Abstract
Microplastics (MPs) have been found in a diverse range of organisms across trophic levels. While a majority of the information on organismal exposure to plastics in the environment comes from gastrointestinal (GI) data, the prevalence of MP particles in other tissues is not well understood. Additionally, many studies have not been able to detect the smallest, most prevalent, MPs (1 µm – 5 mm) that are the most likely to distribute to tissues in the body. To address these knowledge gaps, MPs in the GI tract and muscle of Atlantic killifish (Fundulus heteroclitus) collected from two sites (Falmouth and Bourne) on Buzzards Bay, Cape Cod, MA were quantified down to 2 µm in size. Eight fish from Falmouth and 10 fish Bourne site were analyzed. Fourier-transform infrared spectroscopy and Raman spectroscopy were used to identify all particles. The mean concentrations of MPs in the GI tract and muscle from fish collected from Falmouth was 85.5 ± 70.2 and 11 ± 12.5 particles per gram wet weight, respectively. Fish collected from Bourne site had mean particle concentrations of 12.2 ± 18.1 and 1.69 ± 5.36 particles per gram wet weight. Of the 2,008 particles analyzed in various fish tissue samples, only 3.4% (69 particles) were identified as plastic; polymers included nylon, polyethylene, polypropylene, and polyurethane. MPs detected in the GI tract samples also tended to be more diverse in both size and polymer type than those found in the muscle. We found that MPs < 50 µm, which are often not analyzed in the literature, were the most common in both the GI tract and muscle samples. There was not a significant correlation between the MP content in the muscle compared to the GI tract, indicating that GI tract MP abundance cannot be used to predict non-GI tract tissue MP content; however, MP abundance in muscle correlated with fish total length, suggesting potential bioaccumulation of these small MPs.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s43591-024-00101-w.
Keywords: Microplastics, Fish, Translocation, Fourier-transform infrared spectroscopy, Raman spectroscopy, Bioaccumulation
Introduction
Understanding the fate of plastics in the ocean is challenging, in part, because plastics are an incredibly diverse suite of contaminants [82]. The size, surface chemistry, polymer type, state of degradation, and additives present can all influence the distribution and fate of microplastics (1 μm < particle < 5 mm; MPs) in the environment [82]. The exact influence of these factors on particle abundance and distribution in the environment is still poorly understood due to analytical limitations and a lack of harmonized methods [39].
Plastic ingestion is widespread, having been documented in more than 1,565 aquatic and terrestrial species [86]. Even though ingestion is well-documented, the bioaccumulation potential of microplastics is not well understood. Bioaccumulation is classically defined as the buildup of a material in an organism over its lifetime [62, 89], however, the movement of MPs is likely to be more constrained due to their large particulate nature. The ultimate fate of these ingested MPs is both complex and unknown.
One of the key challenges is that small MPs (smaller than 50 μm), the most prevalent size of environmental plastics [43], are difficult to find and identify in environmental samples [43]. Smaller particles can often be misidentified as MPs due to a lack of distinguishing characteristics as the particles decrease in size [45, 79]. The abundance of MPs is expected to increase with decreasing size, but there is little information regarding the movement and fate of particles smaller than 50 μm in aquatic environments [6, 21, 22, 69]. In addition to being more abundant in the environment, smaller MPs are also more likely to translocate from the gastrointestinal (GI) tract into internal tissues [17, 49, 76]. Therefore, having an accurate size distribution of ingested MPs is critical to understanding exposure and, ultimately, the risk that MPs pose to marine animals.
There is still much to discover regarding the characteristics of particles that govern MP translocation into non-GI tract tissues since the majority of published data examines plastic exclusively in the GI tract [28, 69]. Without this information, the implications of ingestion for bioaccumulation, trophic transfer, and biomagnification of MPs cannot be predicted [71]. Current research suggests that bioaccumulation of MPs is unlikely, but this prediction is limited to particles larger than 100 μm, emphasizing the need for research on the accumulation of smaller MPs in tissues [11].
Smaller MPs are often not quantified in tissues due to analytical challenges [32, 71] and high levels of background contamination [32, 48]. The objective of this study was to quantify the full-size range of MPs using strict quality control procedures to reduce background contamination. Through this approach, we aimed to distinguish differences in characteristics of MPs detected in the GI tract and muscle of Atlantic killifish (Fundulus heteroclitus) and address the bioaccumulation potential of the under-reported small MPs.
Materials and methods
Materials
Pyrex glassware was used whenever possible during this study. Whatman (grade 4; 25 μm pore size) and nitrocellulose (pore size of 1 μm) filter papers (Sigma Aldrich) were used for Fourier-transform infrared spectroscopy (FTIR) and Raman spectroscopy analysis. Polyvinyl chloride (PVC; maximum particle size 250 µm) and polyethylene terephthalate (PET; maximum particle size 300 µm) fragments were obtained from Goodfellow Cambridge limited (Huntingdon, England). Polyethylene (PE; 250–300 µm) microspheres (beads) were obtained from Cospheric Inc. (Somis, CA). Minnow traps were used for collecting Atlantic killifish.
Quality control
We followed the quality control criteria established by Hermsen et al. [32]. All water samples and solutions were filtered through a 0.2 μm filter prior to use. All sample processing apparatus (glass Erlenmeyer flasks and ceramic Buchner funnels) were combusted in a muffle furnace (Fisher Scientific Isotemp Programmable Forced-Draft Furnace) at 500℃ for 5 h. Glassware that could not be combusted in the furnace was rinsed three times with both acetone and filtered water prior to use. All work surfaces were wiped down with ethanol prior to working with samples. Sample manipulation took place in a laminar flow hood (AirClean 600 PCR Workstation). Samples were not exposed to ambient air. When the samples were not being actively manipulated, they were covered with aluminum foil. While working with samples, a 100% cotton jumpsuit was worn to prevent contamination from polyester clothing. During sample processing, occupancy of the room was kept to a single person. Procedural blanks (starting at the digestion stage) were used for every 5 samples.
Fish collection and tissue sampling
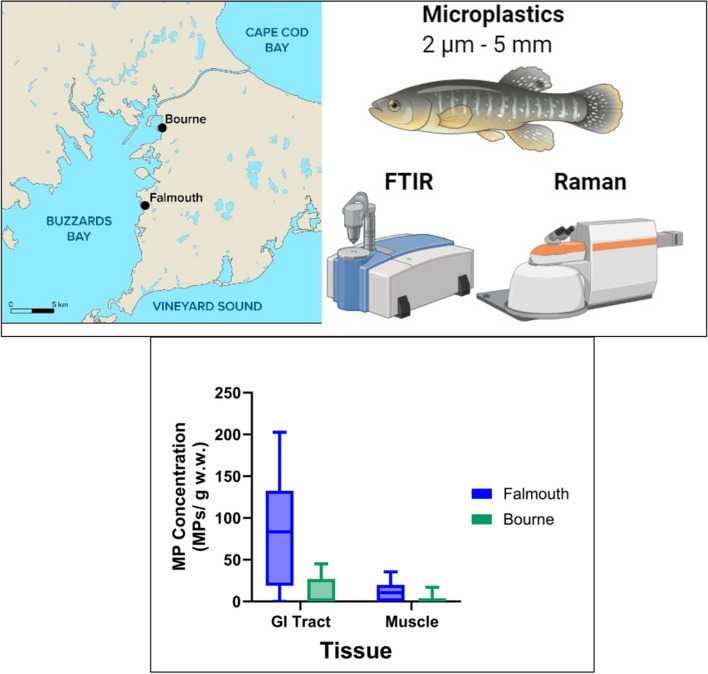
Atlantic killifish were collected in the fall using minnow traps from two locations (Falmouth and Bourne) in Buzzards Bay, Massachusetts (Fig. 1). The sampling dates, coordinates, number of fish collected, and the environmental conditions are shown in Table S1. The killifish was chosen for this study because it is an estuarine species commonly found in coastal and brackish water ecosystems, where microplastic pollution is prevalent [25]. In addition, killifish are omnivores and they are exposed to microplastics both directly through water and indirectly via their diet, allowing for studies of trophic transfer of microplastics and the potential for bioaccumulation across food webs. Fish were immediately euthanized using MS222 (1 g/L) buffered with sodium bicarbonate. Fish were kept in an aluminum-foil lined bucket during transport. We analyzed eight fish from Falmouth site and ten fish from the Bourne site. The total length and weight, sex, and tissue wet weights were recorded upon collection. Prior to dissection, fish were rinsed three times with 0.2 μm filtered water to remove any loose particles on the fish’s skin. The GI tract and a section of the dorsal muscle (without skin) were collected. The total GI tract, including contents, were used in our analysis. Tissues were stored in plastic-free aluminum foil at -80 °C. All collected fish were considered mature as they exceeded 3.2 cm or 3.8 cm for males and females, respectively [2], and they were in good overall health according to the calculated condition factors. Based on length-age relationships [2], the collected fish from Bourne, MA appear to be a couple of years younger than the collected fish from Falmouth, MA.
Fig. 1.
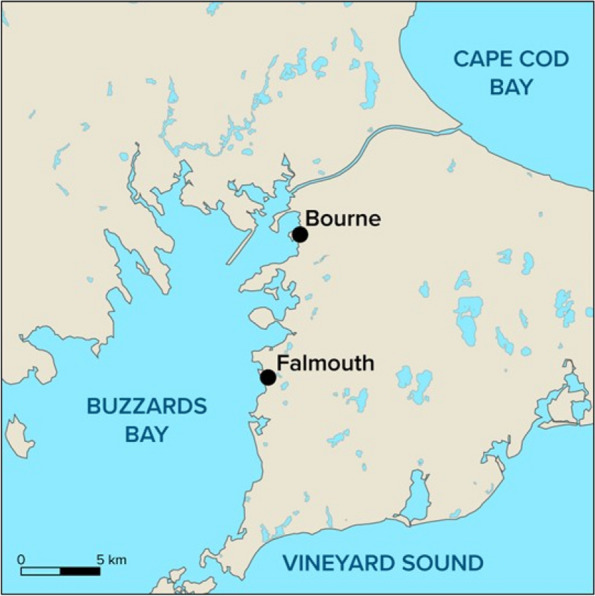
Map of Southeastern Massachusetts showing the two collection sites (Falmouth, MA, Bourne, MA). Geographical coordinates and environmental parameters at the collection sites are provided in supplemental information (Table S1)
At both sampling sites, we collected water samples in 1 L glass jars. The jars were rinsed three times in water from the collection site prior to sample collection. To prevent air contamination, the jars were immersed underwater prior to opening. Duplicate water samples were collected from each site. Samples were stored at room temperature (20—25 ℃). The water samples were collected to estimate the presence of plastic particles in the environment, a potential source of exposure of killifish to microplastics.
Sample digestion
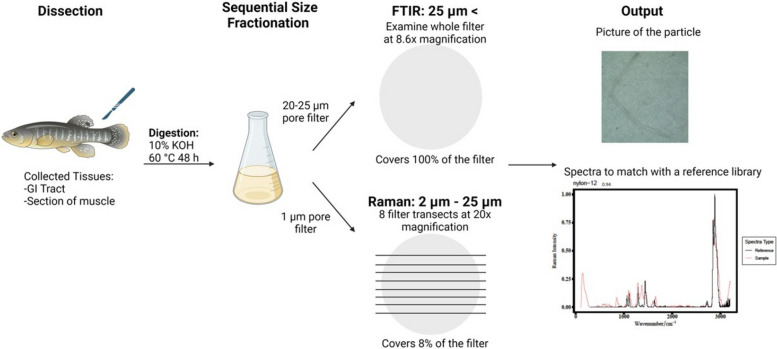
A flow chart of the sample processing and analysis is shown in Fig. 2. Tissue samples were digested with 10% KOH (3 × sample wet weight) at 60 ℃ for 48 h (Fig. 2). KOH digestion at this temperature has previously been shown to not degrade MPs [31]. Digests were neutralized with a combination of sodium bicarbonate (0.05 g/mL) and 10% HCl (0.54 mL HCl/mL KOH) prior to filtration. Particles in the samples were size fractionated by filtering initially through a 25 μm pore-size filter followed by a 1 μm filter (Fig. 2). Filters were stored in plastic-free aluminum tins until analysis. The 25 μm pore-size filter was used for FTIR spectroscopy analysis and the 1 μm filter was used for Raman spectroscopy (Fig. 2). Size fractionation was used to analyze samples more efficiently. Raman spectroscopy is more accurate at identifying particles smaller than 20 μm in length, and FTIR is a more efficient method for particles > 20 μm in length [15].
Fig. 2.
Schematic workflow: Illustration of sample processing and analysis of tissue samples using Fourier-transform infrared spectroscopy (FTIR; particles > 25 µm) and Raman spectroscopy (particles 2—25 µm). Data analysis was done by matching the sample spectra to a reference library using OpenSpecy
Particle recovery experiments
Particle recovery experiments were conducted using PVC and PET fragments and PE beads (250–300 μm) dyed with Nile Red (10 μg/mL). Blue mussel (Mytilus edulis) tissue was used to determine the particle recovery from the MP isolation process from biological tissues. Fifty particles from each polymer type were manually added to whole mussel tissue (Mytilus edulis) in Erlenmeyer flasks. The mussels were then digested for 48 h as in Sect. “Sample Digestion”. The resulting digests were then filtered through 25 μm pore-size filters. Each flask was washed three times with filtered water. The number of particles on the filter were counted under a dissecting microscope. There was 80% recovery of particles regardless of the polymer type (n = 2 experiments per polymer) (Fig. S1). We did not conduct particle recovery experiments with particles smaller than 250 μm.
FTIR spectroscopy sample analysis
The 25 μm filters were scanned for particles under a dissecting microscope at 8.6 × magnification. The whole filter was examined, and any particles found were imaged (ThorCam Imaging Software V.3.5.1.1). Particles were then transferred to a piece of double-sided tape in a glass petri dish and numbered. These particles were analyzed with a diamond attenuated total reflection (ATR) attachment on the Cary 630 FTIR (Agilent Technologies Inc). Particles were placed individually on the detection area. MicroLabPC software was used to collect the spectra. The spectra were collected in absorbance mode with background recorded every 2–5 samples. The spectral range was recorded between 650—4000 cm−1. The spectral resolution was 2 cm−1 with 32 scans averaged.
Raman spectroscopy sample analysis
The 1 μm filter was used for Raman analysis. The particles were analyzed with a Renishaw inVia Raman microscope using Wire 3.4 software to collect images and spectra of the particles. Spectra were collected with a 532 nm excitation laser. The filter was scanned at 20 × magnification. Eight transects were made in a straight line across the filter covering 8% of the filter (Fig. 2). The sub-samples were taken from various parts of the filter, including the edges, middle, and areas where water passed more readily (near holes in filter support). This ensured that both high and low particle concentration areas were covered. Particles were imaged prior to spectra collection. If particles were smaller or larger than the 20 × field of view, 50 × or 5 × magnification was used to image the particle. For spectral collection, no pin hole was used in the back focal plane of the objective. The spectral resolution was 3 nm with a laser intensity of 100 mW with at most approximately 9 mW of which was directed at the sample. A 50 μm slit was used with a grating resolution of 1800 line pairs. Spectra were collected for 3 s. Spectra were the result of the averaging of 3 scans. To minimize risk of burning, sample spectra were collected with 10% laser power or less. In cases where background fluorescence obscured the signal, the spectra was re-collected using a lower laser power setting.
Data validation and analysis
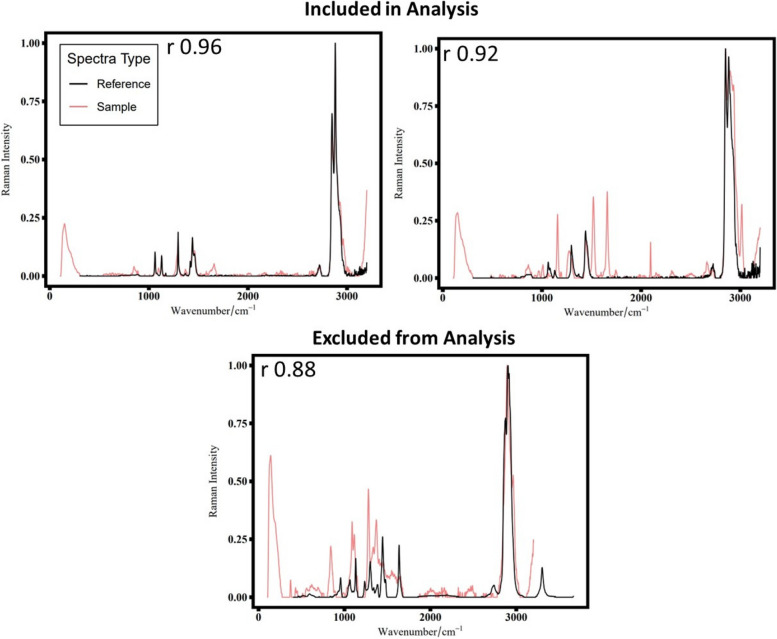
The collected spectra from FTIR and Raman spectroscopy were baseline corrected and smoothed prior to identification. OpenSpecy [13] and a custom library database were used for identifying the particles based on their spectra. Pearson’s correlation coefficient (Pearson’s r) of greater than or equal to 0.8 was used as a statistical cutoff for a good fit between the reference and the particle spectrum [27, 95]. All the plastic particle spectra (Pearson’s r > 0.8) were then manually checked to ensure that the spectral peaks were well matched with the reference peaks from both the libraries [80]. Particles (Pearson’s r > 0.8) were not used in the subsequent analysis if they had peaks that either did not match the overall pattern in the reference spectrum or had a low enough signal-to-noise ratio that it was challenging to interpret the true signal. Examples of spectra that were included or excluded in the analysis are shown in Fig. 3.
Fig. 3.
Examples of spectra. The r value reflects Pearson’s r correlation for the sample and reference spectra. The reference spectra are shown in black, and the sample spectra are shown in coral. Spectra included in analysis had low background noise and few extraneous peaks. Spectra excluded from analysis due to the large number of non-matching peaks
Spectral peaks were identified according to commonly reported Raman shifts in the literature. Plastic degradation or weathering peaks were identified in all of the samples. Non-plastic particles were compared to reference spectra for a variety of different materials, including fur, cellulose, cotton, sand, chitin, and plant material. The non-plastic particles were identified by a Pearson’s r of 0.8 or greater without manually comparing the spectra matches.
Statistics
Fulton’s condition index (K) (Eq. 1) was calculated to determine the overall health of the fish [81].
| 1 |
MP abundance and occurrence data were non-normally distributed, so nonparametric analyses were used. To predict the occurrence of MPs in the muscle, a Random Forest model was generated using the R CARET package’s leave-one-subject-out train() function [41]. Fish length, sex and plastic content in the GI tract were used as input variables. Spearman’s rank correlation was used to determine the correlation between fish total length and GI tract and muscle MP abundance. Graphpad Prism (10.0.2) was used to calculate the Spearman’s rank correlations and to generate the visualizations. Significant correlations were accepted if the p-value of the Spearman’s rank correlation was less than 0.05. A random forest model and Spearman rank correlation were chosen due to the small sample size and structure of our data.
All concentrations are reported as the number of particles per gram of tissue wet weight. Data from the Raman analysis are based on the whole filter plastic count estimates extrapolated from the 8% of the filter that was analyzed. No corrections were made for the FTIR data since the whole filter was analyzed.
We considered applying the important LOD/LOQ approach of Horton et al. [33] to our data, however, the absence of plastic particles in all but one of the blanks (see Results) and the small number of particles in the samples diminished the utility of a full application of the LOD/LOQ approach in this case. Based on this approach, the LOD (which is polymer specific), would be 13 particles/sample for polypropylene (PP). For the other polymers where no contamination was identified, we can estimate that the resolution (LOD) of our analyses were 1 particle per sample for the FTIR analyses and 13 particles per sample for the Raman analyses. Any data indicating 0 particles should be interpreted as below the LOD.
Results
In the eighteen fish analyzed, 69 MPs were identified in the GI tract or the muscle. The fish collected from Falmouth generally contained more MPs than the fish collected from Bourne. Given the developing nature of the field, we describe the different metrics used to verify our sample analysis below. In the next section, the differences in MP morphologies are compared between the GI tract and muscle samples. Following that, we examine the impact of fish total length and sex on MP abundance.
Methodology verification
All encountered particles were analyzed using either Raman or FTIR. Of the 2,008 particles analyzed in various fish tissue samples, only 3.4% (69 particles) were identified as plastic (Table S2). All particles identified as plastic had a Pearson’s r correlation of at least 0.82, and an average correlation of 0.91 (Table S3).
Of the eight fish analyzed from the Falmouth site, seven had MPs in their GI tracts, while only five had MPs in their muscle tissue (Table 1). In terms of particle count, 5 plastic particles were found in the GI tract samples, and nine were detected in the muscle tissue. Among the ten fish analyzed from the Bourne site, MPs were observed in the GI tracts of four fish, and only one fish had MPs in the muscle tissue (Table 1). A total of nine plastic particles were found in the GI tracts, with only one particle detected in the muscle tissue of fish from Bourne site.
Table 1.
Average concentration and size of MPs in fish tissues from Falmouth and Bourne
| Collection site | Falmouth | Bourne | ||
|---|---|---|---|---|
| Tissue | GI Tract | Muscle | GI Tract | Muscle |
| MP Occurrence (# MP samples/total sample number) | 7/8 | 5/8 | 4/10 | 1/10 |
| MP Concentration (Particle Count/g w.w.) | 85.5 ± 70.2 | 11.4 ± 12.5 | 12.2 ± 18.1 | 1.69 ± 5.36 |
| Length (μm) | 24.8 (2.60 – 1291) | 15.5 (4.26 – 28.1) | 24.6 (5.19 – 728) | 8.73 |
| Width (μm) | 12.6 (2.10 – 140) | 9.52 (1.79 – 13.8) | 13.8 (2.35 – 248) | 8.95 |
| Aspect Ratio (Length/Width) | 1.41 (0.61 – 51.0) | 2.13 (0.97 – 10.8) | 2.21 (1.28 – 6.78) | 0.98 |
The mean concentration ± 1 standard deviation is shown
For size characteristics, the medians are shown with range in parentheses
In our procedural blanks, only one particle was identified as plastic (PP). No PP was detected in the fish samples analyzed concurrently with the contaminated procedural blank. Due to the detection of only a single plastic particle out of 11 procedural blanks, no blank correction was performed. Of the 180 particles analyzed with a correlation of at least 0.8, 70 particles were included in the microplastic analysis. Particles not included could be plastic particles that had altered spectra due to particle oxidation, the presence of additives, or a low signal to noise ratio. The analysis we used is highly conservative, due to uncertainty surrounding the 110 particles not included in the analysis, some of which could have been plastic.
We identified a variety of non-plastic particles that are known to be resistant to KOH digestion. Numerous plant-based, chitin and or bone particles were detected with FTIR analysis (Fig. S2A & S2B). Fish collected from Bourne had more plant-based particles in their GI tracts than Falmouth fish (Fig. S2A). Some cellulose and cotton particles were also detected in the samples (Fig. S2). Raman spectroscopy analysis detected primarily minerals, metal compounds, and fragments of soot or black pigment (Fig. S2C & S2D).
Plastic particle identification
Plastic concentrations
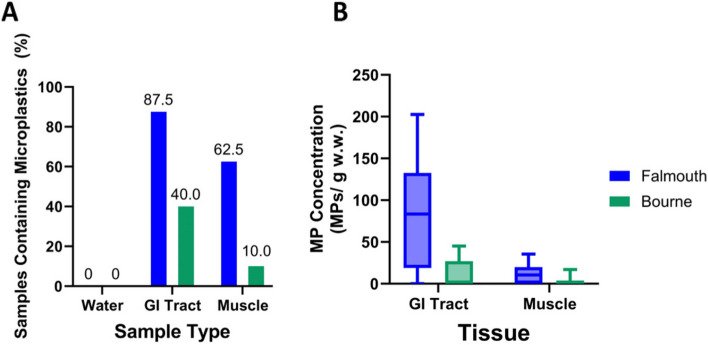
MP occurrence varied widely between the water and fish tissue (GI tract and muscle) samples. The water samples had no detected MPs (Fig. 4A). GI tract samples contained MPs more frequently than the muscle samples (Fig. 4A). Most Falmouth fish contained MPs, with 87.5% of the GI tract samples and 62.5% of muscle samples containing MPs (Fig. 4A). The Bourne sampling site had a lower MP occurrence, with 40.0% of the GI tract samples and 10.0% of muscle samples containing MPs (Fig. 4A).
Fig. 4.
Plastic occurrence and concentration in samples: Samples collected in Falmouth are shown in blue, and samples collected in Bourne are shown in green. A Graph showing percentage of samples that were found to contain microplastics for the three sample types studied (water, GI tract, and muscle). Numbers over bars indicate the percentage found. B Box and whisker plot showing the minimum and maximum number of microplastics found per gram of tissue (wet weight). Data were corrected to account for the whole sample based on the observed 8% of the filter that was analyzed. The solid line indicates the median concentration. N = 8 fish from Falmouth and N = 10 fish from Bourne
MP concentration varied by tissue and sampling site, with the Falmouth GI tract samples containing the largest concentration of plastics (Fig. 4B). The median concentration of MPs in the GI tract of fish from Falmouth was 83 particles/g w.w. while the muscle samples had a median concentration of only 11 particles/g w.w. tissue. Both the GI tract and muscle samples from Bourne fish had a median concentration of 0 particles/g w.w. with the GI tract having a 2.6 × greater range compared to the muscle samples (Fig. 4B). Many fish did not contain any MPs (Table 1), leading to large standard deviations.
Plastic sizes
The MP size distributions were similar between the two collection sites (Table 1), so the data from the sampling sites were combined to more closely examine how MP characteristics differ between tissues. Particles 2 μm—5 mm in length were analyzed in the collected samples. Length is defined as the longest dimension of the particle in a 2-dimensional plane. The particle lengths ranged from 2.6 μm—1291 μm. The median MP length in the GI tract was 24.7 μm, and the median MP length for the muscle samples was 14.5 μm (Fig. 5A). MPs in the GI tract had a greater size range of 1289 μm compared to the muscle MP’s size range of 23.9 μm (Fig. 5A); however, for both tissue types most of the particles (81.2%) were smaller than 50 μm in length (Fig. 5A).
Fig. 5.
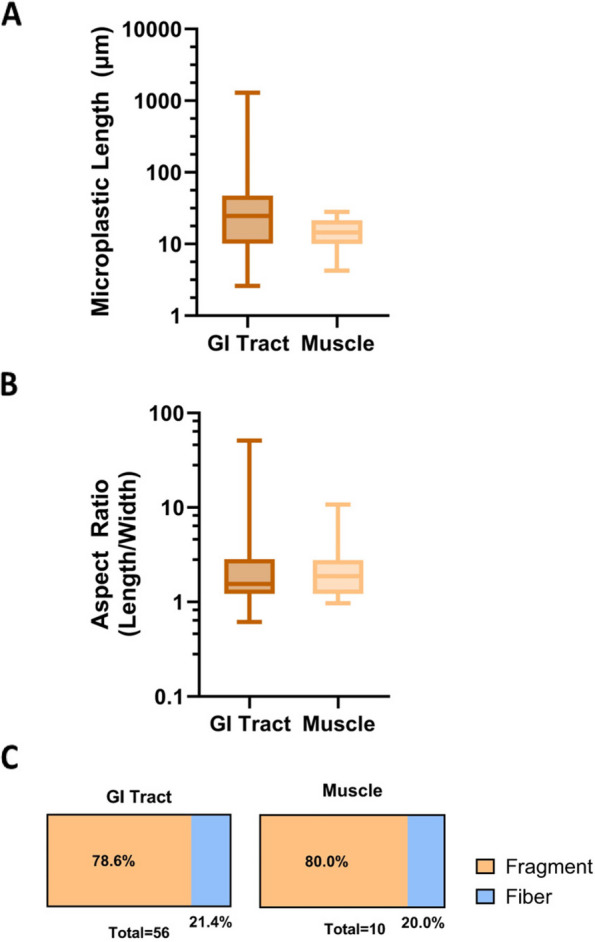
Plastic size distribution: GI tract samples shown in brown. Muscle samples shown in tan. A Full size range showing the lengths of microplastics in microns using a logarithmic scale. B Full range of the aspect ratios (length/width) for microplastics using a logarithmic scale. C Percentage of particles in samples classified as fragment (aspect ratio < 2) or fiber (aspect ratio > 2)
Plastic shape
The particle aspect ratio was determined by dividing the length of the particle by the particle width. The particle aspect ratios varied more widely in the GI tract than in the muscle samples (Fig. 5B). The median aspect ratios for the GI tract and muscle were approximately 1.6 and 1.9, respectively (Fig. 5B).
An aspect ratio of three or greater was used to define a particle as a fiber [46]. Any particles with an aspect ratio below three were defined as a fragment. The identified MPs were predominantly fragments (Fig. 5C). The GI tract and muscle samples had similar percentages of fragments and fibers. The GI tract particles were 78.6% fragments and 21.4% fibers, and the muscle particles were 80.0% fragments and 20.0% fibers (Fig. 5C).
Polymer types
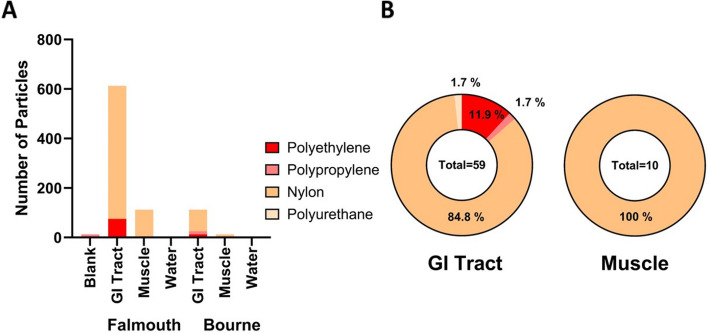
Nylon was the major polymer present in the samples (Fig. 6; Fig. S3). Polyethylene was found in the GI tracts collected from both Falmouth and Bourne sites in roughly equivalent percentages (Fig. 6A). We rarely found polypropylene and polyurethane in the samples (Fig. 6A). The GI tract particle composition consisted of nylon (84.8%), polyethylene (11.9%), polypropylene (1.7%), and polyurethane (1.7%) (Fig. 6B). The MPs found in the muscle samples were all nylon (Fig. 6B).
Fig. 6.
Microplastic Polymer Frequencies: A Total number of microplastics found in the samples color-coded by polymer type. Data were corrected to account for the whole sample based on the observed 8% of the filter that was analyzed. B Percentages of the different polymers present in the GI tract and muscle samples. Data for individual fish can be found in Figure S3 and Supplemental Data File 3
The full range of both the length and aspect ratio for each particle is shown in Figure S4A. The length of the nylon particles was significantly correlated (r = 0.5; p-value < 0.001) with aspect ratio (Fig. S4A & S4B; Linear regression R 2 = 0.74). The length of the polyethylene particles, on the other hand, was not correlated with the aspect ratio (r = 0.42; p-value 0.35; Fig. S4B). The polyethylene particles and nylon particles had similar proportions of fragments (Fig. S4C).
Plastic weathering
Identified MPs frequently had two spectral peaks (800-900 and 1600-1700 cm−1) suggestive of particle oxidation (Fig. S5; [55, 67]). An example of a spectrum displaying these oxidation peaks is illustrated in Figure S5A & S5B along with a picture of the particle and its identified chemical structure. Many particles found in both the GI tract and the muscle samples contained both of those peaks, indicating oxidation of the particles (Fig. S5C). Fewer MPs had the additional 1600 cm−1 peak compared to the 800-900 cm−1 peak (Fig. S5C). It should also be noted that only 10 MPs were detected in the muscle samples, which could skew the reported peak proportions.
Impact of fish length on microplastic abundance
MP abundance correlated with fish total length in the muscle and GI tract; however, key differences between the fish from the different sampling sites complicated this analysis. Only female fish were caught at the Falmouth site (8/8 fish), and mostly male fish were caught at the Bourne site (8/10 fish). The fish collected at the Falmouth site were also larger than those collected at the Bourne site (Fig. 7). These site differences made it challenging to disentangle the impact of sex and fish total length from the different collection sites (Fig. 7). Fish collected from both sites had equivalent Fulton’s condition factors.
Fig. 7.
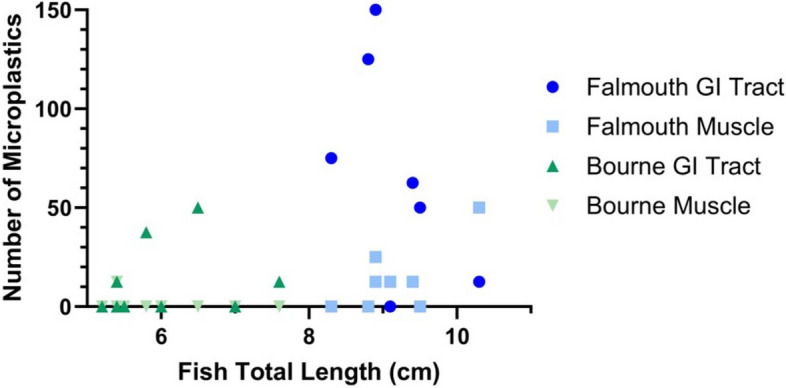
Relationship between fish total length and microplastic abundance: Scatter plot showing the relationship between the total length of a fish and the number of microplastics present. Data were corrected to account for the whole sample based on the observed 8% of the filter that was analyzed. Data was not normalized by wet weight. The Falmouth samples are shown in blue (n = 8), and the Bourne samples (n = 10) are shown in green. GI tract samples are shown in a darker shade than the muscle samples
Additionally, the water samples from both sites had MPs below the limit of detection, indicating that MP prevalence is low at both collection sites. Therefore, for the following analyses the information from both collection sites has been combined.
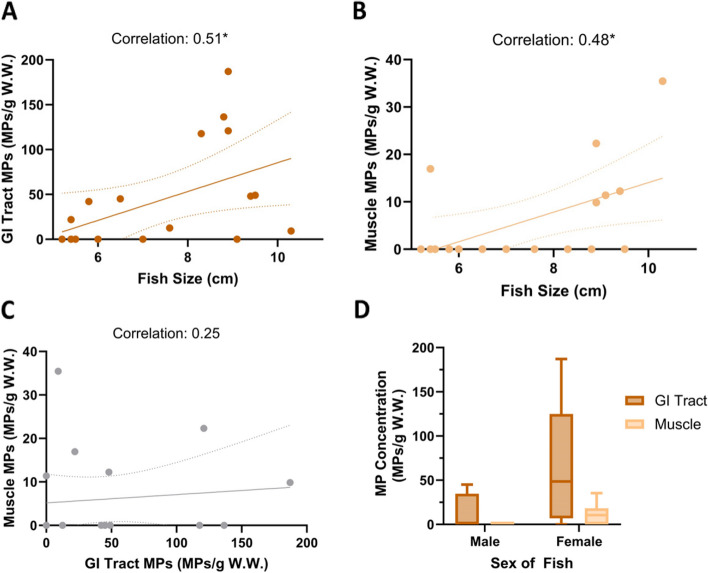
Fish total length correlated with MP abundance in both the GI tract (r = 0.51; p-value 0.03) and the muscle (r = 0.48; p-value 0.04; Fig. 8A & B). Both comparisons showed a moderately positive relationship. GI tract MP abundance did not correlate with muscle MP abundance (r = 0.25; p-value 0.31; Fig. 8C). Female fish had a greater MP abundance than male fish in both the GI tract and muscle (Fig. 8D). In fact, only female fish had MPs present in the muscle (Fig. 8D). The random forest analysis determined that fish total length was the most important variable for predicting muscle MP occurrence, followed by fish sex and then GI tract MP abundance (Table S4).
Fig. 8.
Microplastic Abundance Relationships with Fish Total Length and Sex: Data is corrected to account for the whole sample based on the observed 8% and normalized by tissue wet weight. A-C Linear regression lines with dotted 95% confidence intervals are shown. Correlation refers to the Spearman (nonparametric) correlation analysis between the two variables. A Scatter plot showing the relationship between the total length of a fish and the number of MPs in the GI tract. B Scatter plot showing the relationship between the fish total length and number of MPs in the muscle. C Scatter plot showing the relationship between GI tract MP concentration and Muscle MP concentration. D A box and whisker plot depicting the minimum and maximum concentration of microplastic particles (number of particles/g of tissue w.w.) for the males and females collected. Significance is indicated with an * (p-value < 0.05)
Discussion
The bioaccumulation potential of environmental MPs is not well understood, in part because MP detection is a developing field with little harmonization amongst methods. This study sought to draw on existing recommendations from the field while expanding on both classification techniques and the detectable size range of MPs. Using this rigorous approach, we compare the characteristics of MPs found in the GI tract and muscle. Of particular focus in the following sections are the small size of detected MPs and the correlation of MP abundance and fish total length. Other factors are also discussed to ensure a thorough characterization of the data for future research to draw upon.
Importance of methods in MP assessment
The concentrations and occurrence frequencies of MPs from different field studies are difficult to compare due to the lack of standardized methods across the field. It is unclear how much differences in MP identification approaches [1, 3, 14, 60, 68, 75], collection methods [30, 56, 58, 68, 90], and quality control measures [23, 42, 61, 68, 75, 78, 84, 93, 94] have influenced these results. These differences highlight the often reported [72, 73] need for harmonized methods of MP analysis and improved methods for detecting smaller MPs.
In our study, no significant background contamination was seen, with only one MP detected from the procedural blanks. Recovery assessments with 250 μm particles yielded an 80% recovery rate for three different polymers tested. All of the tested polymers were fragments or spheres, so the recovery rate for fibers in this study is unknown. Particles smaller than 250 μm were not tested due to difficulty in small particle manipulation. Protocols to assess the recovery of different sizes and shapes of MPs are essential to increase the reproducibility of these results.
MP sub-sampling was done in this study to reduce the analysis time of each sample; however, the use of sub-sampling can introduce misestimates of MP concentration [9]. Sub-sampling recommendations are still being developed, especially for MPs smaller than 50 μm. Previous recommendations suggest that our results should be representative of MPs present but might not capture MP polymers that occurred rarely in our sampling environment [15, 20].
Comparison of MPs in muscle and GI tract
We predicted that the size of the MPs is the most important parameter for potential bioaccumulation, but we also considered other factors that are known to impact particle uptake, such as particle shape, polymer composition, and degradation status.
Concentrations of MPs
We detected no plastic particles in our water samples using an established method for detecting small MPs in water [12, 18, 29, 75, 84]. Despite our water samples having non-detectable levels of MPs, MPs were detected in the sampled fish, indicating MP presence in our sample sites.
Both collection site and tissue type affected MP occurrence frequencies. MPs occurred more frequently in the GI tract than in the muscle samples. This trend is generally agreed upon in the literature [36, 60, 65] with one study finding the opposite trend [18].
The present study’s estimated GI tract MP concentration (45 particles/g w.w) is higher than those in other studies (Table 2). The muscle samples had a lower MP concentration than the GI tract samples, but the muscle sample concentration was still greater than most of those previously reported (Table 2). A difference in size limits of detection could partly explain this difference, as we were able to detect smaller particles than most previous studies.
Table 2.
MP concentrations in fish
| Study | MP size limit of detection (μm) | GI tract concentration (MPs/g W.W.) | Muscle concentration (MPs/g W.W.) | Digestion and chemical characterization method |
|---|---|---|---|---|
| Current Study | 2 | 45 ± 60 | 6 ± 10 |
KOH Raman and FTIR |
| [19] | 1-10 | - | 0.14-0.27 |
KOH Raman |
| [88] | 20 | 0.1 ± 0.1 – 8.8 ± 7.4 | N.D |
NaOH FTIR |
| [66] | 20 | 3.42 ± 3.2 | - |
KOH and H2O2 FTIR |
| [90] | 100 | 0.04 ± 0.05 – 0.47 ± 0.28 | - |
KOH FTIR |
| [94] | 100 | 0.02 ± 0.05 | 0.01 |
KOH Micro Raman |
A comparison of the average concentrations of MPs found in the GI tract and muscle. Concentrations are shown as the average number of MPs per gram of wet weight ± 1 standard deviation. If a range is present, multiple species were sampled in the study. A dash indicates the tissue was not analyzed. N.D not detected
The absorption of MPs into the GI tract epithelium cannot be determined from our results since both GI tract and GI tract contents were digested together. It has previously been shown that GI tract contents contain higher MP concentrations than just the GI tract epithelium [14, 83], suggesting that few particles are absorbed by the epithelium from ingested food,however, uptake is known to be in part size-dependent, and the retention and rate of tissue translocation of the smaller MPs found in our study are unknown.
Plastic sizes
The size of MPs found in both the environment and in biota ranges widely throughout the literature. MPs range from 1 μm—5 mm in commonly agreed definitions [82], however, few studies measure the full-size range of these particles. It is common for studies to have minimum size limits of detection that are much higher than the 1 μm theoretical minimum size, or to not report the size limits of detection (Reviewed in [69]). For example, we found that in studies reporting a minimum size detection limit, the median value was 206 μm, with most studies not reporting a minimum size detection limit [69]. These differences in size detection limits make it challenging to compare the abundance of MP sizes detected in fish tissues, such as the muscle, which is predicted to preferentially accumulate smaller MPs. The current study was able to measure particles 2 μm—5 mm in size.
Studies often find that smaller particles are the most prevalent in the atmosphere [47], water [87], and organisms [91]. Our study found the GI tract and muscle MP median lengths to be 24.7 μm and 14.5 μm respectively, sizes that are smaller than the most commonly reported minimum size detection limits for biological samples [5, 10, 54, 57, 59, 93]. Other studies in non-GI tract tissues have found MPs larger than those found in the current study [1, 14, 23, 30, 53, 57, 59–61, 84, 90, 94], but the uptake pathway used by these larger particles is unclear, as in some cases, they are above previously established uptake limits [49, 76].
The MPs found in the muscle were both smaller in size and size range than those found in the GI tract, implying a size restriction for particles to enter the muscle. It has been suggested that particle translocation from the GI tract through a mechanism called persorption has an upper size limit of 150 μm [49]. Persorption is the paracellular movement of particles in the desquamation region of epithelial cells [92]. This mechanism of transport has been previously critiqued as being unlikely and has not been demonstrated since its inception [35, 63]. The actual upper limit of translocation is uncertain, but previous research suggests that only particles smaller than 21 μm are capable of translocating into tissues [16, 34, 37, 49].
In the present study, eight (out of ten) of the MPs detected in muscle were smaller than 21 μm. The maximum size for particles found in the muscle was 28 μm. How these particles reached the muscle is unclear, but some phagocytic cells are 25—30 μm in size and might be capable of engulfing and transporting these particles [44]. GI tract absorption of particles is the most likely route of uptake, as particles larger than 10 μm are unlikely to be internalized from the lungs or gills [70]. We suggest that the MPs detected in the muscle were moved there following phagocytic cell engulfment from the GI tract.
The present study is the first to look for the full-size range of MPs in non-GI tract tissues without significant contamination issues. Previous environmental studies with size detection limits lower than 21 μm either did not look for larger MPs [19, 24] or had a significant amount of background contamination [4, 77, 88],however, it is clear from previous studies that particle translocation is governed by factors other than size, as particles theoretically small enough to translocate have not moved through the intestinal wall [38, 74].
Particle shape
The higher proportion of fragments, versus fibers, in our samples compared to previous studies could be due to the smaller size of MPs detected in our study versus these earlier studies [4, 30, 51, 53, 56–58, 68, 85]. Previously, fibers have tended to predominate in larger size fractions of MPs, while fragments make up the majority of particles < 100 μm [47, 68]. On the other hand, some studies that quantified particles down to 20 μm in size have detected primarily fibers, emphasizing that size is not necessarily the major predicting factor for determining particle shape [42, 65, 66, 84, 94, 95].
Previously, it has been speculated that fibers are more likely to translocate or be internalized by cells compared to other MP shapes [76],however, our study contradicts that finding as fibers were not found at a higher frequency in muscle.
Polymer types
Nylon was the predominant polymer (80-88%) in the GI tract samples. While nylon has occasionally been reported as the major polymer in samples spanning a large range of trophic levels [5, 11, 61, 85], polypropylene and polyethylene are usually the most common polymers reported in marine organisms [18, 19, 27, 40, 50, 54, 66, 95]. Polypropylene and polyethylene are less dense than water, and so species found near the surface of the water could be more likely to encounter these polymer types. Nylon, on the other hand, is slightly denser than seawater, and so would be more likely to sink to the benthic and benthopelagic environment where killifish dwell.
In contrast to the GI tracts, nylon was the only polymer found in the muscle samples. One other study has found nylon at an increased proportion in muscle compared to the GI tract [90] with other studies seeing no difference [58]. Our results suggest that nylon more readily penetrates into muscle than polyethylene.
Implications for MP bioaccumulation
We did not calculate a bioaccumulation factor from our results due to a lack of information regarding the water and sediment concentrations of MPs at the collection sites. Our results suggest that few MPs are ultimately internalized to the muscle. MP deposition in the muscle was potentially impacted by the sex and total length of the fish.
Relationship between fish length and MP abundance
The long-term sequestration of larger MPs (> 100 μm) has previously been shown to be unlikely [11, 27, 53, 57, 91], reviewed in [69]. The bioaccumulation potential, especially for smaller MPs (< 100 μm, has remained unclear, largely due to a lack of information about the distribution and abundance of MPs in non-GI tract tissues; however, our study has started to address this knowledge gap.
A positive relationship was observed between fish length and the MP abundance in the GI tract. This finding agrees with the majority of studies [23, 30, 36, 51, 52, 56, 57] despite some contradictory evidence [18, 40, 66, 90]. It has been suggested that one reason studies report a positive correlation could be that as fish grow, they can ingest a wider size range of MPs and, consequently, are exposed to more MPs in the environment [8]. Larger fish also have increased dietary needs, and so might ingest more prey, thus increasing their incidental MP ingestion.
A positive relationship was also seen between fish length and MP abundance and occurrence in the muscle. This contrasts with most of the existing literature, which did not see this trend for non-GI tract tissues [30, 52, 57]. The lowest reported MP size for some of these studies was approximately 63 μm, which is 31.5 times larger than our lowest reported MP size [30, 57]. It seems likely that MP size influences trends seen regarding fish length. Our results suggest that small MPs, which are often not quantified, are the primary MPs to be found in tissues like the muscle. Killifish increase in length as they age [2], so the correlation between fish total length and muscle MP abundance also could be indicative of bioaccumulation.
Relationship between MP abundance in the GI tract and the muscle
There was no observed relationship between the abundance of MPs in the GI tract and in the muscle. This finding agrees with the majority of previous studies [7, 26, 78] with a few conflicting reports [57, 61]. Generally, this suggests that the MP residence times differ between these tissues, and therefore one tissue cannot be used to predict the content of another. GI tract residence time is short, with previous studies showing rapid elimination of larger MPs (> 250 μm) in killifish [64]. Given the short residence time of MP in the GI tract and the lack of correlation between GI tract and muscle MP abundance, bioaccumulation assessments should not include GI tract data when considering the long-term sequestration potential of MPs.
Other factors
There were several differences between the sites that potentially contributed to the different patterns in MP abundance that we saw including: proximity to a residential area, connection to the ocean, differences in recent weather patterns, and differences in the characteristics of the collected fish; however, the MP water concentrations were not detectable from both collection sites, indicating similar low contamination levels.
Only female fish had MPs in muscle samples, and they also had greater GI tract MP abundance. This finding could be confounded by several biases, such as the collected females being predominantly from the Falmouth site and that female fish are larger than male fish; however, a couple of other studies have observed the same trend [23, 56]. Overall, relatively few studies have compared male and female fish in terms of the MP content of GI tract and tissues. In the future, more research is needed to investigate the origins of this trend.
Conclusions
There continues to be a dearth of information regarding the distribution of smaller MPs to non-GI tract tissues. This study only detected smaller MPs in the muscle tissues, suggesting a potential upper limit on the sizes of MPs in muscle tissue. This MP abundance was positively correlated with fish total length, indicating that the smaller MPs detected in this study might bioaccumulate. Future studies should focus MP quantification efforts on small MPs in non-GI tract tissues to further investigate this trend.
Supplementary Information
Acknowledgements
Not applicable.
Authors’ contributions
JAP: Conceptualization, Formal analysis, Funding acquisition, Methodology, Visualization, Investigation, Writing - original draft, Writing - review & editing. SMG: Methodology, Software, Funding acquisition, Resources, Writing – Review & Editing; SY: Methodology, Software, Writing – Review & Editing; APMM: Methodology, Resources, Writing – Review & Editing; MEH: Conceptualization, Funding acquisition, Methodology, Supervision, Writing - review & editing; NA: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Writing - review & editing.
Funding
This research was supported in part by Woods Hole Sea Grant (Award No. NA18OAR4170104, project R/P–89) to M.E.H., N.A., and J.A.P. and by an NSF Graduate Research Fellowship to J.A.P. Additional support was provided by Woods Hole Sea Grant (Award No. NA18OAR4170104, project R/O-59 to S.M.G.) and by the Woods Hole Center for Oceans and Human Health (NIH/NIEHS grant P01ES028938 and NSF grant OCE-1840381 to M.E.H. and N.A.).
Data availability
The data supporting the findings of this study are available within the paper and its supplementary material and data files. Raw data files (Raman and FTIR spectra) are available from the corresponding author upon reasonable request.
Supplementary Data files:
43591_2024_101_MOESM1_ESM.docx
Data File 1 Raman Spectroscopy Match Information.xlsx.
Data File 2 Plastic Particle Size Measurements.xlsx.
Data File 3 Plastic Particles per Fish.xlsx.
Declarations
Ethics approval and consent to participate
All procedures performed in the study were in accordance with the ethical principles described in the Guide for the Care and Use of Laboratory Animals (U.S. National Research Council, Eighth Edition, 2011). Euthanasia was performed according to the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2013 Edition). The research protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Woods Hole Oceanographic Institution (Assurance D16-00381 from the Office of Laboratory Animal Welfare (OLAW) at the U.S. National Institutes of Health).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Abbasi S, Soltani N, Keshavarzi B, Moore F, Turner A, Hassanaghaei M. Microplastics in different tissues of fish and prawn from the Musa Estuary, Persian Gulf. Chemosphere. 2018;205:80–7. 10.1016/J.CHEMOSPHERE.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 2.Abraham BJ. Species profiles. Life histories and environmental requirements of coastal fishes and invertebrates (Mid-Atlantic). Mummichog and Striped Killifish. 1985.
- 3.Akhbarizadeh R, Moore F, Keshavarzi B. Investigating a probable relationship between microplastics and potentially toxic elements in fish muscles from northeast of Persian Gulf. Environ Pollut. 2018;232:154–63. 10.1016/J.ENVPOL.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 4.Akoueson F, Sheldon LM, Danopoulos E, Morris S, Hotten J, Chapman E, Li J, Rotchell JM. A preliminary analysis of microplastics in edible versus non-edible tissues from seafood samples. Environ Pollut. 2020;263:114452. 10.1016/J.ENVPOL.2020.114452. [DOI] [PubMed] [Google Scholar]
- 5.Amini-Birami F, Keshavarzi B, Esmaeili HR, Moore F, Busquets R, Saemi-Komsari M, Zarei M, Zarandian A. Microplastics in aquatic species of Anzali wetland: an important freshwater biodiversity hotspot in Iran. Environ Pollut. 2023;330:121762. 10.1016/J.ENVPOL.2023.121762. [DOI] [PubMed] [Google Scholar]
- 6.Andrady AL. Plastics and the Ocean: origin, characterization, fate, and impacts. Hoboken, NJ: John Wiley & Sons, Inc; 2022. p. 484. Impacts. Hoboken, NJ: John Wiley & Sons, Inc. 484p. 10.1002/9781119768432. [Google Scholar]
- 7.Arafat ST, Tanoiri H, Yokota M, Nakano H, Arakawa H, Terahara T, Kobayashi T. Microplastic pollution in the gastrointestinal tract of giant river catfish Sperata seenghala (Sykes, 1839) from the Meghna River, Bangladesh. Environ Sci Pollut Res. 2023;1:1–11. 10.1007/S11356-023-28750-Z/FIGURES/4. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjee Sh, Mandal B, Das R, Bhattacharyya S, Chaudhuri P, Mukherjee A. Microplastics in gastro-intestinal tract of estuarine fish from the mangrove ecosystem of Indian Sundarbans. Iran J Fish Sci. 2023;22(2):317–38. 10.22092/IJFS.2023.129046. [Google Scholar]
- 9.Brandt J, Fischer F, Kanaki E, Enders K, Labrenz M, Fischer D. Assessment of Subsampling Strategies in Microspectroscopy of Environmental Microplastic Samples. Front Environ Sci. 2021;8:288. 10.3389/FENVS.2020.579676. [Google Scholar]
- 10.Collard F, Gilbert B, Compère P, Eppe G, Das K, Jauniaux T, Parmentier E. Microplastics in livers of European anchovies (Engraulis encrasicolus, L.). Environ Pollut. 2017;229:1000–5. 10.1016/J.ENVPOL.2017.07.089. [DOI] [PubMed] [Google Scholar]
- 11.Covernton GA, Cox KD, Fleming WL, Buirs BM, Davies HL, Juanes F, Dudas SE, Dower JF. Large size (>100-μm) microplastics are not biomagnifying in coastal marine food webs of British Columbia. Can Ecol Appl. 2022;32(7):e2654. 10.1002/EAP.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Covernton GA, Pearce CM, Gurney-Smith HJ, Chastain SG, Ross PS, Dower JF, Dudas SE. Size and shape matter: A preliminary analysis of microplastic sampling technique in seawater studies with implications for ecological risk assessment. Sci Total Environ. 2019;667:124–32. 10.1016/J.SCITOTENV.2019.02.346. [DOI] [PubMed] [Google Scholar]
- 13.Cowger W, Steinmetz Z, Gray A, Munno K, Lynch J, Hapich H, Primpke S, De Frond H, Rochman C, Herodotou O. Microplastic Spectral Classification Needs an Open Source Community: Open Specy to the Rescue! Anal Chem. 2021;93(21):7543–8. 10.1021/ACS.ANALCHEM.1C00123/SUPPL_FILE/AC1C00123_SI_005.ZIP. [DOI] [PubMed] [Google Scholar]
- 14.Curtean-Bănăduc A, Mihuţ C, Burcea A, Mccall GS, Matei C, Bănăduc D. Screening for microplastic uptake in an urbanized freshwater ecosystem: chondrostoma nasus (Linnaeus, 1758) case study. Water. 2023;15(8):1578. 10.3390/W15081578. [Google Scholar]
- 15.De Frond H, O’Brien AM, Rochman CM. Representative subsampling methods for the chemical identification of microplastic particles in environmental samples. Chemosphere. 2023;310:136772. 10.1016/J.CHEMOSPHERE.2022.136772. [DOI] [PubMed] [Google Scholar]
- 16.Deng Y, Zhang Y, Lemos B, Ren H. Tissue accumulation of microplastics in mice and biomarker responses suggest widespread health risks of exposure. Sci Rep. 2017;1:1–10. 10.1038/srep46687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delon L, Gibson RJ, Prestidge CA, Thierry B. Mechanisms of uptake and transport of particulate formulations in the small intestine. J Control Release. 2022;343:584–99. 10.1016/J.JCONREL.2022.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Dent AR, Chadwick DDA, Eagle LJB, Gould AN, Harwood M, Sayer CD, Rose NL. Microplastic burden in invasive signal crayfish (Pacifastacus leniusculus) increases along a stream urbanization gradient. Ecol Evol. 2023;13(5):e10041. 10.1002/ECE3.10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Giacinto F, Di Renzo L, Mascilongo G, Notarstefano V, Gioacchini G, Giorgini E, Bogdanović T, Petričević S, Listeš E, Brkljača M, Conti F, Profico C, Zambuchini B, Di Francesco G, Giansante C, Diletti G, Ferri N, Berti M. Detection of microplastics, polymers and additives in edible muscle of swordfish (Xiphias gladius) and bluefin tuna (Thunnus thynnus) caught in the Mediterranean Sea. J Sea Res. 2023;192:102359. 10.1016/J.SEARES.2023.102359. [Google Scholar]
- 20.El Khatib D, Langknecht TD, Cashman MA, Reiss M, Somers K, Allen H, Ho KT, Burgess RM. Assessment of filter subsampling and extrapolation for quantifying microplastics in environmental samples using Raman spectroscopy. Mar Pollut Bull. 2023;192:115073. 10.1016/J.MARPOLBUL.2023.115073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enders K, Lenz R, Stedmon CA, Nielsen TG. Abundance, size and polymer composition of marine microplastics ≥ 10 μm in the Atlantic Ocean and their modelled vertical distribution. Mar Pollut Bull. 2015;100(1):70–81. 10.1016/J.MARPOLBUL.2015.09.027. [DOI] [PubMed] [Google Scholar]
- 22.Erni-Cassola G, Gibson MI, Thompson RC, Christie-Oleza JA. Lost, but Found with Nile Red: A Novel Method for Detecting and Quantifying Small Microplastics (1 mm to 20 μm) in Environmental Samples. Environ Sci Technol. 2017;51(23):13641–8. 10.1021/ACS.EST.7B04512/SUPPL_FILE/ES7B04512_SI_001.PDF. [DOI] [PubMed] [Google Scholar]
- 23.Esmaeilbeigi M, Kazemi A, Gholizadeh M, Rezaeiyeh RD. Microplastics and heavy metals contamination in Atropus atropos and associated health risk assessment in the northwest of the Persian Gulf. Iran Regional Stud Marine Sci. 2023;57:102750. 10.1016/J.RSMA.2022.102750. [Google Scholar]
- 24.Ferrante M, Pietro Z, Allegui C, Maria F, Antonio C, Pulvirenti E, Favara C, Chiara C, Grasso A, Omayma M, Gea OC, Banni M. Microplastics in fillets of Mediterranean seafood. A risk assessment study Environmental Research. 2022;204:112247. 10.1016/J.ENVRES.2021.112247. [DOI] [PubMed] [Google Scholar]
- 25.Fulfer VM, Walsh JP. Extensive estuarine sedimentary storage of plastics from city to sea: Narragansett Bay, Rhode Island, USA. Sci Rep. 2023;13(1):1–11. 10.1038/s41598-023-36228-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gedik K, Eryaşar AR, Emanet M, Şahin C, Ceylan Y. Monthly microplastics change in European anchovy’s (Engraulis encrasicolus) gastrointestinal tract in the Black Sea. Mar Pollut Bull. 2023;194:115303. 10.1016/J.MARPOLBUL.2023.115303. [DOI] [PubMed] [Google Scholar]
- 27.Giani D, Andolina C, Baini M, Panti C, Sciandra M, Vizzini S, Fossi MC. Trophic niche influences ingestion of micro- and mesoplastics in pelagic and demersal fish from the Western Mediterranean Sea. Environ Pollut. 2023;328:121632. 10.1016/J.ENVPOL.2023.121632. [DOI] [PubMed] [Google Scholar]
- 28.Gouin T. Toward an Improved Understanding of the Ingestion and Trophic Transfer of Microplastic Particles: Critical Review and Implications for Future Research. Environ Toxicol Chem. 2020;39(6):1119–37. 10.1002/ETC.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green DS, Kregting L, Boots B, Blockley DJ, Brickle P, da Costa M, Crowley Q. A comparison of sampling methods for seawater microplastics and a first report of the microplastic litter in coastal waters of Ascension and Falkland Islands. Mar Pollut Bull. 2018;137:695–701. 10.1016/J.MARPOLBUL.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Guilhermino L, Martins A, Lopes C, Raimundo J, Vieira LR, Barboza LGA, Costa J, Antunes C, Caetano M, Vale C. Microplastics in fishes from an estuary (Minho River) ending into the NE Atlantic Ocean. Mar Pollut Bull. 2021;173:113008. 10.1016/J.MARPOLBUL.2021.113008. [DOI] [PubMed] [Google Scholar]
- 31.Gulizia AM, Brodie E, Daumuller R, Bloom SB, Corbett T, Santana MMF, Motti CA, Vamvounis G, Gulizia AM, Brodie E, Daumuller R, Bloom SB, Corbett T, Santana MMF, Vamvounis G, Motti CA. Evaluating the Effect of Chemical Digestion Treatments on Polystyrene Microplastics: Recommended Updates to Chemical Digestion Protocols. Macromol Chem Phys. 2022;223(13):2100485. 10.1002/MACP.202100485. [Google Scholar]
- 32.Hermsen E, Mintenig SM, Besseling E, Koelmans AA. Quality Criteria for the Analysis of Microplastic in Biota Samples: A Critical Review. Environ Sci Technol. 2018;52(18):10230–40. 10.1021/ACS.EST.8B01611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horton AA, Cross RK, Read DS, Jürgens MD, Ball HL, Svendsen C, Vollertsen J, Johnson AC. Semi-automated analysis of microplastics in complex wastewater samples. Environ Pollut. 2021;268:115841. 10.1016/J.ENVPOL.2020.115841. [DOI] [PubMed] [Google Scholar]
- 34.Huang T, Zhang W, Lin T, Liu S, Sun Z, Liu F, Yuan Y, Xiang X, Kuang H, Yang B, Zhang D. Maternal exposure to polystyrene nanoplastics during gestation and lactation induces hepatic and testicular toxicity in male mouse offspring. Food Chem Toxicol. 2022;160:112803. 10.1016/J.FCT.2021.112803. [DOI] [PubMed] [Google Scholar]
- 35.Hussain N, Jaitley V, Florence AT. Recent advances in the understanding of uptake of microparticulates across the gastrointestinal lymphatics. Adv Drug Deliv Rev. 2001;50(1–2):107–42. 10.1016/S0169-409X(01)00152-1. [DOI] [PubMed] [Google Scholar]
- 36.Jeyasanta KI, Laju RL, Patterson J, Jayanthi M, Bilgi DS, Sathish N, Edward JKP. Microplastic pollution and its implicated risks in the estuarine environment of Tamil Nadu. India Science of The Total Environment. 2023;861:160572. 10.1016/J.SCITOTENV.2022.160572. [DOI] [PubMed] [Google Scholar]
- 37.Jin H, Ma T, Sha X, Liu Z, Zhou Y, Meng X, Chen Y, Han X, Ding J. Polystyrene microplastics induced male reproductive toxicity in mice. J Hazard Mater. 2021;401:123430. 10.1016/J.JHAZMAT.2020.123430. [DOI] [PubMed] [Google Scholar]
- 38.Kim J, Poirier DG, Helm PA, Bayoumi M, Rochman CM. No evidence of spherical microplastics (10–300 μm) translocation in adult rainbow trout (Oncorhynchus mykiss) after a two-week dietary exposure. PLoS One. 2020;15(9):e0239128. 10.1371/JOURNAL.PONE.0239128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koelmans AA, Mohamed Nor NH, Hermsen E, Kooi M, Mintenig SM, De France J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019;155:410–22. 10.1016/J.WATRES.2019.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kor K, Jannat B, Ershadifar H, Ghazilou A. Microplastic occurrence in finfish and shellfish from the mangroves of the northern Gulf of Oman. Mar Pollut Bull. 2023;189:114788. 10.1016/J.MARPOLBUL.2023.114788. [DOI] [PubMed] [Google Scholar]
- 41.Kuhn M. Building predictive models in R using the caret package. J Statist Softw. 2008;28(5):1–26. 10.18637/JSS.V028.I05. [Google Scholar]
- 42.Kumari N, Yadav DK, Yasha Khan, P. K., & Kumar, R. Occurrence of plastics and their characterization in wild caught fish species (Labeo rohita, Wallago attu and Mystus tengara) of River Ganga (India) compared to a commercially cultured species (L rohita). Environmental Pollution. 2023;334. 10.1016/J.ENVPOL.2023.122141. [DOI] [PubMed] [Google Scholar]
- 43.Lebreton L, Egger M, Slat B. A global mass budget for positively buoyant macroplastic debris in the ocean. Sci Rep. 2019;9(1):1–10. 10.1038/s41598-019-49413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lendeckel U, Venz S, Wolke C. Macrophages: shapes and functions. ChemTexts. 2022;8(2):1–12. 10.1007/S40828-022-00163-4/TABLES/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenz R, Enders K, Stedmon CA, MacKenzie DMA, Nielsen TG. A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar Pollut Bull. 2015;100(1):82–91. 10.1016/J.MARPOLBUL.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Li Y, Shao L, Wang W, Zhang M, Feng X, Li W, Zhang D. Airborne fiber particles: Types, size and concentration observed in Beijing. Sci Total Environ. 2020;705:135967. 10.1016/J.SCITOTENV.2019.135967. [DOI] [PubMed] [Google Scholar]
- 47.Liao Z, Ji X, Ma Y, Lv B, Huang W, Zhu X, Fang M, Wang Q, Wang X, Dahlgren R, Shang X. Airborne microplastics in indoor and outdoor environments of a coastal city in Eastern China. J Hazard Mater. 2021;417:126007. 10.1016/J.JHAZMAT.2021.126007. [DOI] [PubMed] [Google Scholar]
- 48.Löder MGJ, Imhof HK, Ladehoff M, Löschel LA, Lorenz C, Mintenig S, Piehl S, Primpke S, Schrank I, Laforsch C, Gerdts G. Enzymatic Purification of Microplastics in Environmental Samples. Environ Sci Technol. 2017;51(24):14283–92. 10.1021/ACS.EST.7B03055. [DOI] [PubMed] [Google Scholar]
- 49.Lusher A, Hollman P, Mendoza-Hill J. Microplastics in fisheries and aquaculture: status of knowledge on their occurrence and implications for aquatic organisms and food safety. Rome: FAO; 2017.
- 50.Mahu E, Datsomor WG, Folorunsho R, Fisayo J, Crane R, Marchant R, Montford J, Boateng MC, Edusei Oti M, Oguguah MN, Gordon C. Human health risk and food safety implications of microplastic consumption by fish from coastal waters of the eastern equatorial Atlantic Ocean. Food Control. 2023;145:109503. 10.1016/J.FOODCONT.2022.109503. [Google Scholar]
- 51.Mai H, Thao Nhi Ngo T, Thien Nguyen D, Hoi Bui V, Duong Dao T, Thom Dang T, Do Manh V, Quoc Viet H, Giay C, Noi H, Nam V. Preliminary assessment of microplastic pollution in commercial freshwater fish species collected from four districts in Bac Ninh province. Vietnam J Sci Technol. 2023;61(4):629–39. 10.15625/2525-2518/17926. [Google Scholar]
- 52.Makhdoumi P, Hossini H, Nazmara Z, Mansouri K, Pirsaheb M. Occurrence and exposure analysis of microplastic in the gut and muscle tissue of riverine fish in Kermanshah province of Iran. Mar Pollut Bull. 2021;173:112915. 10.1016/J.MARPOLBUL.2021.112915. [DOI] [PubMed] [Google Scholar]
- 53.Matias RS, Gomes S, Barboza LGA, Salazar-Gutierrez D, Guilhermino L, Valente LMP. Microplastics in water, feed and tissues of European seabass reared in a recirculation aquaculture system (RAS). Chemosphere. 2023;335:139055. 10.1016/J.CHEMOSPHERE.2023.139055. [DOI] [PubMed] [Google Scholar]
- 54.Matluba M, Ahmed MK, Chowdhury KMA, Khan N, Ashiq MAR, Islam MS. The pervasiveness of microplastic contamination in the gastrointestinal tract of fish from the western coast of Bangladesh. Mar Pollut Bull. 2023;193:115145. 10.1016/J.MARPOLBUL.2023.115145. [DOI] [PubMed] [Google Scholar]
- 55.Matsui H, Arrivo SM, Valentini JJ, Weber JN. Resonance Raman studies of photoinduced decomposition of nylon-6, 6: product identification and mechanistic determination. Macromolecules. 2000;33(15):5655–64. [Google Scholar]
- 56.Matupang DM, Zulkifli HI, Arnold J, Lazim AM, Ghaffar MA, Musa SM. Tropical sharks feasting on and swimming through microplastics: First evidence from Malaysia. Mar Pollut Bull. 2023;189:114762. 10.1016/J.MARPOLBUL.2023.114762. [DOI] [PubMed] [Google Scholar]
- 57.McIlwraith HK, Kim J, Helm P, Bhavsar SP, Metzger JS, Rochman CM. Evidence of Microplastic Translocation in Wild-Caught Fish and Implications for Microplastic Accumulation Dynamics in Food Webs. Environ Sci Technol. 2021;55(18):12372–82. 10.1021/ACS.EST.1C02922/SUPPL_FILE/ES1C02922_SI_002.XLSX. [DOI] [PubMed] [Google Scholar]
- 58.Menéndez D, Blanco-Fernandez C, Machado-Schiaffino G, Ardura A, Garcia-Vazquez E. High microplastics concentration in liver is negatively associated with condition factor in the Benguela hake Merluccius polli. Ecotoxicol Environ Saf. 2023;262:115135. 10.1016/J.ECOENV.2023.115135. [DOI] [PubMed] [Google Scholar]
- 59.Milne MH, Helm PA, Munno K, Bhavsar SP, Rochman CM. Microplastics and Anthropogenic Particles in Recreationally Caught Freshwater Fish from an Urbanized Region of the North American Great Lakes. Environmental Health Perspectives. 2024;132(7). 10.1289/EHP13540. [DOI] [PMC free article] [PubMed]
- 60.My TTA, Dat ND, Hung NQ. Occurrence and Characteristics of Microplastics in Wild and Farmed Shrimps Collected from Cau Hai Lagoon. Central Vietnam Molecules. 2023;28(12):4634. 10.3390/MOLECULES28124634/S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nawar N, Rahman MM, Chowdhury FN, Marzia S, Ali MM, Akbor MA, Siddique MAB, Khatun MA, Shahjalal M, Huque R, Malafaia G. Characterization of microplastic pollution in the Pasur river of the Sundarbans ecosystem (Bangladesh) with emphasis on water, sediments, and fish. Sci Total Environ. 2023;868:161704. 10.1016/J.SCITOTENV.2023.161704. [DOI] [PubMed] [Google Scholar]
- 62.Nordberg GF. Historical perspectives on cadmium toxicology. Toxicol Appl Pharmacol. 2009;238(3):192–200. 10.1016/J.TAAP.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 63.O’hagan, D. T. The intestinal uptake of particles and the implications for drug and antigen delivery. J Anal. 1996;189:477–82. [PMC free article] [PubMed] [Google Scholar]
- 64.Ohkubo N, Ito M, Hano T, Kono K, Mochida K. Estimation of the uptake and gut retention of microplastics in juvenile marine fish: Mummichogs (Fundulus heteroclitus) and red seabreams (Pagrus major). Mar Pollut Bull. 2020;160:111630. 10.1016/J.MARPOLBUL.2020.111630. [DOI] [PubMed] [Google Scholar]
- 65.Pandey N, Verma R, Patnaik S, Anbumani S. Abundance, characteristics, and risk assessment of microplastics in indigenous freshwater fishes of India. Environ Res. 2023;218:115011. 10.1016/J.ENVRES.2022.115011. [DOI] [PubMed] [Google Scholar]
- 66.Park B, Kim SK, Joo S, Kim JS, Jo K, Song NS, Im J, Lee HJ, Kim SW, Lee SB, Kim S, Lee Y, Kim BY, Kim TW. Microplastics in large marine animals stranded in the Republic of Korea. Mar Pollut Bull. 2023;189:114734. 10.1016/J.MARPOLBUL.2023.114734. [DOI] [PubMed] [Google Scholar]
- 67.Phan S, Padilla-Gamiño JL, Luscombe CK. The effect of weathering environments on microplastic chemical identification with Raman and IR spectroscopy: Part I. polyethylene and polypropylene. Polymer Test. 2022;116. 10.1016/J.POLYMERTESTING.2022.107752. [Google Scholar]
- 68.Piskuła P, Astel AM. Microplastics in Commercial Fishes and By-Catch from Selected FAO Major Fishing Areas of the Southern Baltic Sea. Animals. 2023;13(3):458. 10.3390/ANI13030458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pitt JA, Aluru N, Hahn ME. Microplastics in Marine Food Webs. In S. Shumway & E. Ward (Eds.), Plastics in the Sea: Occurrence and Impacts. Cambridge: Academic Press; 2024.
- 70.Prata JC. Microplastics and human health: Integrating pharmacokinetics. 2023;53(16):1489–1511. 10.1080/10643389.2023.2195798.
- 71.Provencher JF, Ammendolia J, Rochman CM, Mallory ML. Assessing plastic debris in aquatic food webs: what we know and don’t know about uptake and trophic transfer. Environ Rev. 2019;27(3):304–17. 10.1139/er-2018-0079. [Google Scholar]
- 72.Provencher JF, Bond AL, Avery-Gomm S, Borrelle SB, Bravo Rebolledo EL, Hammer S, Kühn S, Lavers JL, Mallory ML, Trevail A, Van Franeker JA. Quantifying ingested debris in marine megafauna: a review and recommendations for standardization. Anal Methods. 2017;9(9):1454–69. 10.1039/C6AY02419J. [Google Scholar]
- 73.Provencher JF, Covernton GA, Moore RC, Horn DA, Conkle JL, Lusher AL. Proceed with caution: The need to raise the publication bar for microplastics research. Sci Total Environ. 2020;748:141426. 10.1016/J.SCITOTENV.2020.141426. [DOI] [PubMed] [Google Scholar]
- 74.Pyl M, Taylor A, Oberhänsli F, Swarzenski P, Hussamy L, Besson M, Danis B, Metian M. Size-dependent transfer of microplastics across the intestinal wall of the echinoid Paracentrotus lividus. Aquat Toxicol. 2022;250:106235. 10.1016/J.AQUATOX.2022.106235. [DOI] [PubMed] [Google Scholar]
- 75.Qaiser N, Sidra S, Javid A, Iqbal A, Amjad M, Azmat H, Arooj F, Farooq K, Nimra A, Ali Z. Microplastics abundance in abiotic and biotic components along aquatic food chain in two freshwater ecosystems of Pakistan. Chemosphere. 2023;313:137177. 10.1016/J.CHEMOSPHERE.2022.137177. [DOI] [PubMed] [Google Scholar]
- 76.Ramsperger AFRM, Bergamaschi E, Panizzolo M, Fenoglio I, Barbero F, Peters R, Undas A, Purker S, Giese B, Lalyer CR, Tamargo A, Moreno-Arribas MV, Grossart HP, Kühnel D, Dietrich J, Paulsen F, Afanou AK, Zienolddiny-Narui S, Eriksen Hammer S, Laforsch C. Nano- and microplastics: a comprehensive review on their exposure routes, translocation, and fate in humans. Nano Impact. 2023;29:100441. 10.1016/J.IMPACT.2022.100441. [DOI] [PubMed]
- 77.Rasta M, Sattari M, Taleshi MS, Namin JI. Microplastics in different tissues of some commercially important fish species from Anzali Wetland in the Southwest Caspian Sea. Northern Iran Marine Poll Bull. 2021;169:112479. 10.1016/J.MARPOLBUL.2021.112479. [DOI] [PubMed] [Google Scholar]
- 78.Raza MH, Jabeen F, Ikram S, Zafar S. Characterization and implication of microplastics on riverine population of the River Ravi, Lahore. Pakistan Environ Sci Poll Res. 2023;30(3):6828–48. 10.1007/S11356-022-22440-Y/FIGURES/17. [DOI] [PubMed] [Google Scholar]
- 79.Remy F, Collard F, Gilbert B, Compère P, Eppe G, Lepoint G. When Microplastic Is Not Plastic: The Ingestion of Artificial Cellulose Fibers by Macrofauna Living in Seagrass Macrophytodetritus. Environ Sci Technol. 2015;49(18):11158–66. 10.1021/ACS.EST.5B02005. [DOI] [PubMed] [Google Scholar]
- 80.Renner G, Nellessen A, Schwiers A, Wenzel M, Schmidt TC, Schram J. Data preprocessing & evaluation used in the microplastics identification process: A critical review & practical guide. TrAC, Trends Anal Chem. 2019;111:229–38. 10.1016/J.TRAC.2018.12.004. [Google Scholar]
- 81.Ricker WE. Computation and interpretation of biological statistics of fish populations. Fish Res Board Can Bull. 1975;191:1–382. https://cir.nii.ac.jp/crid/1573950400225789696. [Google Scholar]
- 82.Rochman CM, Brookson C, Bikker J, Djuric N, Earn A, Bucci K, Athey S, Huntington A, McIlwraith H, Munno K, De Frond H, Kolomijeca A, Erdle L, Grbic J, Bayoumi M, Borrelle SB, Wu T, Santoro S, Werbowski LM, Hung C. Rethinking microplastics as a diverse contaminant suite. Environ Toxicol Chem. 2019;38(4):703–11. 10.1002/ETC.4371. [DOI] [PubMed] [Google Scholar]
- 83.Rosas BRC, Sakthi JS, Barjau-González E, Rodríguez-González F, Galván-Magaña F, Ramírez SF, Gómez-Chávez F, Sarkar SK, Jonathan MP. First account of microplastics in pelagic sporting dolphinfish from the eastern Mexican coast of Baja California Sur. Environ Toxicol Pharmacol. 2023;100:104153. 10.1016/J.ETAP.2023.104153. [DOI] [PubMed] [Google Scholar]
- 84.Sabilillah AM, Palupi FR, Adji BK, Nugroho AP. Health risk assessment and microplastic pollution in streams through accumulation and interaction by heavy metals. Glob J Environ Sci Manag. 2023;9(4):719–40. 10.22034/GJESM.2023.04.05. [Google Scholar]
- 85.Sánchez-Guerrero-Hernández MJ, González-Fernández D, Sendra M, Ramos F, Yeste MP, González-Ortegón E. Contamination from microplastics and other anthropogenic particles in the digestive tracts of the commercial species Engraulis encrasicolus and Sardina pilchardus. Sci Total Environ. 2023;860:160451. 10.1016/J.SCITOTENV.2022.160451. [DOI] [PubMed] [Google Scholar]
- 86.Santos RG, Machovsky-Capuska GE, Andrades R. Plastic ingestion as an evolutionary trap: Toward a holistic understanding. Science. 2021;373(6550):56–60. 10.1126/SCIENCE.ABH0945/SUPPL_FILE/ABH0945_SANTOS_SM.PDF. [DOI] [PubMed] [Google Scholar]
- 87.Shim WJ, Kim SK, Lee J, Eo S, Kim JS, Sun C. Toward a long-term monitoring program for seawater plastic pollution in the north Pacific Ocean: Review and global comparison. Environ Pollut. 2022;311:119911. 10.1016/J.ENVPOL.2022.119911. [DOI] [PubMed] [Google Scholar]
- 88.Su L, Deng H, Li B, Chen Q, Pettigrove V, Wu C, Shi H. The occurrence of microplastic in specific organs in commercially caught fishes from coast and estuary area of east China. J Hazard Mater. 2019;365:716–24. 10.1016/J.JHAZMAT.2018.11.024. [DOI] [PubMed] [Google Scholar]
- 89.Suedel BC, Boraczek JA, Peddicord RK, Clifford PA, Dillon TM. Trophic transfer and biomagnification potential of contaminants in aquatic ecosystems. Rev Environ Contam Toxicol. 1994;136:21–89. 10.1007/978-1-4612-2656-7_2/COVER. [DOI] [PubMed] [Google Scholar]
- 90.Sultan MB, Rahman MM, Khatun MA, Shahjalal M, Akbor MA, Siddique MAB, Huque R, Malafaia G. Microplastics in different fish and shellfish species in the mangrove estuary of Bangladesh and evaluation of human exposure. Sci Total Environ. 2023;858:159754. 10.1016/J.SCITOTENV.2022.159754. [DOI] [PubMed] [Google Scholar]
- 91.Valente T, Costantini ML, Careddu G, Berto D, Piermarini R, Rampazzo F, Sbrana A, Silvestri C, Ventura D, Matiddi M. Tracing the route: Using stable isotope analysis to understand microplastic pathways through the pelagic-neritic food web of the Tyrrhenian Sea (Western Mediterranean). Sci Total Environ. 2023;885:163875. 10.1016/J.SCITOTENV.2023.163875. [DOI] [PubMed] [Google Scholar]
- 92.Volkheimer G. Passage of particles through the wall of the gastrointestinal tract. Environ Health Perspect. 1974;9:215–25. 10.1289/EHP.749215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Widyastuti S, Susmana Abidin A, Hikmaturrohmi H, Tri B, Ilhami K, Sofian N, Kurniawan H, Jupri A, Candri DA, Frediansyah A, Prasedya ES. Microplastic Contamination in Different Marine Species of Bintaro Fish Market, Indonesia. Sustainability. 2023;15(12):9836. 10.3390/SU15129836. [Google Scholar]
- 94.Wu L, Dai X, Xu J, Ou D, Wang L, Lin H, He W, Lin H, Du R, Huang H, Li W, Pan Z. Assessment of microplastic contamination in an eastern Pacific tuna (Katsuwonus pelamis) and evaluation of its health risk implication through molecular docking and metabolomics studies. Food Chem. 2023;426:136507. 10.1016/J.FOODCHEM.2023.136507. [DOI] [PubMed] [Google Scholar]
- 95.Zhu W, Liu W, Chen Y, Liao K, Yu W, Jin H. Microplastics in Antarctic krill (Euphausia superba) from Antarctic region. Sci Total Environ. 2023;870:161880. 10.1016/J.SCITOTENV.2023.161880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the paper and its supplementary material and data files. Raw data files (Raman and FTIR spectra) are available from the corresponding author upon reasonable request.
Supplementary Data files:
43591_2024_101_MOESM1_ESM.docx
Data File 1 Raman Spectroscopy Match Information.xlsx.
Data File 2 Plastic Particle Size Measurements.xlsx.
Data File 3 Plastic Particles per Fish.xlsx.