherapy‐induced nausea, improve lymphoma chemotherapy outcomes, and reduce inflammation during immunotherapy. 349 , 350 However, these drugs may diminish the efficacy of certain adjuvant therapies and, in some cases, promote cancer progression. 351 , 352 , 353 For instance, in non‐small cell lung cancer, glucocorticoids have been linked to lower response rates to ICIs and shorter survival times. Psychological interventions can mitigate stress, preventing adverse effects from medications, particularly for patients with contraindications. Stress management strategies are recommended during critical treatment phases, such as the perioperative and adjuvant treatment periods, to assess their effectiveness compared to other time points. Additionally, in vitro studies typically co‐culture neurons with cancer cells, overlooking other crucial components of the TME. More advanced 3D organoid culture methods, which incorporate nerve cells, glial cells, immune cells, and cancer‐associated fibroblasts, offer a more accurate representation of the in vivo tumor environment. 354 , 355 , 356 Ongoing clinical trials with patient‐derived organoids may lead to personalized neuro‐targeted therapies by identifying an individual's unique “neuro‐molecular signature.”
6.3. Collaborative and multidisciplinary research
Researchers utilize advanced high‐resolution imaging techniques, including electron microscopy, two‐photon and three‐photon microscopy, and multiphoton laser scanning microscopy, to investigate the complex interactions between neurons and cancer cells. 357 , 358 , 359 These technologies allow for detailed observation of neural networks within the TME, as well as the overall tumor architecture. Positron emission tomography is particularly valuable for tracking cholinergic neural activity to assess tumor spread, especially in prostate cancer. 360 These imaging methods aid in selecting candidates for neuromodulatory therapies. Further advancements, such as time‐lapse microscopy, fluorescence molecular tomography, and in vivo microscopy, deepen our understanding of the interactions between neurons, cancer cells, and glial cells. 361 , 362 High‐resolution microscopy, including electron and atomic force microscopy, reveals intricate details of neuronal processes, glial cell extensions, and their interactions with cancer cells. 362 Techniques such as light‐sheet microscopy and tissue clearing enable the generation of 3D neuroanatomical maps of tumors and organs, which provide valuable insights for refining local tumor control strategies. 363 , 364
Single‐cell RNA sequencing offers a detailed view of neurotransmitter receptor gene expression, shedding light on synaptic connections between neurons and glioma cells and clarifying the molecular mechanisms that link cancer and the nervous system. 365 Spatial transcriptomics further identifies novel therapeutic targets, overcoming the limitations of bulk tissue analyses that may obscure specific neural subtypes. 366 , 367 , 368 Multi‐omics sequencing technologies allow for the identification of cell‐specific molecular changes, providing a deeper understanding of neuron‒cancer interactions. AI and machine learning have become crucial tools in studying these interactions, accelerating data analysis, pattern recognition, and integrating mathematical modeling with AI to drive advancements in tumor neuroscience. Collaboration between tumor researchers and oncologists, alongside the sharing of technologies and models, is essential for promoting an interdisciplinary approach to precision medicine. Ultimately, this approach—especially when applied to large patient cohorts—aims to improve treatment outcomes in cancer patients.
AUTHOR CONTRIBUTIONS
Xin‐Hua Liang and Ya‐Ling Tang: designed the study. Hua‐Yang Fan: drafted the manuscript. Xin‐Hua Liang and Ya‐Ling Tang: revised the manuscript. All authors have approved the submitted version of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no conflicts of interest.
ETHICS STATEMENT
Not applicable.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China grants (nos. 82073000, 82173326, and 82473058). All figures were originally created by Keynote.
Fan H‐Y, Liang X‐H, Tang Y‐L. Neuroscience in peripheral cancers: tumors hijacking nerves and neuroimmune crosstalk. MedComm. 2024;5:e784. 10.1002/mco2.784
Contributor Information
Xin‐Hua Liang, Email: lxh88866@scu.edu.cn.
Ya‐Ling Tang, Email: tangyaling@scu.edu.cn.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Mancusi R, Monje M. The neuroscience of cancer. Nature. 2023;618(7965):467‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanahan D, Monje M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell. 2023;41(3):573‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Winkler F, Venkatesh HS, Amit M, et al. Cancer neuroscience: state of the field, emerging directions. Cell. 2023;186(8):1689‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu J, Heidelberg RE, Gajjar A. Adolescents and young adults with cancer: CNS tumors. J Clin Oncol. 2024;42(6):686‐695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Magnon C, Hondermarck H. The neural addiction of cancer. Nat Rev Cancer. 2023;23(5):317‐334. [DOI] [PubMed] [Google Scholar]
- 6. Segarra M, Aburto MR, Hefendehl J, Acker‐Palmer A. Neurovascular interactions in the nervous system. Annu Rev Cell Dev Biol. 2019;35:615‐635. [DOI] [PubMed] [Google Scholar]
- 7. Liu Q, Ma Z, Cao Q, et al. Perineural invasion‐associated biomarkers for tumor development. Biomed Pharmacother. 2022;155:113691. [DOI] [PubMed] [Google Scholar]
- 8. Schmitd LB, Beesley LJ, Russo N, et al. Redefining perineural invasion: integration of biology with clinical outcome. Neoplasia. 2018;20(7):657‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ayala GE, Dai H, Powell M, et al. Cancer‐related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14(23):7593‐7603. [DOI] [PubMed] [Google Scholar]
- 10. Albo D, Akay CL, Marshall CL, et al. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer. 2011;117(21):4834‐4845. [DOI] [PubMed] [Google Scholar]
- 11. Park H, Lee CH. The contribution of the nervous system in the cancer progression. BMB Rep. 2024;57(4):167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pundavela J, Roselli S, Faulkner S, et al. Nerve fibers infiltrate the tumor microenvironment and are associated with nerve growth factor production and lymph node invasion in breast cancer. Mol Oncol. 2015;9(8):1626‐1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Griffin N, Faulkner S, Jobling P, Hondermarck H. Targeting neurotrophin signaling in cancer: the renaissance. Pharmacol Res. 2018;135:12‐17. [DOI] [PubMed] [Google Scholar]
- 14. Hung YH, Hou YC, Hsu SH, et al. Pancreatic cancer cell‐derived semaphorin 3A promotes neuron recruitment to accelerate tumor growth and dissemination. Am J Cancer Res. 2023;13(8):3417‐3432. [PMC free article] [PubMed] [Google Scholar]
- 15. Hu J, Chen W, Shen L, Chen Z, Huang J. Crosstalk between the peripheral nervous system and breast cancer influences tumor progression. Biochim Biophys Acta Rev Cancer. 2022;1877(6):188828. [DOI] [PubMed] [Google Scholar]
- 16. Hernandez S, Serrano AG. The role of nerve fibers in the tumor immune microenvironment of solid tumors. Adv Biol. 2022;6(9):e2200046. [DOI] [PubMed] [Google Scholar]
- 17. Khanmammadova N, Islam S, Sharma P, Amit M. Neuro‐immune interactions and immuno‐oncology. Trends Cancer. 2023;9(8):636‐649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lyu Y, Xie F, Chen B, et al. The nerve cells in gastrointestinal cancers: from molecular mechanisms to clinical intervention. Oncogene. 2024;43(2):77‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han J, Jiang Q, Ma R, et al. Norepinephrine‐CREB1‐miR‐373 axis promotes progression of colon cancer. Mol Oncol. 2020;14(5):1059‐1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He B, Gao R, Lv S, et al. Cancer cell employs a microenvironmental neural signal trans‐activating nucleus‐mitochondria coordination to acquire stemness. Signal Transduct Target Ther. 2023;8(1):275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hayakawa Y, Sakitani K, Konishi M, et al. Nerve growth factor promotes gastric tumorigenesis through aberrant cholinergic signaling. Cancer Cell. 2017;31(1):21‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu Y, Shen K, Wang H, et al. Alpha5 nicotine acetylcholine receptor subunit promotes intrahepatic cholangiocarcinoma metastasis. Signal Transduct Target Ther. 2024;9(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Montoya A, Varela‐Ramirez A, Dickerson E, et al. The beta adrenergic receptor antagonist propranolol alters mitogenic and apoptotic signaling in late stage breast cancer. Biomed J. 2019;42(3):155‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Satilmis H, Verheye E, Vlummens P, et al. Targeting the β(2) ‐adrenergic receptor increases chemosensitivity in multiple myeloma by induction of apoptosis and modulating cancer cell metabolism. J Pathol. 2023;259(1):69‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nuevo‐Tapioles C, Santacatterina F, Stamatakis K, et al. Coordinate β‐adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth. Nat Commun. 2020;11(1):3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kang Y, Nagaraja AS, Armaiz‐Pena GN, et al. Adrenergic stimulation of DUSP1 impairs chemotherapy response in ovarian cancer. Clin Cancer Res. 2016;22(7):1713‐1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eng JW, Reed CB, Kokolus KM, et al. Housing temperature‐induced stress drives therapeutic resistance in murine tumour models through β2‐adrenergic receptor activation. Nat Commun. 2015;6:6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ray R, Al Khashali H, Haddad B, et al. Regulation of cisplatin resistance in lung cancer cells by nicotine, BDNF, and a β‐adrenergic receptor blocker. Int J Mol Sci. 2022;23(21):12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Globig AM, Zhao S, Roginsky J, et al. The β(1)‐adrenergic receptor links sympathetic nerves to T cell exhaustion. Nature. 2023;622(7982):383‐392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fjæstad KY, Rømer AMA, Goitea V, et al. Blockade of beta‐adrenergic receptors reduces cancer growth and enhances the response to anti‐CTLA4 therapy by modulating the tumor microenvironment. Oncogene. 2022;41(9):1364‐1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chang A, Botteri E, Gillis RD, et al. Beta‐blockade enhances anthracycline control of metastasis in triple‐negative breast cancer. Sci Transl Med. 2023;15(693):eadf1147. [DOI] [PubMed] [Google Scholar]
- 32. Kraboth Z, Kalman B. ß‐Adrenoreceptors in human cancers. Int J Mol Sci. 2023;24(4):3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Massalee R, Cao X. Repurposing beta‐blockers for combinatory cancer treatment: effects on conventional and immune therapies. Front Pharmacol. 2023;14:1325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nilsson MB, Le X, Heymach JV. β‐Adrenergic signaling in lung cancer: a potential role for beta‐blockers. J Neuroimmune Pharmacol. 2020;15(1):27‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nie M, Chen N, Pang H, et al. Targeting acetylcholine signaling modulates persistent drug tolerance in EGFR‐mutant lung cancer and impedes tumor relapse. J Clin Invest. 2022;132(20):e160152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuol N, Davidson M, Karakkat J, et al. Blocking muscarinic receptor 3 attenuates tumor growth and decreases immunosuppressive and cholinergic markers in an orthotopic mouse model of colorectal cancer. Int J Mol Sci. 2022;24(1):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wen YC, Tram VTN, Chen WH, et al. CHRM4/AKT/MYCN upregulates interferon alpha‐17 in the tumor microenvironment to promote neuroendocrine differentiation of prostate cancer. Cell Death Dis. 2023;14(5):304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gandhi S, Pandey MR, Attwood K, et al. Phase I clinical trial of combination propranolol and pembrolizumab in locally advanced and metastatic melanoma: safety, tolerability, and preliminary evidence of antitumor activity. Clin Cancer Res. 2021;27(1):87‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Filippi L, Bruno G, Domazetovic V, Favre C, Calvani M. Current therapies and new targets to fight melanoma: a promising role for the β3‐adrenoreceptor. Cancers (Basel). 2020;12(6):1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Li W, Wan J, Chen C, et al. Dissecting the role of cell signaling versus CD8(+) T cell modulation in propranolol antitumor activity. J Mol Med. 2022;100(9):1299‐1306. [DOI] [PubMed] [Google Scholar]
- 41. Ferraguti G, Terracina S, Tarani L, et al. Nerve growth factor and the role of inflammation in tumor development. Curr Issues Mol Biol. 2024;46(2):965‐989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gasparini G, Pellegatta M, Crippa S, et al. Nerves and pancreatic cancer: new insights into a dangerous relationship. Cancers (Basel). 2019;11(7):893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Romon R, Adriaenssens E, Lagadec C, Germain E, Hondermarck H, Le Bourhis X. Nerve growth factor promotes breast cancer angiogenesis by activating multiple pathways. Mol Cancer. 2010;9:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Renz BW, Takahashi R, Tanaka T, et al. β2 adrenergic‐neurotrophin feedforward loop promotes pancreatic cancer. Cancer Cell. 2018;33(1):75‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simó M, Navarro X, Yuste VJ, Bruna J. Autonomic nervous system and cancer. Clin Auton Res. 2018;28(3):301‐314. [DOI] [PubMed] [Google Scholar]
- 46. Zahalka AH, Frenette PS. Nerves in cancer. Nat Rev Cancer. 2020;20(3):143‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kamiya A, Hiyama T, Fujimura A, Yoshikawa S. Sympathetic and parasympathetic innervation in cancer: therapeutic implications. Clin Auton Res. 2021;31(2):165‐178. [DOI] [PubMed] [Google Scholar]
- 48. Cui Q, Jiang D, Zhang Y, Chen C. The tumor‒nerve circuit in breast cancer. Cancer Metastasis Rev. 2023;42(2):543‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Erin N, Akman M, Aliyev E, Tanrıöver G, Korcum AF. Olvanil activates sensory nerve fibers, increases T cell response and decreases metastasis of breast carcinoma. Life Sci. 2022;291:120305. [DOI] [PubMed] [Google Scholar]
- 50. Darragh LB, Nguyen A, Pham TT, et al. Sensory nerve release of CGRP increases tumor growth in HNSCC by suppressing TILs. Med. 2024;5(3):254‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holland AM, Bon‐Frauches AC, Keszthelyi D, Melotte V, Boesmans W. The enteric nervous system in gastrointestinal disease etiology. Cell Mol Life Sci. 2021;78(10):4713‐4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Vaes N, Schonkeren SL, Rademakers G, et al. Loss of enteric neuronal Ndrg4 promotes colorectal cancer via increased release of Nid1 and Fbln2. EMBO Rep. 2021;22(6):e51913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhao CM, Hayakawa Y, Kodama Y, et al. Denervation suppresses gastric tumorigenesis. Sci Transl Med. 2014;6(250):250ra115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Amit M, Takahashi H, Dragomir MP, et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature. 2020;578(7795):449‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Perez‐Pacheco C, Schmitd LB, Furgal A, et al. Increased nerve density adversely affects outcome in oral cancer. Clin Cancer Res. 2023;29(13):2501‐2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hojman P, Gehl J, Christensen JF, Pedersen BK. Molecular mechanisms linking exercise to cancer prevention and treatment. Cell Metab. 2018;27(1):10‐21. [DOI] [PubMed] [Google Scholar]
- 57. Kurz E, Hirsch CA, Dalton T, et al. Exercise‐induced engagement of the IL‐15/IL‐15Rα axis promotes anti‐tumor immunity in pancreatic cancer. Cancer Cell. 2022;40(7):720‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wei Y, Liang Y, Lin H, Dai Y, Yao S. Autonomic nervous system and inflammation interaction in endometriosis‐associated pain. J Neuroinflammation. 2020;17(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Eichmann A, Brunet I. Arterial innervation in development and disease. Sci Transl Med. 2014;6(252):252ps9. [DOI] [PubMed] [Google Scholar]
- 60. Willems JL, Buylaert WA, Lefebvre RA, Bogaert MG. Neuronal dopamine receptors on autonomic ganglia and sympathetic nerves and dopamine receptors in the gastrointestinal system. Pharmacol Rev. 1985;37(2):165‐216. [PubMed] [Google Scholar]
- 61. Scott‐Solomon E, Boehm E, Kuruvilla R. The sympathetic nervous system in development and disease. Nat Rev Neurosci. 2021;22(11):685‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. 2007;87(3):873‐904. [DOI] [PubMed] [Google Scholar]
- 63. Gibbons CH. Basics of autonomic nervous system function. Handb Clin Neurol. 2019;160:407‐418. [DOI] [PubMed] [Google Scholar]
- 64. VanPatten S, Al‐Abed Y. The challenges of modulating the ‘rest and digest’ system: acetylcholine receptors as drug targets. Drug Discov Today. 2017;22(1):97‐104. [DOI] [PubMed] [Google Scholar]
- 65. Cole SW, Nagaraja AS, Lutgendorf SK, Green PA, Sood AK. Sympathetic nervous system regulation of the tumour microenvironment. Nat Rev Cancer. 2015;15(9):563‐572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Maestroni GJM. Adrenergic modulation of hematopoiesis. J Neuroimmune Pharmacol. 2020;15(1):82‐92. [DOI] [PubMed] [Google Scholar]
- 67. Insel PA. Structure and function of alpha‐adrenergic receptors. Am J Med. 1989;87(2):12s‐18s. [DOI] [PubMed] [Google Scholar]
- 68. Sofuoglu M, Mooney M. Cholinergic functioning in stimulant addiction: implications for medications development. CNS Drugs. 2009;23(11):939‐952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kolmus K, Tavernier J, Gerlo S. β2‐Adrenergic receptors in immunity and inflammation: stressing NF‐κB. Brain Behav Immun. 2015;45:297‐310. [DOI] [PubMed] [Google Scholar]
- 70. Simpson RJ, Boßlau TK, Weyh C, et al. Exercise and adrenergic regulation of immunity. Brain Behav Immun. 2021;97:303‐318. [DOI] [PubMed] [Google Scholar]
- 71. Halder N, Lal G. Cholinergic system and its therapeutic importance in inflammation and autoimmunity. Front Immunol. 2021;12:660342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Dhawan S, Cailotto C, Harthoorn LF, de Jonge WJ. Cholinergic signalling in gut immunity. Life Sci. 2012;91(21‐22):1038‐1042. [DOI] [PubMed] [Google Scholar]
- 73. Shah N, Khurana S, Cheng K, Raufman JP. Muscarinic receptors and ligands in cancer. Am J Physiol Cell Physiol. 2009;296(2):C221‐C232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jensen AWP, Carnaz Simões AM, Thor Straten P, Holmen Olofsson G. Adrenergic signaling in immunotherapy of cancer: friend or foe? Cancers (Basel). 2021;13(3):394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Farooq MA, Ajmal I, Hui X, Chen Y, Ren Y, Jiang W. β2‐Adrenergic receptor mediated inhibition of T cell function and its implications for CAR‐T cell therapy. Int J Mol Sci. 2023;24(16):12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li XQ, Peng WT, Shan S, et al. β‐Arrestin2 regulating β2‐adrenergic receptor signaling in hepatic stellate cells contributes to hepatocellular carcinoma progression. J Cancer. 2021;12(24):7287‐7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Palm D, Lang K, Niggemann B, et al. The norepinephrine‐driven metastasis development of PC‐3 human prostate cancer cells in BALB/c nude mice is inhibited by beta‐blockers. Int J Cancer. 2006;118(11):2744‐2749. [DOI] [PubMed] [Google Scholar]
- 78. Zhi X, Li B, Li Z, et al. Adrenergic modulation of AMPK‑dependent autophagy by chronic stress enhances cell proliferation and survival in gastric cancer. Int J Oncol. 2019;54(5):1625‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang X, Zhang Y, He Z, et al. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Dis. 2019;10(11):788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu H, Wang C, Xie N, et al. Activation of adrenergic receptor β2 promotes tumor progression and epithelial mesenchymal transition in tongue squamous cell carcinoma. Int J Mol Med. 2018;41(1):147‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Nagaraja AS, Dorniak PL, Sadaoui NC, et al. Sustained adrenergic signaling leads to increased metastasis in ovarian cancer via increased PGE2 synthesis. Oncogene. 2016;35(18):2390‐2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Moretti S, Massi D, Farini V, et al. β‐Adrenoceptors are upregulated in human melanoma and their activation releases pro‐tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Invest. 2013;93(3):279‐290. [DOI] [PubMed] [Google Scholar]
- 83. Liu J, Qu L, Wan C, et al. A novel β2‐AR/YB‐1/β‐catenin axis mediates chronic stress‐associated metastasis in hepatocellular carcinoma. Oncogenesis. 2020;9(9):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Reavis HD, Gysler SM, McKenney GB, et al. Norepinephrine induces anoikis resistance in high‐grade serous ovarian cancer precursor cells. JCI Insight. 2024;9(5):e170961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Madden KS, Szpunar MJ, Brown EB. β‐Adrenergic receptors (β‐AR) regulate VEGF and IL‐6 production by divergent pathways in high β‐AR‐expressing breast cancer cell lines. Breast Cancer Res Treat. 2011;130(3):747‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Qin JF, Jin FJ, Li N, et al. Adrenergic receptor β2 activation by stress promotes breast cancer progression through macrophages M2 polarization in tumor microenvironment. BMB Rep. 2015;48(5):295‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Caulfield MP. Muscarinic receptors–characterization, coupling and function. Pharmacol Ther. 1993;58(3):319‐379. [DOI] [PubMed] [Google Scholar]
- 88. Schaaf CP. Nicotinic acetylcholine receptors in human genetic disease. Genet Med. 2014;16(9):649‐656. [DOI] [PubMed] [Google Scholar]
- 89. Zahalka AH, Arnal‐Estapé A, Maryanovich M, et al. Adrenergic nerves activate an angio‐metabolic switch in prostate cancer. Science. 2017;358(6361):321‐326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Wang J, Wei J, Pu T, et al. Cholinergic signaling via muscarinic M1 receptor confers resistance to docetaxel in prostate cancer. Cell Rep Med. 2024;5(2):101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lobbes LA, Schütze MA, Droeser R, et al. Muscarinic acetylcholine receptor M3 expression and survival in human colorectal carcinoma—an unexpected correlation to guide future treatment? Int J Mol Sci. 2023;24(9):8198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bele T, Turk T, Križaj I. Nicotinic acetylcholine receptors in cancer: limitations and prospects. Biochim Biophys Acta Mol Basis Dis. 2024;1870(1):166875. [DOI] [PubMed] [Google Scholar]
- 93. Schaal CM, Bora‐Singhal N, Kumar DM, Chellappan SP. Regulation of Sox2 and stemness by nicotine and electronic‐cigarettes in non‐small cell lung cancer. Mol Cancer. 2018;17(1):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. He Z, Xu Y, Rao Z, et al. The role of α7‐nAChR‐mediated PI3K/AKT pathway in lung cancer induced by nicotine. Sci Total Environ. 2024;912:169604. [DOI] [PubMed] [Google Scholar]
- 95. Takahashi R, Ijichi H, Fujishiro M. The role of neural signaling in the pancreatic cancer microenvironment. Cancers (Basel). 2022;14(17):4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dragomir MP, Moisoiu V, Manaila R, et al. A holistic perspective: exosomes shuttle between nerves and immune cells in the tumor microenvironment. J Clin Med. 2020;9(11):3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Jennings MR, Munn D, Blazeck J. Immunosuppressive metabolites in tumoral immune evasion: redundancies, clinical efforts, and pathways forward. J Immunother Cancer. 2021;9(10):e003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Koop LK, Tadi P. Neuroanatomy, sensory nerves. StatPearls. StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC; 2024. [PubMed] [Google Scholar]
- 99. Leroux A, Roque M, Casas E, et al. The effect of CGRP and SP and the cell signaling dialogue between sensory neurons and endothelial cells. Biol Res. 2024;57(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Carr R, Frings S. Neuropeptides in sensory signal processing. Cell Tissue Res. 2019;375(1):217‐225. [DOI] [PubMed] [Google Scholar]
- 101. Salio C, Lossi L, Ferrini F, Merighi A. Neuropeptides as synaptic transmitters. Cell Tissue Res. 2006;326(2):583‐598. [DOI] [PubMed] [Google Scholar]
- 102. Mashaghi A, Marmalidou A, Tehrani M, Grace PM, Pothoulakis C, Dana R. Substance P and pain chronicity. Cell Tissue Res. 2016;73(22):4249‐4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zieglgänsberger W. Substance P and pain chronicity. Cell Tissue Res. 2019;375(1):227‐241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Russo AF, Hay DL. Physiol Rev. 2023;103(2):1565‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Coveñas R, Muñoz M. Cancer progression and substance P. Histol Histopathol. 2014;29(7):881‐890. [DOI] [PubMed] [Google Scholar]
- 106. Padmanaban V, Keller I, Seltzer ES, Ostendorf BN, Kerner Z, Tavazoie SF. Neuronal substance P drives metastasis through an extracellular RNA‐TLR7 axis. Nature. 2024;633(8028):207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yu H, Huang T, Lu WW, Tong L, Chen D. Osteoarthritis pain. Int J Mol Sci. 2022;23(9):4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Balood M, Ahmadi M, Eichwald T, et al. Nociceptor neurons affect cancer immunosurveillance. Nature. 2022;611(7935):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Erin N, Szallasi A. Carcinogenesis and metastasis: focus on TRPV1‐positive neurons and immune cells. Biomolecules. 2023;13(6):983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Szkutnik‐Fiedler D. Pharmacokinetics, pharmacodynamics and drug‒drug interactions of new anti‐migraine drugs‐lasmiditan, gepants, and calcitonin‐gene‐related peptide (CGRP) receptor monoclonal antibodies. Pharmaceutics. 2020;12(12):1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tu NH, Inoue K, Lewis PK, et al. Calcitonin related polypeptide alpha mediates oral cancer pain. Cells. 2023;12(13):1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Mardelle U, Bretaud N, Daher C, Feuillet V. From pain to tumor immunity: influence of peripheral sensory neurons in cancer. Front Immunol. 2024;15:1335387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Peng L, Agogo GO, Guo J, Yan M. Substance P and fibrotic diseases. Neuropeptides. 2019;76:101941. [DOI] [PubMed] [Google Scholar]
- 114. Wallrapp A, Chiu IM. Neuroimmune interactions in the intestine. Annu Rev Immunol. 2024;42(1):489‐519. [DOI] [PubMed] [Google Scholar]
- 115. Spencer NJ, Hu H. Enteric nervous system: sensory transduction, neural circuits and gastrointestinal motility. Nat Rev Gastroenterol Hepatol. 2020;17(6):338‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Niesler B, Kuerten S, Demir IE, Schäfer KH. Disorders of the enteric nervous system—a holistic view. Nat Rev Gastroenterol Hepatol. 2021;18(6):393‐410. [DOI] [PubMed] [Google Scholar]
- 117. Rademakers G, Vaes N, Schonkeren S, Koch A, Sharkey KA, Melotte V. The role of enteric neurons in the development and progression of colorectal cancer. Biochim Biophys Acta Rev Cancer. 2017;1868(2):420‐434. [DOI] [PubMed] [Google Scholar]
- 118. Wang K, Zhao XH, Liu J, Zhang R, Li JP. Nervous system and gastric cancer. Biochim Biophys Acta Rev Cancer. 2020;1873(1):188313. [DOI] [PubMed] [Google Scholar]
- 119. Schonkeren SL, Thijssen MS, Vaes N, Boesmans W, Melotte V. The emerging role of nerves and glia in colorectal cancer. Cancers (Basel). 2021;13(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Liebl F, Demir IE, Mayer K, et al. The impact of neural invasion severity in gastrointestinal malignancies: a clinicopathological study. Ann Surg. 2014;260(5):900‐907. discussion 907‐908. [DOI] [PubMed] [Google Scholar]
- 121. Schorn S, Demir IE, Haller B, et al. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma—a systematic review and meta‐analysis. Surg Oncol. 2017;26(1):105‐115. [DOI] [PubMed] [Google Scholar]
- 122. Zauszkiewicz‐Pawlak A, Godlewski J, Kwiatkowski P, Kmiec Z. Ultrastructural characteristics of myenteric plexus in patients with colorectal cancer. Folia Histochem Cytobiol. 2017;55(1):6‐10. [DOI] [PubMed] [Google Scholar]
- 123. Ceyhan GO, Demir IE, Altintas B, et al. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun. 2008;374(3):442‐447. [DOI] [PubMed] [Google Scholar]
- 124. Duchalais E, Guilluy C, Nedellec S, et al. Colorectal cancer cells adhere to and migrate along the neurons of the enteric nervous system. Cell Mol Gastroenterol Hepatol. 2018;5(1):31‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Deborde S, Omelchenko T, Lyubchik A, et al. Schwann cells induce cancer cell dispersion and invasion. J Clin Invest. 2016;126(4):1538‐1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Renz BW, Tanaka T, Sunagawa M, et al. Cholinergic signaling via muscarinic receptors directly and indirectly suppresses pancreatic tumorigenesis and cancer stemness. Cancer Discov. 2018;8(11):1458‐1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Partecke LI, Käding A, Trung DN, et al. Subdiaphragmatic vagotomy promotes tumor growth and reduces survival via TNFα in a murine pancreatic cancer model. Oncotarget. 2017;8(14):22501‐22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. He K, Wang H, Huo R, Jiang SH, Xue J. Schwann cells and enteric glial cells: emerging stars in colorectal cancer. Biochim Biophys Acta Rev Cancer. 2024;1879(5):189160. [DOI] [PubMed] [Google Scholar]
- 129. Orange ST, Leslie J, Ross M, Mann DA, Wackerhage H. The exercise IL‐6 enigma in cancer. Trends Endocrinol Metab. 2023;34(11):749‐763. [DOI] [PubMed] [Google Scholar]
- 130. Koelwyn GJ, Zhuang X, Tammela T, Schietinger A, Jones LW. Exercise and immunometabolic regulation in cancer. Nat Metab. 2020;2(9):849‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Goh J, Kirk EA, Lee SX, Ladiges WC. Exercise, physical activity and breast cancer: the role of tumor‐associated macrophages. Exerc Immunol Rev. 2012;18:158‐176. [PubMed] [Google Scholar]
- 132. Gustafson MP, Wheatley‐Guy CM, Rosenthal AC, et al. Exercise and the immune system: taking steps to improve responses to cancer immunotherapy. J Immunother Cancer. 2021;9(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Wennerberg E, Lhuillier C, Rybstein MD, et al. Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget. 2020;11(4):452‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gomes‐Santos IL, Amoozgar Z, Kumar AS, et al. Exercise training improves tumor control by increasing CD8(+) T‐cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol Res. 2021;9(7):765‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Schnyder S, Handschin C. Skeletal muscle as an endocrine organ: PGC‐1α, myokines and exercise. Bone. 2015;80:115‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Kim S, Kim K. The effects of exercise and conjugated linoleic acid intake on IGF‐1 and pro‐inflammatory cytokines in atrophied skeletal muscle of rats. Integr Med Res. 2013;2(4):166‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Huang KY, Upadhyay G, Ahn Y, et al. Neuronal innervation regulates the secretion of neurotrophic myokines and exosomes from skeletal muscle. Proc Natl Acad Sci U S A. 2024;121(19):e2313590121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Zeng Q, Cheng Y, Zhu Q, et al. The relationship between overexpression of glial cell‐derived neurotrophic factor and its RET receptor with progression and prognosis of human pancreatic cancer. J Int Med Res. 2008;36(4):656‐664. [DOI] [PubMed] [Google Scholar]
- 139. Cintrón‐Colón AF, Almeida‐Alves G, Boynton AM, Spitsbergen JM. GDNF synthesis, signaling, and retrograde transport in motor neurons. Cell Tissue Res. 2020;382(1):47‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Kostrzewa RM, Jacobowitz DM. Pharmacological actions of 6‐hydroxydopamine. Pharmacol Rev. 1974;26(3):199‐288. [PubMed] [Google Scholar]
- 141. Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. [DOI] [PubMed] [Google Scholar]
- 142. Raju B, Haug SR, Ibrahim SO, Heyeraas KJ. Sympathectomy decreases size and invasiveness of tongue cancer in rats. Neuroscience. 2007;149(3):715‐725. [DOI] [PubMed] [Google Scholar]
- 143. Atherton MA, Park S, Horan NL, et al. Sympathetic modulation of tumor necrosis factor alpha‐induced nociception in the presence of oral squamous cell carcinoma. Pain. 2023;164(1):27‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Szpunar MJ, Belcher EK, Dawes RP, Madden KS. Sympathetic innervation, norepinephrine content, and norepinephrine turnover in orthotopic and spontaneous models of breast cancer. Brain Behav Immun. 2016;53:223‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Horvathova L, Padova A, Tillinger A, Osacka J, Bizik J, Mravec B. Sympathectomy reduces tumor weight and affects expression of tumor‐related genes in melanoma tissue in the mouse. Stress. 2016;19(5):528‐534. [DOI] [PubMed] [Google Scholar]
- 146. Shiralkar J, Anthony T, McCallum GA, Durand DM. Neural recordings can differentiate between spontaneously metastasizing melanomas and melanomas with low metastatic potential. PLoS One. 2024;19(2):e0297281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85(1‐3):1‐17. [DOI] [PubMed] [Google Scholar]
- 148. Erin N, Zhao W, Bylander J, Chase G, Clawson G. Capsaicin‐induced inactivation of sensory neurons promotes a more aggressive gene expression phenotype in breast cancer cells. Breast Cancer Res Treat. 2006;99(3):351‐364. [DOI] [PubMed] [Google Scholar]
- 149. Stopczynski RE, Normolle DP, Hartman DJ, et al. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014;74(6):1718‐1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Schwartz ES, Christianson JA, Chen X, et al. Synergistic role of TRPV1 and TRPA1 in pancreatic pain and inflammation. Gastroenterology. 2011;140(4):1283‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Schwartz ES, La JH, Scheff NN, Davis BM, Albers KM, Gebhart GF. TRPV1 and TRPA1 antagonists prevent the transition of acute to chronic inflammation and pain in chronic pancreatitis. J Neurosci. 2013;33(13):5603‐5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Saloman JL, Albers KM, Li D, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A. 2016;113(11):3078‐3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Foster SL, Seehus CR, Woolf CJ, Talbot S. Sense and immunity: context‐dependent neuro‐immune interplay. Front Immunol. 2017;8:1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhang Y, Lin C, Liu Z, et al. Cancer cells co‐opt nociceptive nerves to thrive in nutrient‐poor environments and upon nutrient‐starvation therapies. Cell Metab. 2022;34(12):1999‐2017. [DOI] [PubMed] [Google Scholar]
- 155. Baral P, Udit S, Chiu IM. Pain and immunity: implications for host defence. Nat Rev Immunol. 2019;19(7):433‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tsuru S, Ito Y, Matsuda H, et al. RAMP1 signaling in immune cells regulates inflammation‐associated lymphangiogenesis. Lab Invest. 2020;100(5):738‐750. [DOI] [PubMed] [Google Scholar]
- 157. Vitorakis N, Gargalionis AN, Papavassiliou KA, Adamopoulos C, Papavassiliou AG. Precision targeting strategies in pancreatic cancer: the role of tumor microenvironment. Cancers (Basel). 2024;16(16):2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhu YH, Zheng JH, Jia QY, et al. Immunosuppression, immune escape, and immunotherapy in pancreatic cancer: focused on the tumor microenvironment. Cell Oncol. 2023;46(1):17‐48. [DOI] [PubMed] [Google Scholar]
- 159. Fan JQ, Wang MF, Chen HL, Shang D, Das JK, Song J. Current advances and outlooks in immunotherapy for pancreatic ductal adenocarcinoma. Mol Cancer. 2020;19(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Liu Y, Tian S, Ning B, Huang T, Li Y, Wei Y. Stress and cancer: the mechanisms of immune dysregulation and management. Front Immunol. 2022;13:1032294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Machelska H, Celik M. Immune cell‐mediated opioid analgesia. Immunol Lett. 2020;227:48‐59. [DOI] [PubMed] [Google Scholar]
- 162. Gysler SM, Drapkin R. Tumor innervation: peripheral nerves take control of the tumor microenvironment. J Clin Invest. 2021;131(11):e147276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Heine M, Heck J, Ciuraszkiewicz A, Bikbaev A. Dynamic compartmentalization of calcium channel signalling in neurons. Neuropharmacology. 2020;169:107556. [DOI] [PubMed] [Google Scholar]
- 164. Prevarskaya N, Skryma R, Shuba Y. Ion channels in cancer: are cancer hallmarks oncochannelopathies? Physiol Rev. 2018;98(2):559‐621. [DOI] [PubMed] [Google Scholar]
- 165. Fan JJ, Huang X. Ion channels in cancer: orchestrators of electrical signaling and cellular crosstalk. Rev Physiol Biochem Pharmacol. 2022;183:103‐133. [DOI] [PubMed] [Google Scholar]
- 166. Kondratskyi A, Kondratska K, Skryma R, Prevarskaya N. Ion channels in the regulation of apoptosis. Biochim Biophys Acta. 2015;1848(pt B):2532‐2546. [DOI] [PubMed] [Google Scholar]
- 167. Anderson KJ, Cormier RT, Scott PM. Role of ion channels in gastrointestinal cancer. World J Gastroenterol. 2019;25(38):5732‐5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Liu H, Weng J, Huang CL, Jackson AP. Voltage‐gated sodium channels in cancers. Biomark Res. 2024;12(1):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Giammello F, Biella C, Priori EC, et al. Modulating voltage‐gated sodium channels to enhance differentiation and sensitize glioblastoma cells to chemotherapy. Cell Commun Signal. 2024;22(1):434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Li RQ, Zhao XH, Zhu Q, et al. Exploring neurotransmitters and their receptors for breast cancer prevention and treatment. Theranostics. 2023;13(3):1109‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Erin N, Shurin GV, Baraldi JH, Shurin MR. Regulation of carcinogenesis by sensory neurons and neuromediators. Cancers (Basel). 2022;14(9):2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Li D, Hu LN, Zheng SM, et al. High nerve density in breast cancer is associated with poor patient outcome. FASEB Bioadv. 2022;4(6):391‐401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Schmitd LB, Perez‐Pacheco C, D'Silva NJ. Nerve density in cancer: less is better. FASEB Bioadv. 2021;3(10):773‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Bednarsch J, Kather J, Tan X, et al. Nerve fibers in the tumor microenvironment as a novel biomarker for oncological outcome in patients undergoing surgery for perihilar cholangiocarcinoma. Liver Cancer. 2021;10(3):260‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Eckerling A, Ricon‐Becker I, Sorski L, Sandbank E, Ben‐Eliyahu S. Stress and cancer: mechanisms, significance and future directions. Nat Rev Cancer. 2021;21(12):767‐785. [DOI] [PubMed] [Google Scholar]
- 176. Weeden CE, Hill W, Lim EL, Grönroos E, Swanton C. Impact of risk factors on early cancer evolution. Cell. 2023;186(8):1541‐1563. [DOI] [PubMed] [Google Scholar]
- 177. Wu S, Zhu W, Thompson P, Hannun YA. Evaluating intrinsic and non‐intrinsic cancer risk factors. Nat Commun. 2018;9(1):3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Vicente‐Dueñas C, Hauer J, Cobaleda C, Borkhardt A, Sánchez‐García I. Epigenetic priming in cancer initiation. Trends Cancer. 2018;4(6):408‐417. [DOI] [PubMed] [Google Scholar]
- 179. Tiwari RK, Sharma V, Pandey RK, Shukla SS. Nicotine addiction: neurobiology and mechanism. J Pharmacopuncture. 2020;23(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Le Foll B, Piper ME, Fowler CD, et al. Tobacco and nicotine use. Nat Rev Dis Primers . 2022;8(1):19. [DOI] [PubMed] [Google Scholar]
- 181. Wittenberg RE, Wolfman SL, De Biasi M, Dani JA. Nicotinic acetylcholine receptors and nicotine addiction: a brief introduction. Neuropharmacology. 2020;177:108256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Zhang L, Pan J, Chen W, Jiang J, Huang J. Chronic stress‐induced immune dysregulation in cancer: implications for initiation, progression, metastasis, and treatment. Am J Cancer Res. 2020;10(5):1294‐1307. [PMC free article] [PubMed] [Google Scholar]
- 183. Lupu M, Caruntu A, Caruntu C, et al. Neuroendocrine factors: the missing link in non‑melanoma skin cancer (review). Oncol Rep. 2017;38(3):1327‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Gaillard H, García‐Muse T, Aguilera A. Replication stress and cancer. Nat Rev Cancer. 2015;15(5):276‐289. [DOI] [PubMed] [Google Scholar]
- 185. Yasuda MT, Sakakibara H, Shimoi K. Estrogen‐ and stress‐induced DNA damage in breast cancer and chemoprevention with dietary flavonoid. Genes Environ. 2017;39:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lamboy‐Caraballo R, Ortiz‐Sanchez C, Acevedo‐Santiago A, Matta J, Monteiro ANA, Armaiz‐Pena GN. Norepinephrine‐induced DNA damage in ovarian cancer cells. Int J Mol Sci. 2020;21(6):2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Reeder A, Attar M, Nazario L, et al. Stress hormones reduce the efficacy of paclitaxel in triple negative breast cancer through induction of DNA damage. Br J Cancer. 2015;112(9):1461‐1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hara MR, Kovacs JJ, Whalen EJ, et al. A stress response pathway regulates DNA damage through β2‐adrenoreceptors and β‐arrestin‐1. Nature. 2011;477(7364):349‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Hara MR, Sachs BD, Caron MG, Lefkowitz RJ. Pharmacological blockade of a β(2)AR‐β‐arrestin‐1 signaling cascade prevents the accumulation of DNA damage in a behavioral stress model. Cell Cycle. 2013;12(2):219‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Flint MS, Baum A, Chambers WH, Jenkins FJ. Induction of DNA damage, alteration of DNA repair and transcriptional activation by stress hormones. Psychoneuroendocrinology. 2007;32(5):470‐479. [DOI] [PubMed] [Google Scholar]
- 191. Pfitzinger PL, Fangmann L, Wang K, et al. Indirect cholinergic activation slows down pancreatic cancer growth and tumor‐associated inflammation. J Exp Clin Cancer Res. 2020;39(1):289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Shehwana H, Keskus AG, Ozdemir SE, et al. CHRNA5 belongs to the secondary estrogen signaling network exhibiting prognostic significance in breast cancer. Cell Oncol. 2021;44(2):453‐472. [DOI] [PubMed] [Google Scholar]
- 193. Koh M, Takahashi T, Kurokawa Y, et al. Propranolol suppresses gastric cancer cell growth by regulating proliferation and apoptosis. Gastric Cancer. 2021;24(5):1037‐1049. [DOI] [PubMed] [Google Scholar]
- 194. Rousseau B, Murugan S, Palagani A, Sarkar DK. Beta 2 adrenergic receptor and mu opioid receptor interact to potentiate the aggressiveness of human breast cancer cell by activating the glycogen synthase kinase 3 signaling. Breast Cancer Res. 2022;24(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Sood AK, Armaiz‐Pena GN, Halder J, et al. Adrenergic modulation of focal adhesion kinase protects human ovarian cancer cells from anoikis. J Clin Invest. 2010;120(5):1515‐1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zhang D, Ma Q, Wang Z, et al. β2‐Adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFκB pathway. Mol Cancer. 2011;10:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Chin CC, Li JM, Lee KF, et al. Selective β2‐AR blockage suppresses colorectal cancer growth through regulation of EGFR‐Akt/ERK1/2 signaling, G1‐phase arrest, and apoptosis. J Cell Physiol. 2016;231(2):459‐472. [DOI] [PubMed] [Google Scholar]
- 198. Wu FQ, Fang T, Yu LX, et al. ADRB2 signaling promotes HCC progression and sorafenib resistance by inhibiting autophagic degradation of HIF1α. J Hepatol. 2016;65(2):314‐324. [DOI] [PubMed] [Google Scholar]
- 199. Raufman JP, Chen Y, Cheng K, Compadre C, Compadre L, Zimniak P. Selective interaction of bile acids with muscarinic receptors: a case of molecular mimicry. Eur J Pharmacol. 2002;457(2‐3):77‐84. [DOI] [PubMed] [Google Scholar]
- 200. Yu H, Xia H, Tang Q, et al. Acetylcholine acts through M3 muscarinic receptor to activate the EGFR signaling and promotes gastric cancer cell proliferation. Sci Rep. 2017;7:40802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Pan C, Wu J, Zheng S, et al. Depression accelerates gastric cancer invasion and metastasis by inducing a neuroendocrine phenotype via the catecholamine/β(2)‒AR/MACC1 axis. Cancer Commun. 2021;41(10):1049‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Zhou Z, Shu Y, Bao H, et al. Stress‐induced epinephrine promotes epithelial‐to‐mesenchymal transition and stemness of CRC through the CEBPB/TRIM2/P53 axis. J Transl Med. 2022;20(1):262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Armaiz‐Pena GN, Allen JK, Cruz A, et al. Src activation by β‐adrenoreceptors is a key switch for tumour metastasis. Nat Commun. 2013;4:1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Landen CN, Lin YG, Armaiz‐Pena GN, et al. Neuroendocrine modulation of signal transducer and activator of transcription‐3 in ovarian cancer. Cancer Res. 2007;67(21):10389‐10396. [DOI] [PubMed] [Google Scholar]
- 205. Choi MJ, Cho KH, Lee S, et al. hTERT mediates norepinephrine‐induced Slug expression and ovarian cancer aggressiveness. Oncogene. 2015;34(26):3402‐3412. [DOI] [PubMed] [Google Scholar]
- 206. Lin XH, Liu HH, Hsu SJ, et al. Norepinephrine‐stimulated HSCs secrete sFRP1 to promote HCC progression following chronic stress via augmentation of a Wnt16B/β‐catenin positive feedback loop. J Exp Clin Cancer Res. 2020;39(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Du P, Zeng H, Xiao Y, et al. Chronic stress promotes EMT‐mediated metastasis through activation of STAT3 signaling pathway by miR‐337‐3p in breast cancer. Cell Death Dis. 2020;11(9):761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Pu J, Zhang X, Luo H, Xu L, Lu X, Lu J. Adrenaline promotes epithelial‐to‐mesenchymal transition via HuR‐TGFβ regulatory axis in pancreatic cancer cells and the implication in cancer prognosis. Biochem Biophys Res Commun. 2017;493(3):1273‐1279. [DOI] [PubMed] [Google Scholar]
- 209. Ray R, Goel S, Al Khashali H, et al. Regulation of soluble E‐cadherin signaling in non‐small‐cell lung cancer cells by nicotine, BDNF, and β‐adrenergic receptor ligands. Biomedicines. 2023;11(9):2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Lutgendorf SK, Cole S, Costanzo E, et al. Stress‐related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9(12):4514‐4521. [PubMed] [Google Scholar]
- 211. Thaker PH, Han LY, Kamat AA, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12(8):939‐944. [DOI] [PubMed] [Google Scholar]
- 212. Yang EV, Kim SJ, Donovan EL, et al. Norepinephrine upregulates VEGF, IL‐8, and IL‐6 expression in human melanoma tumor cell lines: implications for stress‐related enhancement of tumor progression. Brain Behav Immun. 2009;23(2):267‐275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Shan T, Ma J, Ma Q, et al. β2‐AR‐HIF‐1α: a novel regulatory axis for stress‐induced pancreatic tumor growth and angiogenesis. Curr Mol Med. 2013;13(6):1023‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Xie H, Li C, He Y, Griffin R, Ye Q, Li L. Chronic stress promotes oral cancer growth and angiogenesis with increased circulating catecholamine and glucocorticoid levels in a mouse model. Oral Oncol. 2015;51(11):991‐997. [DOI] [PubMed] [Google Scholar]
- 215. Yang EV, Sood AK, Chen M, et al. Norepinephrine up‐regulates the expression of vascular endothelial growth factor, matrix metalloproteinase (MMP)‐2, and MMP‐9 in nasopharyngeal carcinoma tumor cells. Cancer Res. 2006;66(21):10357‐10364. [DOI] [PubMed] [Google Scholar]
- 216. Xia Y, Wei Y, Li ZY, et al. Catecholamines contribute to the neovascularization of lung cancer via tumor‐associated macrophages. Brain Behav Immun. 2019;81:111‐121. [DOI] [PubMed] [Google Scholar]
- 217. Felten DL, Felten SY. Sympathetic noradrenergic innervation of immune organs. Brain Behav Immun. 1988;2(4):293‐300. [DOI] [PubMed] [Google Scholar]
- 218. Le CP, Nowell CJ, Kim‐Fuchs C, et al. Chronic stress in mice remodels lymph vasculature to promote tumour cell dissemination. Nat Commun. 2016;7:10634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Shi RJ, Ke BW, Tang YL, Liang XH. Perineural invasion: a potential driver of cancer‐induced pain. Biochem Pharmacol. 2023;215:115692. [DOI] [PubMed] [Google Scholar]
- 220. Batsakis JG. Nerves and neurotropic carcinomas. Ann Otol Rhinol Laryngol. 1985;94(4 pt 1):426‐427. [PubMed] [Google Scholar]
- 221. Wang H, Zheng Q, Lu Z, et al. Role of the nervous system in cancers: a review. Cell Death Discov. 2021;7(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Huang D, Su S, Cui X, et al. Nerve fibers in breast cancer tissues indicate aggressive tumor progression. Medicine (Baltimore). 2014;93(27):e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Maru N, Ohori M, Kattan MW, Scardino PT, Wheeler TM. Prognostic significance of the diameter of perineural invasion in radical prostatectomy specimens. Hum Pathol. 2001;32(8):828‐833. [DOI] [PubMed] [Google Scholar]
- 224. Mitsunaga S, Hasebe T, Kinoshita T, et al. Detail histologic analysis of nerve plexus invasion in invasive ductal carcinoma of the pancreas and its prognostic impact. Am J Surg Pathol. 2007;31(11):1636‐1644. [DOI] [PubMed] [Google Scholar]
- 225. Schweizerhof M, Stösser S, Kurejova M, et al. Hematopoietic colony‐stimulating factors mediate tumor‒nerve interactions and bone cancer pain. Nat Med. 2009;15(7):802‐807. [DOI] [PubMed] [Google Scholar]
- 226. Selvaraj D, Gangadharan V, Michalski CW, et al. A functional role for VEGFR1 expressed in peripheral sensory neurons in cancer pain. Cancer Cell. 2015;27(6):780‐796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Hirth M, Gandla J, Höper C, et al. CXCL10 and CCL21 promote migration of pancreatic cancer cells toward sensory neurons and neural remodeling in tumors in mice, associated with pain in patients. Gastroenterology. 2020;159(2):665‐681. [DOI] [PubMed] [Google Scholar]
- 228. Swanson BJ, McDermott KM, Singh PK, Eggers JP, Crocker PR, Hollingsworth MA. MUC1 is a counter‐receptor for myelin‐associated glycoprotein (Siglec‐4a) and their interaction contributes to adhesion in pancreatic cancer perineural invasion. Cancer Res. 2007;67(21):10222‐10229. [DOI] [PubMed] [Google Scholar]
- 229. Okada Y, Takeyama H, Sato M, et al. Experimental implication of celiac ganglionotropic invasion of pancreatic‐cancer cells bearing c‐ret proto‐oncogene with reference to glial‐cell‐line‐derived neurotrophic factor (GDNF). Int J Cancer. 1999;81(1):67‐73. [DOI] [PubMed] [Google Scholar]
- 230. Lian EY, Hyndman BD, Moodley S, Maritan SM, Mulligan LM. RET isoforms contribute differentially to invasive processes in pancreatic ductal adenocarcinoma. Oncogene. 2020;39(41):6493‐6510. [DOI] [PubMed] [Google Scholar]
- 231. Roger E, Martel S, Bertrand‐Chapel A, et al. Schwann cells support oncogenic potential of pancreatic cancer cells through TGFβ signaling. Cell Death Dis. 2019;10(12):886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Na'ara S, Amit M, Gil Z. L1CAM induces perineural invasion of pancreas cancer cells by upregulation of metalloproteinase expression. Oncogene. 2019;38(4):596‐608. [DOI] [PubMed] [Google Scholar]
- 233. Bakst RL, Xiong H, Chen CH, et al. Inflammatory monocytes promote perineural invasion via CCL2‐mediated recruitment and cathepsin B expression. Cancer Res. 2017;77(22):6400‐6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Kershner LJ, Choi K, Wu J, et al. Multiple Nf1 Schwann cell populations reprogram the plexiform neurofibroma tumor microenvironment. JCI Insight. 2022;7(18):e154513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Su D, Guo X, Huang L, et al. Tumor‐neuroglia interaction promotes pancreatic cancer metastasis. Theranostics. 2020;10(11):5029‐5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Li J, Kang R, Tang D. Cellular and molecular mechanisms of perineural invasion of pancreatic ductal adenocarcinoma. Cancer Commun (Lond). 2021;41(8):642‐660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Silverman DA, Martinez VK, Dougherty PM, Myers JN, Calin GA, Amit M. Cancer‐associated neurogenesis and nerve‐cancer cross‐talk. Cancer Res. 2021;81(6):1431‐1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Dobrenis K, Gauthier LR, Barroca V, Magnon C. Granulocyte colony‐stimulating factor off‐target effect on nerve outgrowth promotes prostate cancer development. Int J Cancer. 2015;136(4):982‐988. [DOI] [PubMed] [Google Scholar]
- 239. McIlvried LA, Atherton MA, Horan NL, Goch TN, Scheff NN. Sensory neurotransmitter calcitonin gene‐related peptide modulates tumor growth and lymphocyte infiltration in oral squamous cell carcinoma. Adv Biol. 2022;6(9):e2200019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Pundavela J, Demont Y, Jobling P, et al. ProNGF correlates with Gleason score and is a potential driver of nerve infiltration in prostate cancer. Am J Pathol. 2014;184(12):3156‐3162. [DOI] [PubMed] [Google Scholar]
- 241. Deborde S, Gusain L, Powers A, et al. Reprogrammed Schwann cells organize into dynamic tracks that promote pancreatic cancer invasion. Cancer Discov. 2022;12(10):2454‐2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Mauffrey P, Tchitchek N, Barroca V, et al. Progenitors from the central nervous system drive neurogenesis in cancer. Nature. 2019;569(7758):672‐678. [DOI] [PubMed] [Google Scholar]
- 243. Aggarwal A. Hypothalamo‐pituitary‐adrenal axis and brain during stress, yoga and meditation: a review. Int J Health Clin Res. 2020;3(9):96‐103.
- 244. Ehrhart‐Bornstein M, Bornstein SR. Cross‐talk between adrenal medulla and adrenal cortex in stress. Ann N Y Acad Sci. 2008;1148:112‐117. [DOI] [PubMed] [Google Scholar]
- 245. Kvetnansky R, Sabban EL, Palkovits M. Catecholaminergic systems in stress: structural and molecular genetic approaches. Physiol Rev. 2009;89(2):535‐606. [DOI] [PubMed] [Google Scholar]
- 246. Basarrate S, Monzel AS, Smith JLM, Marsland AL, Trumpff C, Picard M. Glucocorticoid and adrenergic receptor distribution across human organs and tissues: a map for stress transduction. Psychosom Med. 2024;86(2):89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Niedzwiedz CL, Knifton L, Robb KA, Katikireddi SV, Smith DJ. Depression and anxiety among people living with and beyond cancer: a growing clinical and research priority. BMC Cancer. 2019;19(1):943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Nordin K, Berglund G, Glimelius B, Sjödén PO. Predicting anxiety and depression among cancer patients: a clinical model. Eur J Cancer. 2001;37(3):376‐384. [DOI] [PubMed] [Google Scholar]
- 249. PDQ Supportive Palliative Care Editorial Board . Adjustment to cancer: anxiety and distress (PDQ®): health professional version. PDQ Cancer Information Summaries. Bethesda (MD): National Cancer Institute (US; ); 2002. [Google Scholar]
- 250. Brandão T, Tavares R, Schulz MS, Matos PM. Measuring emotion regulation and emotional expression in breast cancer patients: a systematic review. Clin Psychol Rev. 2016;43:114‐127. [DOI] [PubMed] [Google Scholar]
- 251. Giese‐Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29(4):413‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Schrepf A, Clevenger L, Christensen D, et al. Cortisol and inflammatory processes in ovarian cancer patients following primary treatment: relationships with depression, fatigue, and disability. Brain Behav Immun. 2013(suppl 0):S126‐S134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Thomson F, Craighead M. Innovative approaches for the treatment of depression: targeting the HPA axis. Neurochem Res. 2008;33(4):691‐707. [DOI] [PubMed] [Google Scholar]
- 254. Zhang H, Yang Y, Cao Y, Guan J. Effects of chronic stress on cancer development and the therapeutic prospects of adrenergic signaling regulation. Biomed Pharmacother. 2024;175:116609. [DOI] [PubMed] [Google Scholar]
- 255. Cui B, Peng F, Lu J, et al. Cancer and stress: NextGen strategies. Brain Behav Immun. 2021;93:368‐383. [DOI] [PubMed] [Google Scholar]
- 256. Flaherty RL, Falcinelli M, Flint MS. Stress and drug resistance in cancer. Cancer Drug Resist. 2019;2(3):773‐786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Hanns P, Paczulla AM, Medinger M, Konantz M, Lengerke C. Stress and catecholamines modulate the bone marrow microenvironment to promote tumorigenesis. Cell Stress. 2019;3(7):221‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Jia Q, Zhou Y, Song L, et al. Baicalin reduces chronic stress‐induced breast cancer metastasis via directly targeting β2‐adrenergic receptor. J Pharm Anal. 2024;14(7):100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Haist M, Stege H, Grabbe S, Bros M. The functional crosstalk between myeloid‐derived suppressor cells and regulatory T cells within the immunosuppressive tumor microenvironment. Cancers (Basel). 2021;13(2):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260. Neo SY, Tong L, Chong J, et al. Tumor‐associated NK cells drive MDSC‐mediated tumor immune tolerance through the IL‐6/STAT3 axis. Sci Transl Med. 2024;16(747):eadi2952. [DOI] [PubMed] [Google Scholar]
- 261. Tang J, Li Z, Lu L, Cho CH. β‐Adrenergic system, a backstage manipulator regulating tumour progression and drug target in cancer therapy. Semin Cancer Biol. 2013;23(6 pt B):533‐542. [DOI] [PubMed] [Google Scholar]
- 262. Lempesis IG, Georgakopoulou VE, Papalexis P, Chrousos GP, Spandidos DA. Role of stress in the pathogenesis of cancer (review). Int J Oncol. 2023;63(5):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Antoni MH, Dhabhar FS. The impact of psychosocial stress and stress management on immune responses in patients with cancer. Cancer. 2019;125(9):1417‐1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Sandbank E, Eckerling A, Margalit A, Sorski L, Ben‐Eliyahu S. Immunotherapy during the immediate perioperative period: a promising approach against metastatic disease. Curr Oncol. 2023;30(8):7450‐7477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Yan J, Chen Y, Luo M, et al. Chronic stress in solid tumor development: from mechanisms to interventions. J Biomed Sci. 2023;30(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Bakos O, Lawson C, Rouleau S, Tai LH. Combining surgery and immunotherapy: turning an immunosuppressive effect into a therapeutic opportunity. J Immunother Cancer. 2018;6(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Daher C, Vimeux L, Stoeva R, et al. Blockade of β‐adrenergic receptors improves CD8(+) T‐cell priming and cancer vaccine efficacy. Cancer Immunol Res. 2019;7(11):1849‐1863. [DOI] [PubMed] [Google Scholar]
- 268. Nissen MD, Sloan EK, Mattarollo SR. β‐Adrenergic signaling impairs antitumor CD8(+) T‐cell responses to B‐cell lymphoma immunotherapy. Cancer Immunol Res. 2018;6(1):98‐109. [DOI] [PubMed] [Google Scholar]
- 269. Bucsek MJ, Qiao G, MacDonald CR, et al. β‐Adrenergic signaling in mice housed at standard temperatures suppresses an effector phenotype in CD8(+) T cells and undermines checkpoint inhibitor therapy. Cancer Res. 2017;77(20):5639‐5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Ma Y, Kroemer G. The cancer‐immune dialogue in the context of stress. Nat Rev Immunol. 2024;24(4):264‐281. [DOI] [PubMed] [Google Scholar]
- 271. Shi M, Yang Z, Hu M, et al. Catecholamine‐Induced β2‐adrenergic receptor activation mediates desensitization of gastric cancer cells to trastuzumab by upregulating MUC4 expression. J Immunol. 2013;190(11):5600‐5608. [DOI] [PubMed] [Google Scholar]
- 272. Liu D, Yang Z, Wang T, et al. β2‐AR signaling controls trastuzumab resistance‐dependent pathway. Oncogene. 2016;35(1):47‐58. [DOI] [PubMed] [Google Scholar]
- 273. Fearon DT. The expansion and maintenance of antigen‐selected CD8(+) T cell clones. Adv Immunol. 2007;96:103‐139. [DOI] [PubMed] [Google Scholar]
- 274. Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annu Rev Immunol. 2007;25:171‐192. [DOI] [PubMed] [Google Scholar]
- 275. Arens R, Schoenberger SP. Plasticity in programming of effector and memory CD8 T‐cell formation. Immunol Rev. 2010;235(1):190‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Sanders VM, Baker RA, Ramer‐Quinn DS, Kasprowicz DJ, Fuchs BA, Street NE. Differential expression of the beta2‐adrenergic receptor by Th1 and Th2 clones: implications for cytokine production and B cell help. J Immunol. 1997;158(9):4200‐4210. [PubMed] [Google Scholar]
- 277. Mueller SN. Neural control of immune cell trafficking. J Exp Med. 2022;219(3):e20211604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Nakai A, Hayano Y, Furuta F, Noda M, Suzuki K. Control of lymphocyte egress from lymph nodes through β2‐adrenergic receptors. J Exp Med. 2014;211(13):2583‐2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Devi S, Alexandre YO, Loi JK, et al. Adrenergic regulation of the vasculature impairs leukocyte interstitial migration and suppresses immune responses. Immunity. 2021;54(6):1219‐1230. [DOI] [PubMed] [Google Scholar]
- 280. Grebe KM, Hickman HD, Irvine KR, Takeda K, Bennink JR, Yewdell JW. Sympathetic nervous system control of anti‐influenza CD8+ T cell responses. Proc Natl Acad Sci U S A. 2009;106(13):5300‐5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Kohm AP, Sanders VM. Norepinephrine and beta 2‐adrenergic receptor stimulation regulate CD4+ T and B lymphocyte function in vitro and in vivo. Pharmacol Rev. 2001;53(4):487‐525. [PubMed] [Google Scholar]
- 282. Rosas‐Ballina M, Olofsson PS, Ochani M, et al. Acetylcholine‐synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334(6052):98‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Shurin MR, Shurin GV, Zlotnikov SB, Bunimovich YL. The neuroimmune axis in the tumor microenvironment. J Immunol. 2020;204(2):280‐285. [DOI] [PubMed] [Google Scholar]
- 284. Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421(6921):384‐388. [DOI] [PubMed] [Google Scholar]
- 285. Pilatova K, Bencsikova B, Demlova R, Valik D, Zdrazilova‐Dubska L. Myeloid‐derived suppressor cells (MDSCs) in patients with solid tumors: considerations for granulocyte colony‐stimulating factor treatment. Cancer Immunol Immunother. 2018;67(12):1919‐1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Tcyganov E, Mastio J, Chen E, Gabrilovich DI. Plasticity of myeloid‐derived suppressor cells in cancer. Curr Opin Immunol. 2018;51:76‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Law AMK, Valdes‐Mora F, Gallego‐Ortega D. Myeloid‐derived suppressor cells as a therapeutic target for cancer. Cells. 2020;9(3):561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Ma T, Renz BW, Ilmer M, et al. Myeloid‐derived suppressor cells in solid tumors. Cells. 2022;11(2):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Zhao Y, Wu T, Shao S, Shi B, Zhao Y. Phenotype, development, and biological function of myeloid‐derived suppressor cells. Oncoimmunology. 2016;5(2):e1004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Mohammadpour H, MacDonald CR, McCarthy PL, Abrams SI, Repasky EA. β2‐Adrenergic receptor signaling regulates metabolic pathways critical to myeloid‐derived suppressor cell function within the TME. Cell Rep. 2021;37(4):109883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Xiao L, Li X, Fang C, Yu J, Chen T. Neurotransmitters: promising immune modulators in the tumor microenvironment. Front Immunol. 2023;14:1118637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292. Mohammadpour H, MacDonald CR, Qiao G, et al. β2 adrenergic receptor‐mediated signaling regulates the immunosuppressive potential of myeloid‐derived suppressor cells. J Clin Invest. 2019;129(12):5537‐5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Dantzer R. Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev. 2018;98(1):477‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Peng WT, Sun WY, Li XR, Sun JC, Du JJ, Wei W. Emerging roles of G protein‐coupled receptors in hepatocellular carcinoma. Int J Mol Sci. 2018;19(5):1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Nevin JT, Moussa M, Corwin WL, Srivastava PK. Sympathetic nervous tone limits the development of myeloid‐derived suppressor cells. Sci Immunol. 2020;5(51):eaay9368. [DOI] [PubMed] [Google Scholar]
- 296. Singh A, Ranjan A. Adrenergic receptor signaling regulates the CD40‐receptor mediated anti‐tumor immunity. Front Immunol. 2023;14:1141712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Zhang Y, Brekken RA. Direct and indirect regulation of the tumor immune microenvironment by VEGF. J Leukoc Biol. 2022;111(6):1269‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Sorrentino C, Miele L, Porta A, Pinto A, Morello S. Myeloid‐derived suppressor cells contribute to A2B adenosine receptor‐induced VEGF production and angiogenesis in a mouse melanoma model. Oncotarget. 2015;6(29):27478‐27489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Chen SH, Zhang BY, Zhou B, Zhu CZ, Sun LQ, Feng YJ. Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am J Cancer Res. 2019;9(1):1‐21. [PMC free article] [PubMed] [Google Scholar]
- 300. Nguyen TM, Ngoc DTM, Choi JH, Lee CH. Unveiling the neural environment in cancer: exploring the role of neural circuit players and potential therapeutic strategies. Cells. 2023;12(15):1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Wang W, Li L, Chen N, et al. Nerves in the tumor microenvironment: origin and effects. Front Cell Dev Biol. 2020;8:601738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Sloan EK, Priceman SJ, Cox BF, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70(18):7042‐7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303. Partecke LI, Günther C, Hagemann S, et al. Induction of M2‐macrophages by tumour cells and tumour growth promotion by M2‐macrophages: a quid pro quo in pancreatic cancer. Pancreatology. 2013;13(5):508‐516. [DOI] [PubMed] [Google Scholar]
- 304. Cheng Y, Tang XY, Li YX, et al. Depression‐induced neuropeptide Y secretion promotes prostate cancer growth by recruiting myeloid cells. Clin Cancer Res. 2019;25(8):2621‐2632. [DOI] [PubMed] [Google Scholar]
- 305. Zhu Y, Herndon JM, Sojka DK, et al. Tissue‐resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity. 2017;47(2):323‐338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405(6785):458‐562. [DOI] [PubMed] [Google Scholar]
- 307. Dubeykovskaya Z, Si Y, Chen X, et al. Neural innervation stimulates splenic TFF2 to arrest myeloid cell expansion and cancer. Nat Commun. 2016;7:10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Kim JK, Kim YS, Lee HM, et al. GABAergic signaling linked to autophagy enhances host protection against intracellular bacterial infections. Nat Commun. 2018;9(1):4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Huang D, Wang Y, Thompson JW, et al. Cancer‐cell‐derived GABA promotes β‐catenin‐mediated tumour growth and immunosuppression. Nat Cell Biol. 2022;24(2):230‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Spranger S, Dai D, Horton B, Gajewski TF. Tumor‐residing Batf3 dendritic cells are required for effector T cell trafficking and adoptive T cell therapy. Cancer Cell. 2017;31(5):711‐723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Zhang B, Vogelzang A, Miyajima M, et al. B cell‐derived GABA elicits IL‐10(+) macrophages to limit anti‐tumour immunity. Nature. 2021;599(7885):471‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Wu X, Shen Q, Chang H, Li J, Xing D. Promoted CD4(+) T cell‐derived IFN‐γ/IL‐10 by photobiomodulation therapy modulates neurogenesis to ameliorate cognitive deficits in APP/PS1 and 3xTg‐AD mice. J Neuroinflammation. 2022;19(1):253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23‐44. [DOI] [PubMed] [Google Scholar]
- 314. Gungabeesoon J, Gort‐Freitas NA, Kiss M, et al. A neutrophil response linked to tumor control in immunotherapy. Cell. 2023;186(7):1448‐1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Jaillon S, Ponzetta A, Di Mitri D, Santoni A, Bonecchi R, Mantovani A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat Rev Cancer. 2020;20(9):485‐503. [DOI] [PubMed] [Google Scholar]
- 316. Xue R, Zhang Q, Cao Q, et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature. 2022;612(7938):141‐147. [DOI] [PubMed] [Google Scholar]
- 317. Li J, Che M, Zhang B, Zhao K, Wan C, Yang K. The association between the neuroendocrine system and the tumor immune microenvironment: emerging directions for cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2023;1878(6):189007. [DOI] [PubMed] [Google Scholar]
- 318. Shalabi S, Belayachi A, Larrivée B. Involvement of neuronal factors in tumor angiogenesis and the shaping of the cancer microenvironment. Front Immunol. 2024;15:1284629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Sookhai S, Wang JH, McCourt M, O'Connell D, Redmond HP. Dopamine induces neutrophil apoptosis through a dopamine D‐1 receptor‐independent mechanism. Surgery. 1999;126(2):314‐322. [PubMed] [Google Scholar]
- 320. Burfeind KG, Zhu X, Norgard MA, et al. Circulating myeloid cells invade the central nervous system to mediate cachexia during pancreatic cancer. Elife. 2020;9:. e54095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Zha C, Meng X, Li L, et al. Neutrophil extracellular traps mediate the crosstalk between glioma progression and the tumor microenvironment via the HMGB1/RAGE/IL‐8 axis. Cancer Biol Med. 2020;17(1):154‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322. Gilardi M, Ramos M, Hollern D. B cells secrete GABA, which provokes a pro‐tumor immune microenvironment. Cancer Cell. 2022;40(1):17‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Zhang X, Lei B, Yuan Y, et al. Brain control of humoral immune responses amenable to behavioural modulation. Nature. 2020;581(7807):204‐208. [DOI] [PubMed] [Google Scholar]
- 324. Shi DD, Guo JA, Hoffman HI, et al. Therapeutic avenues for cancer neuroscience: translational frontiers and clinical opportunities. Lancet Oncol. 2022;23(2):e62‐e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Demir IE, Reyes CM, Alrawashdeh W, et al. Clinically actionable strategies for studying neural influences in cancer. Cancer Cell. 2020;38(1):11‐14. [DOI] [PubMed] [Google Scholar]
- 326. Qiao G, Chen M, Bucsek MJ, Repasky EA, Hylander BL. Adrenergic signaling: a targetable checkpoint limiting development of the antitumor immune response. Front Immunol. 2018;9:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Joensuu H, Gligorov J. Adjuvant treatments for triple‐negative breast cancers. Ann Oncol. 2012;23(suppl 6):vi40‐vi45. [DOI] [PubMed] [Google Scholar]
- 328. Florent R, Poulain L, N'Diaye M. Drug repositioning of the α(1)‐adrenergic receptor antagonist naftopidil: a potential new anti‐cancer drug? Int J Mol Sci. 2020;21(15):5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Wang J, Lu S, Meng Y, Fu W, Zhou X. Beta adrenergic blockade and clinical outcomes in patients with colorectal cancer: a systematic review and meta‐analysis. Eur J Pharmacol. 2022;929:175135. [DOI] [PubMed] [Google Scholar]
- 330. Puzderova B, Belvoncikova P, Grossmannova K, et al. Promising chemosensitizer and candidate for the combined therapy through disruption of tumor microenvironment homeostasis by decreasing the level of carbonic anhydrase IX. Int J Mol Sci. 2023;24(13):11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Di Fonte R, Strippoli S, Garofoli M, et al. Cervical cancer benefits from trabectedin combination with the β‐blocker propranolol: in vitro and ex vivo evaluations in patient‐derived organoids. Front Cell Dev Biol. 2023;11:1178316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Farhoumand LS, Liu H, Tsimpaki T, et al. Blockade of ß‐adrenergic receptors by nebivolol enables tumor control potential for uveal melanoma in 3D tumor spheroids and 2D cultures. Int J Mol Sci. 2023;24(6):5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Liang J, Seghiri M, Singh PK, et al. The β2‐adrenergic receptor associates with CXCR4 multimers in human cancer cells. Proc Natl Acad Sci U S A. 2024;121(14):e2304897121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Yu J, Li M, Ren B, et al. Unleashing the efficacy of immune checkpoint inhibitors for advanced hepatocellular carcinoma: factors, strategies, and ongoing trials. Front Pharmacol. 2023;14:1261575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T‐cell infiltration. Theranostics. 2021;11(11):5365‐5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Montani L, Petrinovic MM. Targeting axonal regeneration: the growth cone takes the lead. J Neurosci. 2014;34(13):4443‐4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Le TT, Oudin MJ. Understanding and modeling nerve‒cancer interactions. Dis Model Mech. 2023;16(1):dmm049729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Sang Q, Sun D, Chen Z, Zhao W. NGF and PI3K/Akt signaling participate in the ventral motor neuronal protection of curcumin in sciatic nerve injury rat models. Biomed Pharmacother. 2018;103:1146‐1153. [DOI] [PubMed] [Google Scholar]
- 339. Peach CJ, Tonello R, Gomez K, et al. Neuropilin‐1 is a co‐receptor for NGF and TrkA‐evoked pain. bioRxiv. 2024. [Google Scholar]
- 340. Patel TD, Jackman A, Rice FL, Kucera J, Snider WD. Development of sensory neurons in the absence of NGF/TrkA signaling in vivo. Neuron. 2000;25(2):345‐357. [DOI] [PubMed] [Google Scholar]
- 341. Bimonte S, Cascella M, Forte CA, Esposito G, Cuomo A. The role of anti‐nerve growth factor monoclonal antibodies in the control of chronic cancer and non‐cancer pain. J Pain Res. 2021;14:1959‐1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Kelleher JH, Tewari D, McMahon SB. Neurotrophic factors and their inhibitors in chronic pain treatment. Neurobiol Dis. 2017;97:127‐138. [DOI] [PubMed] [Google Scholar]
- 343. Lei Y, Tang L, Xie Y, et al. Gold nanoclusters‐assisted delivery of NGF siRNA for effective treatment of pancreatic cancer. Nat Commun. 2017;8:15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Lillemoe KD, Cameron JL, Kaufman HS, Yeo CJ, Pitt HA, Sauter PK. Chemical splanchnicectomy in patients with unresectable pancreatic cancer. A prospective randomized trial. Ann Surg. 1993;217(5):456‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345. Coarfa C, Florentin D, Putluri N, et al. Influence of the neural microenvironment on prostate cancer. Prostate. 2018;78(2):128‐139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Vudatha V, Herremans KM, Freudenberger DC, Liu C, Trevino JG. In vivo models of pancreatic ductal adenocarcinoma. Adv Cancer Res. 2023;159:75‐112. [DOI] [PubMed] [Google Scholar]
- 347. Guo S, Gao S, Liu R, et al. Oncological and genetic factors impacting PDX model construction with NSG mice in pancreatic cancer. FASEB J. 2019;33(1):873‐884. [DOI] [PubMed] [Google Scholar]
- 348. Lai Y, Wei X, Lin S, Qin L, Cheng L, Li P. Current status and perspectives of patient‐derived xenograft models in cancer research. J Hematol Oncol. 2017;10(1):106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Navari RM, Aapro M. Antiemetic prophylaxis for chemotherapy‐induced nausea and vomiting. N Engl J Med. 2016;374(14):1356‐1367. [DOI] [PubMed] [Google Scholar]
- 350. Boutros C, Tarhini A, Routier E, et al. Safety profiles of anti‐CTLA‐4 and anti‐PD‐1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473‐486. [DOI] [PubMed] [Google Scholar]
- 351. Fucà G, Galli G, Poggi M, et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non‐small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open. 2019;4(1):e000457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death‐1 and programmed death‐ligand 1 blockade in patients with non‐small‐cell lung cancer. J Clin Oncol. 2018;36(28):2872‐2878. [DOI] [PubMed] [Google Scholar]
- 353. Scott SC, Pennell NA. Early use of systemic corticosteroids in patients with advanced NSCLC treated with nivolumab. J Thorac Oncol. 2018;13(11):1771‐1775. [DOI] [PubMed] [Google Scholar]
- 354. Boucherit N, Gorvel L, Olive D. 3D tumor models and their use for the testing of immunotherapies. Front Immunol. 2020;11:603640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Yuan J, Li X, Yu S. Cancer organoid co‐culture model system: novel approach to guide precision medicine. Front Immunol. 2022;13:1061388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Urzì O, Gasparro R, Costanzo E, et al. Three‐dimensional cell cultures: the bridge between in vitro and in vivo models. Int J Mol Sci. 2023;24(15):12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357. Li L, Hanahan D. Hijacking the neuronal NMDAR signaling circuit to promote tumor growth and invasion. Cell. 2013;153(1):86‐100. [DOI] [PubMed] [Google Scholar]
- 358. Venkataramani V, Tanev DI, Strahle C, et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature. 2019;573(7775):532‐538. [DOI] [PubMed] [Google Scholar]
- 359. Venkatesh HS, Morishita W, Geraghty AC, et al. Electrical and synaptic integration of glioma into neural circuits. Nature. 2019;573(7775):539‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Stokholm MG, Høyer S, Borre M, et al. Molecular imaging of cholinergic processes in prostate cancer using ¹¹C‐donepezil and ¹⁸F‐FEOBV. Eur J Nucl Med Mol Imaging. 2016;43(5):906‐910. [DOI] [PubMed] [Google Scholar]
- 361. Werner C, Sauer M, Geis C. Super‐resolving microscopy in neuroscience. Chem Rev. 2021;121(19):11971‐12015. [DOI] [PubMed] [Google Scholar]
- 362. Hierro‐Bujalance C, Bacskai BJ, Garcia‐Alloza M. In vivo imaging of microglia with multiphoton microscopy. Front Aging Neurosci. 2018;10:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Richardson DS, Guan W, Matsumoto K, et al. Tissue clearing. Nat Rev Methods Primers. 2021;1(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Ueda HR, Ertürk A, Chung K, et al. Tissue clearing and its applications in neuroscience. Nat Rev Neurosci. 2020;21(2):61‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Tetzlaff SK, Reyhan E, Bengtson CP, et al. Characterizing and targeting glioblastoma neuron‐tumor networks with retrograde tracing. bioRxiv. 2024. doi: 10.1101/2024.03.18.585565 [DOI] [Google Scholar]
- 366. Perdyan A, Lawrynowicz U, Horbacz M, Kaminska B, Mieczkowski J. Integration of single‐cell RNA sequencing and spatial transcriptomics to reveal the glioblastoma heterogeneity. F1000Res. 2022;11:1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Jin Y, Zuo Y, Li G, et al. Advances in spatial transcriptomics and its applications in cancer research. Mol Cancer. 2024;23(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Masarapu Y, Cekanaviciute E, Andrusivova Z, et al. Spatially resolved multiomics on the neuronal effects induced by spaceflight in mice. Nat Commun. 2024;15(1):4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


