Abstract
This phase 3, observer-blinded, non-inferiority randomized trial (ClinicalTrials.gov: NCT05517642), conducted from September 2022 to May 2023 at three Malaysian sites, involved 540 adults previously vaccinated with three COVID-19 doses. Participants were randomized 1:1 to receive either one dose of inhaled Recombinant COVID-19 Vaccine (Ad5-nCoV-IH) or intramuscular tozinameran (BNT-IM). The study assessed safety, vaccine efficacy (VE) and immunogenicity against SARS-CoV-2 variants. The primary outcome was the non-inferiority of anti-spike protein receptor-binding domain (S-RBD IgG) antibodies, with a 97.5% confidence interval lower limit for the geometric mean concentration (GMC) ratio >0.67. Ad5-nCoV-IH showed lower immunogenicity than BNT-IM, with a GMC ratio of 0.22 and a seroconversion rate difference of -71.91%. Adverse drug reactions (ADRs) were less frequent with Ad5-nCoV-IH (39.26%) compared to BNT-IM (64.68%). No serious vaccine-related adverse events were reported. Both vaccines had comparable efficacy against COVID-19 variants. This study was funded by Tianjin Biomedical Science and Technology Major Project.
Subject terms: DNA vaccines, Viral infection, Randomized controlled trials, Drug delivery
Introduction
The global COVID-19 response has yielded vaccines utilizing mRNA1, viral vector2,3, and inactivated vaccine4 technologies, which are crucial in reducing disease severity and transmission. However, real-world data indicate waning immunity, prompting booster doses to sustain protection5–7. Sub-variants within Omicron further complicate the landscape8–10. Strategies like heterologous vaccines11–14, optimisation of regimens15, and updated immunogens16, are being explored, yet breakthrough infections rise17,18, prompting the need for next-generation vaccines designated to stimulate comprehensive immunity, encompassing both mucosal and systemic immune responses19,20. However, designing vaccines that can effectively trigger strong and protective immune responses in the respiratory mucosa has posed a continual challenge, leading most licensed vaccines to date relying on systemic innate and adaptive immunity, are administered intramuscularly21. A booster regimen using mRNA vaccines has already been deployed by governments around the world. Subsequent studies found the booster dose to be safe, immunogenic22,23, and effective against variants24. Additionally, studies have highlighted the advantages of administering vaccines to the respiratory tract mucosa, inducing both systemic and local immune response25. Specifically, local immune response, including immunoglobulin G (IgG) and secretory immunoglobulin A (SIgA) antibodies and cellular immune response can help prevent infections at the site of entry, and the neutralizing antibody produced can further activate mucosal tissue-resident memory T cells and trained airway macrophages, resulting in localized immunity and fewer systemic side effects26–28. Animal studies have supported the protective efficacy of single mucosal vaccination with adenovirus-vectored COVID-19 vaccines, including Recombinant Covid-19 Vaccine (Adenovirus Type 5 Vector) (Ad5-nCoV), against wild-type SARS-CoV-2 replication in the upper respiratory tract29. Previous clinical trials on Recombinant Covid-19 Vaccine (Adenovirus Type 5 Vector) for Inhalation (Ad5-nCoV-IH) have demonstrated good safety and immunogenicity23,30. Here we conducted this randomized, observer-blinded controlled trial to assess the safety, immunogenicity, and efficacy of Ad5-nCoV-IH by mouth as a second booster vaccination, evaluating its ability to prevent breakthrough infections caused by both wild-type and Omicron variants. A non-inferiority trial design was employed to demonstrate whether Ad5-nCoV-IH can provide comparable clinical outcomes to intramuscular tozinameran vaccine (BNT-IM) while potentially offering additional advantages. Both the vaccines were based on original strain of SARS-CoV-2.
It was hypothesized in terms of non-inferiority immunogenicity hypothesis testing that Ad5-nCoV-IH is not clinically inferior to BNT-IM. With non-inferiority margin of 0.67, if the lower bound of the 97.5% confidence interval (CI) for the ratio of the geometric mean concentration (GMC) of anti-spike protein receptor-binding domain (S-RBD IgG) antibodies induced by Ad5-nCoV-IH to BNT-IM is greater than 0.67, the Ad5-nCoV-IH is considered non-inferior.
Results
Trial population
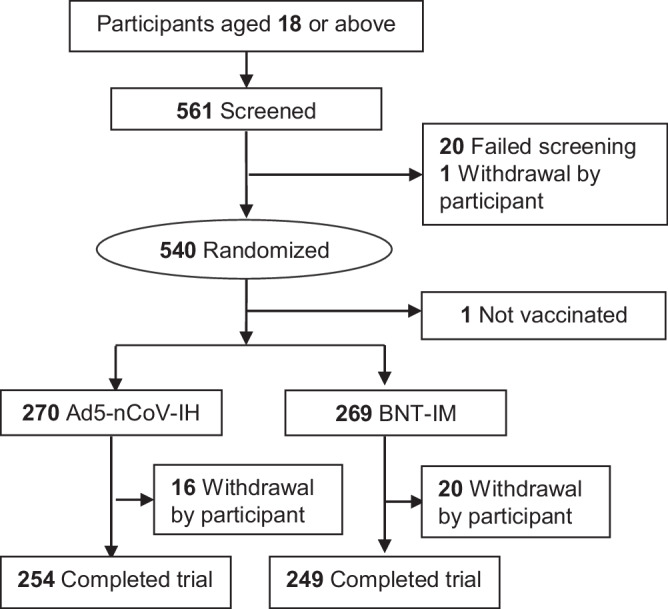
Between September 20 and November 10, 2022, 561 participants were screened, of which 20 were ineligible to take part in the study and one withdrew consent before vaccination. 270 participants received Ad5-nCoV-IH, of which 16 dropped out early and 254 completed the trial among whom the mean age was 34.5 (standard deviation (SD), 10.73) years, with 61.85% male. 270 participants randomized to BNT-IM, with one withdrawn consent before administration of vaccines. 269 participants received BNT-IM, of which 20 dropped out early and 249 completed the trial among whom a mean age was 34.4 (SD, 10.60) years, with 59.48% male (Fig. 1; Table 1). The distribution of gender, age, and race among these two groups was well-balanced. Participants of 25.56% receiving AZ + AZ + X (AZ: COVID-19 Vaccine, ChAdOx1-S, Recombinant; X: the first booster dose of any brand), 39.26% receiving mRNA+mRNA+X (mRNA: tozinameran vaccine), 32.58% receiving ICV + ICV + X (ICV: COVID-19 Vaccine [Vero Cell], Inactivated), and 2.59% having received a COVID-19 vaccine outside these categories in the Ad5-nCoV-IH group. In comparison, the BNT-IM group had percentages of 18.59%, 42.01%, 36.43%, and 2.97% for similar vaccine types, respectively. The median duration from the primary series to the first booster for all participants was 168 days, and that from the first to the second booster was 274 days (Table 1).
Fig. 1. Participant disposition flow chart.
BNT-IM Intramuscular tozinameran vaccine, Ad5-nCoV-IH Recombinant Covid-19 Vaccine (Adenovirus Type 5 Vector) for Inhalation.
Table 1.
Baseline characteristics of analysis population
| Ad5-nCoV-IH | BNT-IM | Total | ||
|---|---|---|---|---|
| Gender | ||||
| Male | n(%) | 167(61.85) | 160(59.48) | 327(60.67) |
| Female | n(%) | 103(38.15) | 109(40.52) | 212(39.33) |
| Total | n(miss) | 270(0) | 269(0) | 539(0) |
| Age | ||||
| Mean(SD) | 34.5(10.73) | 34.4(10.60) | 34.5(10.66) | |
|
Median Q1,Q3 |
32.0 26.0,41.0 |
32.0 26.0,41.0 |
32.0 26.0,41.0 |
|
| Race | ||||
| Malay | n(%) | 205(75.93) | 208(77.32) | 413(76.62) |
| Chinese | n(%) | 42(15.56) | 43(15.99) | 85(15.77) |
| Indian | n(%) | 7(2.59) | 6(2.23) | 13(2.41) |
| Other | n(%) | 16(5.93) | 12(4.46) | 28(5.19) |
| Total | n(miss) | 270(0) | 269(0) | 539(0) |
| History of RT-PCR/RTK-confirmed SARS-CoV-2 infections | ||||
| Yes | n(%) | 145(53.70) | 139(51.67) | 284(52.69) |
| No | n(%) | 125(46.30) | 130(48.33) | 255(47.31) |
| Total | n(miss) | 270(0) | 269(0) | 539(0) |
| Vaccination history grouping | ||||
| AZ + AZ + X | n(%) | 69(25.56) | 50(18.59) | 119(22.08) |
| mRNA+mRNA+X | n(%) | 106(39.26) | 113(42.01) | 219(40.63) |
| ICV + ICV + X | n(%) | 88(32.58) | 98(36.43) | 186(34.52) |
| Others | n(%) | 7(2.59) | 8(2.97) | 15(2.78) |
| Total | n(miss) | 270(0) | 269(0) | 539(0) |
| Interval between second injection in the primary series immunization and the first booster, days | ||||
| n(miss) | 270(0) | 269(0) | 539(0) | |
| Mean(SD) | 167.7(40.39) | 171.9(37.97) | 169.8(39.22) | |
| Median | 166.0 | 169.0 | 168.0 | |
| Q1,Q3 | 139.0,190.0 | 142.0,199.0 | 140.0,196.0 | |
| Min,Max | 58,330 | 83,280 | 58,330 | |
| Interval between first booster and second booster, days | ||||
| n(miss) | 270(0) | 269(0) | 539(0) | |
| Mean(SD) | 279.1(39.81) | 282.1(43.42) | 280.6(41.63) | |
| Median | 274.0 | 275.0 | 274.0 | |
| Q1,Q3 | 256.0,305.0 | 256.0,313.0 | 256.0,312.0 | |
| Min,Max | 116,363 | 149,449 | 116,449 | |
| Pre-existing immunity, GMC of anti-spike RBD IgG antibodies (Wild-type) PPS | ||||
| n(miss) | 270(0) | 268(0) | 539(0) | |
| Mean(SD) | 1273.45 (3.11) | 1231.70 (3.41) | 1252.48 (3.25) | |
| Median | 1335.59 | 1283.77 | 1306.73 | |
| Q1,Q3 | 572.00,2975.97 | 510.775,2930.177 | 563.25,2973.38 | |
| Min,Max | 27.79,16431.60 | 2.67,68418.90 | 2.67,68418.90 | |
| 95%CI | 1111.45,1459.07 | 1062.93,1427.27 | 1133.35,1384.13 | |
| Pre-existing immunity, GMT of neutralizing antibodies against Omicron Pseudovirus variants (BA.4/5) (PPS) | ||||
| n(miss) | 270(0) | 268(0) | 538(0) | |
| Mean(SD) | 286.92(3.76) | 319.38(3.95) | 302.65(3.85) | |
| Median | 301.45 | 322.49 | 313.00 | |
| Q1,Q3 | 124.00,700.00 | 145.00,854.98 | 132.00,751.00 | |
| Min,Max | 15.0,30882.0 | 15.0,45953.0 | 15.0,45953.0 | |
| 95%CI | 244.82, 336.26 | 270.75,376.75 | 269.98,339.28 | |
n actual number of participants observed and the corresponding percentage, RT-PCR/RTK real-time reverse transcription-polymerase chain reaction/rapid test kit, AZ = COVID-19 Vaccine ChAdOx1-S Recombinant, X first booster dose of any vaccine, mRNA tozinameran vaccine, ICV COVID-19 Vaccine (Vero Cell) Inactivated, GMC geometric mean concentration, Anti-spike RBD IgG anti-spike receptor-binding domain immunoglobulin G, PPS per-protocol set, GMT geometric mean titre, Ad5-nCoV-IH Recombinant Covid-19 Vaccine (Adenovirus Type 5 Vector) for Inhalation, BNT-IM intramuscular tozinameran vaccine.
Primary outcomes
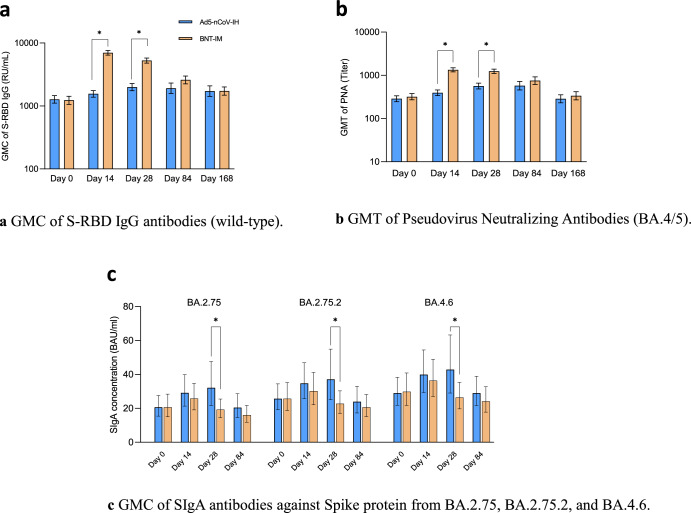
All 539 participants underwent primary immunogenicity assessments, and persistent immunogenicity assessments (at additional visits of week 12 and 24) were conducted on 139 participants in the experimental group and 141 in the control group. Results based on the per-protocol set (PPS) in Fig. 2a (refer to Supplementary Table 1 and 2 for PPS and mITT set, respectively) revealed that the GMC of S-RBD IgG antibodies against the Wild-type in the Ad5-nCoV-IH group rose from 1273.454 (95% CI, 1111.45-1459.07) at the start to 1555.533 (95% CI, 1377.17-1757.00) by day 14, representing a geometric mean increase (GMI) of 1.23 (SD, 2.09). In comparison, the BNT-IM group rose from 1231.701 (95% CI, 1062.93-1427.27) before vaccination to 6975.264 (95% CI, 6407.51-7593.33), with the GMI was 5.74 (SD, 2.74) (Supplementary Table 3). The seroconversion rate (SCR) were 17.10% (95% CI, 12.80%- 22.14%) for the Ad5-nCoV-IH group and 89.02% (95% CI, 84.61%-92.52%) for the BNT-IM group at 14 days (Supplementary Table 4). Analysis showed that the GMC ratio between the Ad5-nCoV-IH and BNT-IM groups was 0.22 (97.5% CI, 0.19-0.26) and a difference in SCR was -71.91% (95% CI, -78.63%-65.19%), suggesting that the Ad5-nCoV-IH vaccine induced lower humoral immune responses compared to the BNT-IM vaccine at 14 days post-vaccination (Supplementary Table 5). However, unlike the rapid response seen with BNT-IM at day 14 post-vaccination, the Ad5-nCoV-IH group reached its peak antibody levels at day 28, with a proportion of 27.61% (95% CI, 22.35%-33.38%). By day 168 post-vaccination, both vaccine groups showed similar levels of antibodies with no significant difference.
Fig. 2. Immune responses elicited by a second booster vaccination.
Ad5-nCoV-IH Recombinant Covid-19 Vaccine (Adenovirus Type 5 Vector) for Inhalation, BNT-IM intramuscular tozinameran vaccine, Anti-spike RBD IgG anti-spike receptor binding domain immunoglobulin G, GMC geometric mean concentration, GMT geometric mean titer, PNA pseudo neutralization assay, SIgA secretory Immunoglobulin A. a GMC of S-RBD IgG antibodies (wild-type). b GMT of Pseudovirus Neutralizing Antibodies (BA.4/5). The blue bar is Ad5-nCoV-IH group among which primary immunogenicity assessments were performed at Day 0, Day 14, and Day 28 for 270 participants, and persistent immunogenicity assessments were performed at Day 84 and Day 168 for 139 participants. The orange bar is BNT-IM group among which primary immunogenicity assessments were performed at Day 0, Day 14, and Day 28 for 268 participants, and persistent immunogenicity assessments were performed at Day 84 and Day 168 for 141 participants. c GMC of SIgA antibodies against Spike protein from BA.2.75, BA.2.75.2, and BA.4.6. The blue bar is Ad5-nCoV-IH group among which immunogenicity assessments were performed at Day 0, Day 14, Day 28, and Day 84 for 44 participants. The orange bar is BNT-IM group of which 44 participants’ immunogenicity assessments were performed at Day 0, Day 14, Day 28 and Day 84. * Indicates statistically significant differences between the Ad5-nCoV-IH group and the BNT-IM group (p < 0.05).
According to the PPS-based analysis, the GMC ratio of the anti-spike RBD IgG antibodies (wild-type) between the Ad5-nCoV-IH and BNT-IM groups was 0.22 (95% CI: 0.19, 0.26) at 14 days after vaccination, with the lower limit of the 97.5% CI < 0.67, which concluded that the antibody GMC non-inferiority hypothesis is not valid.
Secondary outcomes
Neutralizing antibodies (NAb) against Omicron BA.4/5 pseudovirus exhibited a pattern similar to that of S-RBD IgG antibodies. Participants in the experimental group showed a modest increase in geometric mean titre (GMT) from 286.92 (95% CI, 244.82–336.26) before vaccination to 395.3 (95% CI, 340.64–458.62) at day 14, peaking at 574.9 (95% CI, 457.54–722.31) on day 84, while the control group exhibited a sharp rise from 319.38 (95% CI, 270.75–376.75) pre-vaccination to a peak level of 1338.8 (95% CI, 1200.13–1493.50) by day 14, decreasing dramatically to 752.8 (95% CI, 616.45–919.24) at day 84 (Fig. 2b). Supplementary Table 6 and 7 showed the data for mITT and PPS set. The GMIs and SCRs were shown in Supplementary Table 8 and Supplementary Table 9. Collectively, the differences in GMT, GMI and SCR between the two groups were statistically significant at 14 and 28 days but not at 84 and 168 days after vaccination.
Considering the potential of respiratory tract delivery to induce mucosal immunity, our investigation extended to examine SIgA antibodies targeting the spike protein of the wild-type and also nine Variants of Concern (VOCs), B.1.1.529, BA.2.75, BA.2.75.2, BA.4.6, BA.5, BF.7, BQ.1, BQ.1.1, and XBB.1, involving a total of 88 participants with consecutive participant IDs (excluding those with positive COVID-19 cases post-vaccination). Seven participants were excluded due to invalid results, resulting in 42 from the Ad5-nCoV-IH group and 39 from the BNT-IM group. As depicted in Fig. 2c, besides Ad5-nCoV-IH demonstrated certain advantage and significant difference compared to BNT-IM in GMC of SIgA against BA.2.75 (32.072 (95% CI, 21.63–47.56) vs. 19.217 (95% CI, 14.49–25.49)), BA2.75.2 (37.085 (95% CI, 25.06–54.88) vs. 22.696 (95% CI, 16.99–30.33)) and BA.4.6 (42.832 (95% CI, 29.02–63.23) vs. 26.371 (95% CI, 19.70–35.30)) respectively on Day 28 post-vaccination (Supplementary Table 10), a more extensive advantage with significant difference was observed as well in the GMI encompassing BA.2.75, BA.2.75.2, BA.4.6, BA.5, BF.7, BQ.1, BQ.1.1 and XBB.1, respectively on Day 28 (Supplementary Table 11).
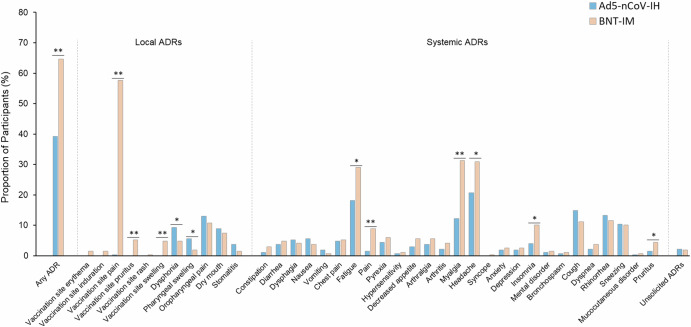
In the Ad5-nCoV-IH group, 127 participants (47.04%) experienced 817 episodes of adverse event (AE) with 106 participants (39.26%) having 515 vaccine related AE, referred to as adverse drug reactions (ADRs), within 28 days post-vaccination. In the BNT-IM group, 189 participants (70.26%) experienced 1171 episodes of AEs with 174 participants (64.68%) reporting 892 ADRs. Although statistical tests for safety parameters were not predefined in the protocol, we conducted them and found statistically significant differences in the number of AEs and ADRs between the two groups (P < 0.001), with the BNT-IM group showing a higher incidence (Fig. 3 for ADRs and Supplementary Table 12 for AEs). As Ad5-nCoV-IH was administered through oral inhalation, oropharyngeal pain, pharyngeal swelling, dysphonia, dry mouth, and stomatitis were specifically attributed to local ADR in the Ad5-nCoV-IH group. In terms of local ADRs, oropharyngeal pain was the most frequently reported in the Ad5-nCoV-IH group at 12.96%. In terms of other local ADRs in this group, dysphonia (9.26%), dry mouth (8.89%), pharyngeal swelling (5.56%), and stomatitis (3.70%) were frequently observed, with only dysphonia and pharyngeal swelling showing a statistically significant difference (P < 0.05) when compared to the BNT-IM group, despite being categorized as systemic reactions. For the BNT-IM group, vaccination site pain was the predominant local ADR at 57.62%. The occurrence rates of pruritus, swelling, erythema, induration, and rash as local ADRs were 5.20%, 4.83%, 1.49%, 1.49%, and 0.37%, respectively. There was a case from the Ad5-nCoV-IH group where vaccination site pain was reported as a local reaction, and the incidence was 0.37%. Ad5-nCoV-IH resulted in fewer systemic ADRs, showing lower frequencies for headache (20.74%), fatigue (18.15%), myalgia (12.22%), insomnia (4.07%), pain (1.48%), and pruritus (1.48%). In comparison, the BNT-IM group reported higher numbers for these systemic ADRs, with rates of 30.86%, 29.00%, 31.23%, 10.04%, 8.92%, and 4.46%, respectively. However, there were no significant differences in the incidence of grade 3 or higher ADRs (P = 0.788) and unsolicited ADRs (P = 0.765). No vaccine related serious adverse events (SAEs), adverse event of special interest (AESIs), withdrawal-related events, or deaths occurred (Supplementary Table 13 and Supplementary Table 14).
Fig. 3. Solicited and unsolicited ADRs emerged in the 0 to 28 Days post booster vaccination (SS).
Percentage of participants experienced ADR(s). The analysis was based on the safety cohort (SS), included all randomized participants who received the booster vaccination and had at least one evaluation data available (270 participants from the Ad5-nCoV-IH group and 269 participants from the BNT-IM group). *P < 0.05, **P < 0.001.
Starting from 14 days after the second booster vaccination, there were 22 confirmed COVID-19 cases in the Ad5-nCoV-IH group compared to 26 cases in the BNT-IM group, with a median follow-up period of 60 days post-vaccination, resulting in an adjusted vaccine efficacy (aVE of 0.19 (95% CI, -0.43–0.55). All positive cases in this study reported at least one symptom, such as sore throat, cough, or chills, according to participant questionnaires for COVID-19. There was no significant difference for efficacy of the Ad5-nCoV-IH and IMB-BNT at follow-up periods of 90 days with aVE of 0.20 (95% CI, -0.35-0.53) and 120 days with aVE of 0.22 (95% CI, -0.30-0.53), as the study was underpowered for this endpoint. The number of COVID-19 cases became comparable between the two groups by the end of the study, yielding an aVE of 0.07 (95% CI, -0.49-0.42) with a median follow-up period of 169 days (Table 2 and Supplementary Fig. 1). An exploratory analysis of vaccine effectiveness against breakthrough infections was assessed based on participants’ infection and vaccination histories (Supplementary Table 15). The study was not designed to detect a statistically significant difference for this endpoint. Those with a history of infection (COVID-19 confirmed by PCR/ rapid test kit (RTK) test) in the Ad5-nCoV-IH group consistently demonstrated no statistical difference with aVE values of 0.31 (95% CI, -0.57-0.7), 0.32 (95% CI, -0.42-0.67), 0.34 (95% CI, -0.34-0.67), and 0.12 (95% CI, -0.64-0.53) compared to the BNT-IM group at median follow-up periods of 60, 90, 120 and 169 days, respectively. Individuals without a prior infection history exhibited similar efficacy between the two groups. Another exploratory analysis of aVE was performed based on vaccination history, when Ad5-nCoV-IH served as a second booster dose following previous vaccination of mRNA+mRNA+X, it demonstrated no statistical difference in aVE compared to BNT-IM over a median follow-up period of 60 days (0.38, 95% CI, -0.56-0.75), 90 days (0.33, 95% CI, -0.53-0.71) and 120 days (0.33, 95% CI, -0.53, 0.71). Among individuals with a vaccination history of ICV + ICV + X, Ad5-nCoV-IH exhibited an aVE of 0.04 (95% CI, -1.88-0.68) at a 60-day follow-up, 0.16 (95% CI, -1.3-0.69) at 90-day follow-up, and 0.29 (95% CI, -0.78- 0.71) at 169-day follow-up. Overall, the efficacy results indicated that the Ad5-nCoV-IH group showed similar protective efficacy in preventing breakthrough infections.
Table 2.
Vaccine efficacy of recombinant COVID-19 vaccine (Ad5-nCoV-IH) in preventing virologically confirmed COVID-19 cases at 14 days after vaccination
| Median follow-up days | Ad5-nCoV-IH n/N (%) | BNT-IM n/N (%) | VE (95% CI) | aVE (95% CI) |
|---|---|---|---|---|
| 60 days | 22/270 (8.1%) | 26/269 (9.7%) | 0.17(-0.46-0.53) | 0.19(-0.43-0.55) |
| 90 days | 26/270 (9.6%) | 31/269 (11.5%) | 0.18(-0.38-0.51) | 0.2(-0.35-0.53) |
| 120 days | 27/270 (10.0%) | 33/269 (12.3%) | 0.2(-0.32-0.52) | 0.22(-0.3-0.53) |
| 169 days (full time) | 34/270 (13.7%) | 35/269 (13.0%) | 0.06(-0.51-0.41) | 0.07(-0.49-0.42) |
N number of participants in each data set, n actual number of COVID-19 cases, Ad5-nCoV-IH Recombinant Covid-19 Vaccine (Adenovirus Type 5 Vector) for Inhalation, BNT-IM intramuscular tozinameran vaccine, VE vaccine efficacy, aVE adjusted vaccine efficacy.
We conducted supplementary examinations on the genetic composition of circulating variants throughout the entire study duration. Out of the nasopharyngeal samples from 69 endpoint cases, 64 were eligible for sequencing. All identified variants were of the Omicron lineage, including BA.2.10.1, BA.2, BA.5.2, XBB.1, BM.1.1, BN.1.1, BQ.1.1, and others. Additionally, eight cases remained unassigned (Supplementary Table 16).
Discussion
The focus of COVID-19 vaccination has shifted from achieving herd immunity or sterile immunity to preventing severe outcomes of the infection31, particularly among vulnerable populations such as elderly or severely immunocompromised individuals32,33. Unlike immunocompetent individuals, severely immunocompromised individuals face heightened risks from COVID-19 infection, as even mild infections could lead to severe outcomes34. Thus, preventing infection altogether is crucial for this group.
According to the PPS-based analysis, the GMC ratio of the anti-spike RBD IgG antibodies (wild-type) between the Ad5-nCoV-IH and BNT-IM groups was 0.22 (95% CI: 0.19, 0.26) at 14 days post-vaccination. Since the lower limit of the 97.5% confidence interval is below 0.67, it was concluded that the hypothesis of non-inferiority for the antibody GMC is not valid. Unlike BNT-IM, Ad5-nCoV-IH not only able to induce systemic immunity, but also mucosal immunity to form a defensive lining at the lung level35 (Fig. 2). Although the IgG induced by Ad5-nCoV-IH were not as high as BNT-IM shown in the first 28 days post booster dose, the level seems to reduce to around the same level starting from Day 84 to Day 168. Moreover, the higher IgG level induced by BNT-IM did not translate to better vaccine effectiveness as depicted in this study.
Although the aVE shown in the study was underpowered to demonstrate a statistically significant difference with a wide 95% confidence interval, given the sample size was just 540 with limited incidences, the Ad5-nCoV-IH showed a similar aVE at 60-, 90-, and 120-days post booster dose when compared to BNT-IM. The aVE becomes more comparable between the two arms after 169 days post booster dose. Participants with history of COVID-19 infection receiving Ad5-nCoV-IH exhibited a similar protection from COVID-19 infection albeit with point estimate of approximately 30% higher when compared to BNT-IM.
The sIgA was reportedly comparable in both arms, with only a modest increase only in Day-28 post vaccination. This suggests the potential of Ad5-nCoV-IH in stimulating mucosal immunity. Additionally, the broader cross-reactive immunity of SIgA induced by wild-type SARS-CoV-2-based vaccines underscores the positive impact of this innovative administration method.
Integrating the exploratory analysis of humoral and mucosal immunity with the efficacy results suggests that serum antibody concentrations alone may not be the sole criterion for assessing VE. The study indicates that in participants with already high levels of pre-existing systemic immunity, a boost of approximately 20% versus 500% in systemic immunity of IgG may not provide any further meaningful protection from COVID-19 infection. On the contrary, the inducement of mucosal immunity through Ad5-nCoV-IH appears to provide similar protection from COVID-19 infection. This is comparable to findings from a human infection model study using influenza A/California/2009 (H1N1) as the challenge agent, where pre-existing mucosal IgA, rather than systemic IgG, proved effective in protecting against more severe disease36.
For individuals who had prior infection or existing vaccination history of ICV + ICV + X or mRNA+mRNA+X (Supplementary Table 15), result showed that Ad5-nCoV-IH consistently exhibited higher point estimates against breakthrough infections compared to BNT-IM across various time spans, while BNT-IM outperformed Ad5-nCoV-IH in individuals with a vaccination history of AZ + AZ + X, suggesting heterogenous immunization using cross-platform may be beneficial to protection from infections. However, due to the limited sample size, the study did not have sufficient statistical power to establish a significant difference for this outcome.
Perhaps owning to the intricate and complex system with a large array of immune cells in the respiratory tract37, only a lower dosage of antigen is required. For example, the dosage of Ad5-nCoV-IH is just 0.1 ml (1.0×1010 viral particles), which also contributes to reducing the risk of side effects often associated with higher doses. Therefore, in terms of safety profiles, participants in the Ad5-nCoV-IH group experienced a significantly lower percentage of ADRs compared to BNT-IM group. With the improved tolerability and similar protection profiles brought by the booster Ad5-nCoV-IH, it is anticipated that this innovative delivery method offers benefits for vaccines targeting respiratory tract infections caused by airborne pathogens.
This study has several limitations, so caution is warranted in interpreting the results. One key limitation is its single-blind design. While observers were kept unaware of group assignments and participants were instructed not to reveal their treatment arm, the risk of bias remains. Blinded observers evaluated AEs and conducted COVID-19 investigations, only a few authorized staff with confidentiality agreements had access to group information. Despite these precautions, the single-blind design could still influence the study’s results. Another limitation that could introduce potential bias in determining efficacy endpoints is the observation of only RTK results in three cases. Among these three cases, two were prevented from undergoing the necessary confirmation real-time reverse transcription-polymerase chain reaction (RT-PCR) test due to travel restrictions while the other declined to perform the test. Additionally, the wide CIs in some estimates suggest some level of uncertainty in the precise efficacy and mucosal immunity, emphasising the need for continued monitoring and research to enhance our comprehension of Ad5-nCoV-IH’s effectiveness and the new delivery method for immunization. Furthermore, the history of prior infection may not be accurate, as one may have asymptomatic COVID-19 without realising it.
In conclusion, Ad5-nCoV-IH exhibited inferiority of anti-spike RBD IgG antibodies immunogenicity to BNT-IM. However, Ad5-nCoV-IH demonstrated better tolerability characteristics and suggested similar vaccine efficacy against emerging variants, despite showing lower stimulation of immunogenicity in individuals with existing high immunity to SARS-CoV-2 compared to BNT-IM.
Methods
The clinical trial was reviewed and approved by Medical Research and Ethics Committee (NMRR-22-01132-8KT).
Study design
This is a phase 3, randomized, parallel-controlled, observer-blinded, non-inferiority trial to evaluate the safety, immunogenicity, and efficacy of Ad5-nCoV-IH, as a second booster dose against Omicron and other VOCs of SARS-CoV-2 to prevent breakthrough infections. Recruitment occurred at three hospitals across Malaysia.
Participants
A total of 540 eligible adults aged 18 or older, and in good health as determined by study clinician, who completed a course of primary and first booster vaccination at least 16 weeks prior, after signing informed consent form, were enrolled in the study. Key exclusion criteria were history of SARS and middle east respiratory syndrome (MERS) infection, confirmed or suspected cases of COVID-19 at the time of screening, receipt of any SARS-CoV-2 vaccine after the first booster vaccination, any history of anaphylaxis, any history of allergic disease or reactions to vaccine ingredients, any bleeding disorder, current use of anticoagulants, pregnancy, or intent to conceive, and severe, or uncontrolled comorbidities.
Randomization and Blinding
Randomization was carried in eligible participants using the Interactive Web Response System (IWRS), they were randomly assigned in 1:1 ratio using block randomization with block size of 10 to receive either one dose of Ad5-nCoV-IH (0.1 ml, 1.0 × 1010 viral particles) through inhaling aerosol generated from the vaccine liquid using a nebuliser (Aerogen) by mouth or BNT-IM (0.3 ml) injected into their non-dominant arm. This study used a single-blind design, and the observers remained blinded before unblinding and did not know which group the participant belonged to. Participants were instructed not to disclose their treatment arm to blinded observers. Participant grouping information was only available to authorised unblinded administrators or investigators who signed confidentiality statements to withhold participant grouping information and ensure the blind study’s integrity. The blinded observers were delegated to evaluate AEs, SAEs and AESIs throughout the study.
Interventions
Study visits were scheduled at 2 and 4 weeks post-second booster dose for immunogenicity and safety assessments. Saliva and blood samples were collected for specific SIgA antibodies, IgG antibodies against SARS-CoV-2 spike-RBD protein, pseudovirus neutralization assays against Omicron BA.4/5 variants. A subgroup of participants (the last 140 participants in each arm) had additional visits at 12 and 24 weeks post-second booster dose for persistent immunogenicity assessment. Study personnel contacted participants virtually every 2 weeks after week 4 post booster for the duration of the study (6 months). Weekly rapid SARS-CoV-2 antigen tests began at week-2, with positive results leading to immediate confirmatory RT-PCR tests for efficacy assessment. Participants reported symptoms and weekly RTK test results through a web-based app. Upon enrolment, participants were provided information and given access to electronic diary (e-diary) to record AEs. Each AE was described by its nature, onset date, grading and duration. E-diaries were followed up by the blinded observers regularly for further assessments. Samples were shipped to local laboratory and laboratory in China for testing.
Outcomes
The primary outcome was immunogenicity assessments of geometric mean ratio (GMR) of anti-spike RBD IgG antibodies against wild-type, NAb against Omicron BA.4/5 pseudovirus and specific SIgA antibodies at baseline, week 2 and 4, and persistent immunogenicity assessments (at an additional visit of week 12 and 24). The non-inferiority of immunogenicity at Day 14 post vaccination would be analysed. The secondary outcomes were safety parameters of the incidence of solicited AEs and ADRs, within 28 days post-vaccination and SAEs and AESIs throughout the study, and efficacy parameters consisting of RT-PCR/RTK confirmed COVID-19 infection in any clinical setting whether symptomatic or not, occurring at least 14 days after the second booster dose vaccination, along with the genotyping assay-confirmed variant-specific COVID-19 infection. The GMI and SCR rates were exploratory measures that were not pre-defined in the protocol.
Immunogenicity assessment methods
The anti-SARS-CoV-2 RBD-specific IgG was measured using an ELISA kit (Nanjing Vazyme Biotech Co., Ltd., Nanjing, China). Samples were diluted and added to enzyme-labelled plate wells, followed by the addition of an RBD calibrator, expressed in RU/ml, equivalent to BAU/ml according to WHO International Standard (NIBSC code: 20/136). The limit of Quantification is 20.00 RU/ml. The plates were then incubated at 37 °C for 30 minutes. After incubation, the wells were washed, and an enzyme-labelled reagent working solution was added. The plates were then incubated for an additional 30 minutes. Post-incubation, the wells were washed, and the liquid was removed using a microplate dewatering centrifuge. A chromogenic solution was then added to the wells, and optical density (OD) values were measured using a microplate photometer (Thermo Fisher Scientific, Waltham, MA, USA) at dual wavelengths of 450 nm and 630 nm.
Neutralising antibodies against SARS-CoV-2 BA.4/5 pesudovirus were measured with a validated pseudovirion neutralisation assay in which a HIV-1 virus carrying a luciferase reporter gene, with the SARS-CoV-2 Spike protein expressed on the viral envelope, to evaluate neutralisation capacity of antibodies in participants’ serum. With serials dilution, cell control with only cells and virus control with pseudovirus SARS-CoV-2 BA.4/5 were set. Titres were calculated according to the raw data of relative light units (RLU) from CC, VC, and samples. The lower limit titer is 30.
The Meso Scale Discovery (MSD) electrochemiluminescence assay was used to measure secretory IgA (SIgA) levels elicited by the vaccines. The procedure involved diluting MSD Buffer (20×) to a 1× working solution and preparing Blocker A solution. Standards were thawed and diluted fourfold across seven concentrations, with a blank included. Antibodies were then diluted and prepared. After washing the plate, 150 µL of GOLD Read Buffer B was added to each well for detection using the V-PLEX SARS-CoV-2 Panel 32 (IgA) Kit (MSD, Rockville, MD, US). The lower limits of quantification for the B.1.1.529, B.2.75, BA.2.75.2, BA.5, BA.4.6, BF.7, BQ.1, BQ.1.1, wildtype, and XBB.1 variants were 0.043, 0.003, 0.003, 0.003, 0.003, 0.003, 0.003, 0.003, 0.003, and 0.004 AU/ml, respectively.
Participants who tested positive on the RTK had oropharyngeal swabs collected by medical staff within 3 days. The swabs were immediately placed into viral transport medium tubes after collection and then transferred to the designated laboratory for sequencing. RNA extraction was performed according to the standard protocol provided in the Quick-DNA/RNA Viral MagBead kit (Zymo Research). Sequencing was conducted using the Illumina COVIDSeq Test (RUO) Kit to confirm the genotype of the isolated virus from positive cases.
Sample size and statistical analysis
Sample sizes were determined by accepting a 1.5-fold difference in GMC, with a log10 transformed SD of 0.6, and a true ratio of 1.0, power = 0.85, and the non-inferiority margin Δ = 0.67, 215 participants were needed in each group. Considering a 20% dropout rate, the sample size was set at 270 per group. If, following 14 days post-vaccination, the lower limit of the 97.5% CI for GMC of S-RBD IgG antibody exceeded 0.67, the experimental group vaccine was considered non-inferior to the control group vaccine (one-sided CI approach). GMR at day 14 post-vaccination were tested using a two-sided alpha level of 0.025 for immunogenicity hypothesis testing. With the non-inferiority margin being set as Δ = -10%, when the lower limit of 97.5% CI for the SCR difference was greater than -10% after 14 days post-vaccination, the non-inferiority of the experimental group would be considered to be true. The analysis set consisted of modified intention-to-treat (mITT) and safety set (SS), which included all participants who received the booster vaccination. No imputation for missing information was applied. The PPS comprised individuals who received the vaccine without any major protocol deviation. All AEs were coded using the latest available version of the Medical Dictionary for Regulatory Activities (MedDRA version 26.0), and were presented by group, system organ and class, and preferred term. To compare the frequency indicators between groups, the χ2 or Fisher’s exact test was used. For the immunogenicity analysis, GMT and 95% CI were presented, along with the differences in antibody responses between groups being evaluated with the use of an analysis of covariance (ANCOVA) model (with antibody titres after booster as the dependent variable and study vaccine as the fixed effect) that was adjusted for age groups and pre-booster antibody titres. SCR was defined as 4-fold increase from baseline. For protective efficacy analysis, the day of the second booster dose was defined as day 0. The primary efficacy endpoint was the number of positive RT-PCR/RTK COVID-19 incidences 14 or more days post-vaccination. The crude vaccine efficacy (VE) was estimated as 1-hazard ratio (HR) and a 4 proportional risk model was used to adjust the VE estimate to account for baseline imbalance between the 2 groups. The covariates of the Cox model included age, sex, race, primary vaccination, prior SARS-CoV-2 infection status, first booster vaccine, time between second injection in the primary series and the first, and time between first booster injection and the second booster. VE was calculated based on a median follow-up period of 60 days, 90 days, 120 days, and the full course of study, respectively. Statistical analyses were performed using SAS Version 9.4 (SAS Institute Inc., Cary, NC, US).
Supplementary information
Acknowledgements
The study was funded by Tianjin Biomedical Science and Technology Major Project [Project number 21ZXSYSY00040]. The vaccine was developed and the study was sponsored by CanSino Biologics Inc. CanSino Biologics Inc. contributed to the trial design and provided the investigational product. Study site investigators, contracted by CanSino Biologics Inc., contributed to the study conduct, data collection and interpretation, Shanghai ImStat Medical Technology Co. Ltd., was involved in data management and statistical analysis for the study, and Immunotherapeutcis Laboratory and Department of Medicine, Faculty of Medicine, University Malaya, was responsible for local laboratory testing. The study vaccine Ad5-nCoV-IH was developed and manufactured by CanSino Biologics Inc. The BNT-IM of control arm, manufactured by Pfizer-BioNTech was sponsored by Ministry of Health, Malaysia. All author had final responsibility for the decision to submit the manuscript for publication. We extend our sincere appreciation for the contributions of all trial participants. Furthermore, we would like to acknowledge the permission granted by the Director General of Health Malaysia for publication and the invaluable support from team members across different institutes.
Author contributions
J.G. and S.Ng. had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. R.W. and C.C.K. contributed equally as co-first authors; J.G. and S.Ng. contributed equally as co-corresponding authors. Study concept and design: J.G., T.O.L. Acquisition, analysis, or interpretation of data: D.N., C.C.K, S.B., R.Y., N.Z, S.S.C, L.H.P., A.M.N., W.H.W.M., M.R.M.D., W.M.R.W.A.K., M.H.T. and A.A.A. Drafting of the manuscript: R.W. and C.C.K. Statistical analysis: X.W. Obtained funding: R.W., H.H, X.Y.Z., J.G., and T.Z. Administrative, technical, or material support: T.Z., S.Ng., R.R., S.W., Z.Z., L.H., S.A., X.Y.Z., L.W., M.Y., Y.L., V.K.C., G.Y.C, K.Y.C., Y.L.L., X.X. and J.S.T. Supervision: J.G. and S.Ng. Critical review of the manuscript for important intellectual content: All authors.
Data availability
The study protocol is provided in the Supplementary Research Protocol. Anonymised participant data supporting the results reported in this article will be made available when the trial is published. The data will be accessible upon requests directed to the corresponding authors. All data will be made available for a minimum of 5 years from the trial’s conclusion.
Competing interests
T.Z., J.G., R. W., S.A, H.H., X.Y.Z, L.W., M.Y., Y.L. and V.K.C. reported being employed by CanSino Biologics Inc. which manufacture the study vaccine, during the conduct of the study and outside the submitted work. J.G. and T.Z. reported holding stock in CanSino Biologics Inc. T.O.L. reported serving as a paid senior scientific advisor to CanSino Biologics Inc. S.Ng., D.N., S.B., R.Y., N.Z., S.S.C., A.A.A., C.C.K. and, L.H.P. reported receiving research grants via Clinical Research Malaysia (CRM) through a contract with CanSino Biologics Inc. during the conduct of the study and outside the submitted work. R.R. and X.W. received financial support from CanSino Biologics Inc. for contracted work outside the submitted work. X.X.X and J.S.T. received financial support from CanSino Biologics Inc. for contracted work outside the submitted work. G.Y.C., A.M.N, K.Y.C, Y.L.L, W.H.W.M, M.R.M.D., W.M.R.W.A.K. and M.H.T. reported receiving research grants from Clinical Research Malaysia (CRM) through a contract with CanSino Biologics Inc. No other disclosures were reported.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Chun K. Chew, Ruijie Wang.
Contributor Information
Jinbo Gou, Email: jinbo.gou@cansinotech.com.
Sharon S. M. Ng, Email: sharonng@moh.gov.my
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-024-01003-x.
References
- 1.Polack, F. P. et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med.383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halperin, S. A. et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: an international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet399, 237–248 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falsey, A. R. et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 Vaccine. N. Engl. J. Med.385, 2348–2360 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Kaabi, N. et al. Effect of 2 Inactivated SARS-CoV-2 Vaccines on Symptomatic COVID-19 Infection in Adults: A Randomized Clinical Trial. JAMA326, 35–45 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arora, P. et al. Augmented neutralisation resistance of emerging omicron subvariants BA.2.12.1, BA.4, and BA.5. Lancet Infect. Dis.22, 1117 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feikin, D. R. et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet399, 924–944 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiolet, T., Kherabi, Y., MacDonald, C. J., Ghosn, J. & Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: a narrative review. Clin. Microbiol. Infect.28, 202–221 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao, Y. et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nat.608, 593–602 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu, Z., Liu, K. & Gao, G. F. Omicron variant of SARS-CoV-2 imposes a new challenge for the global public health. Biosaf. Heal.4, 147–149 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang, M. et al. Atlas of currently available human neutralizing antibodies against SARS-CoV-2 and escape by Omicron sub-variants BA.1/BA.1.1/BA.2/BA.3. Immunity55, 1501–1514.e3 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larkin, H. D. New Data on Heterologous COVID-19 Vaccine Combinations. JAMA328, 916–917 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Tan, S. H. X. et al. Association of Homologous and Heterologous Vaccine Boosters With COVID-19 Incidence and Severity in Singapore. JAMA327, 1181–1182 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mattoo, SulS. & Myoung, J. A Promising Vaccination Strategy against COVID-19 on the Horizon: Heterologous Immunization. J. Microbiol. Biotechnol.31, 601–1614 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa Clemens, S. A. et al. Heterologous versus homologous COVID-19 booster vaccination in previous recipients of two doses of CoronaVac COVID-19 vaccine in Brazil (RHH-001): a phase 4, non-inferiority, single blind, randomised study. Lancet399, 521–529 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao, X. et al. Effects of a Prolonged Booster Interval on Neutralization of Omicron Variant. N. Engl. J. Med.386, 894–896 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu, K. et al. Protective prototype-Beta and Delta-Omicron chimeric RBD-dimer vaccines against SARS-CoV-2. Cell185, 2265–2278.e14 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Accorsi, E. K. et al. Association Between 3 Doses of mRNA COVID-19 Vaccine and Symptomatic Infection Caused by the SARS-CoV-2 Omicron and Delta Variants. JAMA327, 639–651 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Altarawneh, H. N. et al. Protection against the Omicron Variant from Previous SARS-CoV-2 Infection. N. Engl. J. Med.386, 1288–1290 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afkhami, S., Kang, A., Jeyanathan, V., Xing, Z. & Jeyanathan, M. Adenoviral-vectored next-generation respiratory mucosal vaccines against COVID-19. Curr. Opin. Virol.61, 101334 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.García-Silva, I., Govea-Alonso, D. O. & Rosales-Mendoza, S. Current status of mucosal vaccines against SARS-CoV2: a hope for protective immunity. Expert. Opin. Biol. Ther.23, 207–222 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Wahl, I. & Wardemann, H. Sterilizing immunity: Understanding COVID-19. Immunity55, 2231 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yechezkel, M. et al. Safety of the fourth COVID-19 BNT162b2 mRNA (second booster) vaccine: a prospective and retrospective cohort study. Lancet Respir. Med.11, 139–150 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang, R. et al. Safety and immunogenicity of aerosolised Ad5-nCoV, intramuscular Ad5-nCoV, or inactivated COVID-19 vaccine CoronaVac given as the second booster following three doses of CoronaVac: a multicentre, open-label, phase 4, randomised trial. Lancet Respir. Med.11, 613–623 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Regev-Yochay, G. et al. Efficacy of a Fourth Dose of Covid-19 mRNA Vaccine against Omicron. N. Engl. J. Med.386, 1377–1380 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heida, R., Hinrichs, W. L. J. & Frijlink, H. W. Inhaled vaccine delivery in the combat against respiratory viruses: a 2021 overview of recent developments and implications for COVID-19. Expert. Rev. Vaccines.21, 957–974 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Afkhami, S. et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell185, 896–915.e19 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu, S. et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat. Commun.11, 4081 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xing, Z. et al. Innate immune memory of tissue-resident macrophages and trained innate immunity: Re-vamping vaccine concept and strategies. J. Leukoc. Biol.108, 825–834 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Feng, L. et al. An adenovirus-vectored COVID-19 vaccine confers protection from SARS-COV-2 challenge in rhesus macaques. Nat. Commun.11, 4207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu, S. et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis.21, 1654–1664 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell, R. et al. Trends in Severe Outcomes Among Adult and Pediatric Patients Hospitalized With COVID-19 in the Canadian Nosocomial Infection Surveillance Program, March 2020 to May 2022. Jama. Netw. Open.6, e239050 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anand, S. T. et al. Severe COVID-19 in Vaccinated Adults With Hematologic Cancers in the Veterans Health Administration. Jama. Netw. Open.7, e240288 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auvigne, V. et al. Severe hospital events following symptomatic infection with Sars-CoV-2 Omicron and Delta variants in France, December 2021-January 2022: A retrospective, population-based, matched cohort study. EClinicalMedicine48, 101455 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tan, W. C. et al. COVID-19 Severity and Waning Immunity After up to 4 mRNA Vaccine Doses in 73 608 Patients With Cancer and 621 475 Matched Controls in Singapore: A Nationwide Cohort Study. Jama. Oncol.9, 1221–1229 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh, J. E. et al. Intranasal priming induces local lung-resident B cell populations that secrete protective mucosal antiviral IgA. Sci. Immunol.6, eabj5129 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gould, V. M. W. et al. Nasal IgA provides protection against human influenza challenge in volunteers with low serum influenza antibody titre. Front. Microbiol.8, 900 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mettelman, R. C., Allen, E. K. & Thomas, P. G. Mucosal immune responses to infection and vaccination in the respiratory tract. Immunity55, 749 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol is provided in the Supplementary Research Protocol. Anonymised participant data supporting the results reported in this article will be made available when the trial is published. The data will be accessible upon requests directed to the corresponding authors. All data will be made available for a minimum of 5 years from the trial’s conclusion.