Abstract
Asthma and atopic dermatitis (AD) are representative chronic diseases in childhood. This study aimed to investigate the impact of preterm birth on the incidence and severity of asthma and AD in children, as well as to identify neonatal risk factors for asthma and AD. We used health claims data recorded between 2007 and 2014 in the Korean National Health Insurance Service database. We recruited 2,224,476 infants born between 2007 and 2014 and divided them into three groups: 3518 of extremely preterm (EP) infants (< 28 weeks of gestational age (GA)), 82,579 of other preterm (OP) infants (28–36 weeks of GA), and 2,138,379 of full-term (FT) infants (> 37 weeks of GA). We defined asthma as > 3 episodes of clinical visits in a year before 6 years of age, early asthma as onset at < 2 years of age, and severe asthma as > 1 event of status asthmaticus or admission to a hospital via an emergency room. AD was defined as ≥ 3 diagnoses in a year before 6 years of age, early AD as onset at < 2 years of age, and severe AD as prescription of high-potency topical steroids or immunosuppressants. An association of preterm birth with asthma and AD was assessed using inverse probability of treatment-weighted multivariable Cox regression analysis. Cardiorespiratory conditions, such as respiratory distress syndrome, bronchopulmonary dysplasia, patent ductus arteriosus, and pulmonary hypertension, significantly increased the risk of asthma. Specifically, bronchopulmonary dysplasia emerged as a significant risk factor for both severe and early-onset asthma (odds ratio (OR) 1.36, 95% CI 1.21–1.37 for severe asthma; OR 1.55, 95% CI 1.30–1.85 for early asthma), while it was associated with a decreased risk of AD (OR 0.86, 95% CI 0.80–0.92). Neonatal sepsis, jaundice, and retinopathy of prematurity were also identified as significant risk factors for later asthma. A stepwise increase in the risk of asthma with an increasing degree of prematurity was observed, with the OP group showing an adjusted hazard ratio (aHR) of 1.24 (95% CI: 1.22–1.26) and the EP group showing an aHR of 1.51 (95% CI: 1.41–1.63). Conversely, preterm birth was inversely associated with the risk of AD, with aHRs of 0.73 (95% CI: 0.67–0.79) for the OP group and 0.88 (95% CI: 0.87–0.89) for the EP group. Conclusion Preterm children have a significantly higher risk of asthma and lower risk of AD, with cardiorespiratory conditions significantly increasing the risk of asthma. Thus, we highlight the need for targeted respiratory management strategies for this high-risk population.
|
What is Known: •Asthma and atopic dermatitis are prevalent chronic diseases in childhood, reducing the quality of life of children. •Preterm birth was associated with an increased risk of asthma, but few large nationwide studies. •Research on the relationship between preterm birth and pediatric atopic dermatitis is controversial, with few large nationwide studies. | |
|
What is New: • Preterm children, especially born before 28 weeks of gestational age, had a significantly higher risk of asthma and lower risk of atopic dermatitis. • Cardiorespiratory comorbidities such as RDS, BPD, PDA, and pulmonary hypertension in neonatal period are prominent risk factors for asthma. • Preterm children are vulnerable to both early-onset and severe asthma. |
Supplementary Information
The online version contains supplementary material available at 10.1007/s00431-024-05747-5.
Keywords: Asthma, Atopic dermatitis, Preterm birth, Nationwide study
Introduction
The incidence of preterm birth, defined as delivery before 37 weeks of gestational age (GA), has been on a steady incline in Korea [1]. It is attributed to such factors as advanced maternal age, smoking during pregnancy, and increased multiple births resulting from assisted reproductive technologies [2]. Concurrently, advancements in neonatology have significantly improved the survival rates of preterm infants [3], sparking a heightened interest in the long-term health outcomes of these children.
Asthma and atopic dermatitis (AD) are representative chronic respiratory and skin diseases, respectively, occurring in children due to various causes. Asthma represents the most common chronic respiratory disease in children, characterized by reversible airway obstructions [4]. The prevalence of asthma has been shown to vary among studies in Korea. Studies in other countries show a decreasing trend in the prevalence of asthma [5]; however, Korean studies show that the recent asthma incidence is stagnant or increasing without significant change. Some studies demonstrate that asthma incidence has not changed in all ages since 2008, except for the 20 s, which showed a steady increase [6]. The Korea Community Health Survey database shows that the overall prevalence of asthma increased. The National Health Insurance Service-National Sample Cohort indicated a gradual increase in annual asthma prevalence from 4.5 to 6.2% [7]. Nationally, children aged < 18 years exhibit the highest rates of asthma medication prescriptions, with the annual incidence of pediatric asthma on an upward trajectory [8, 9]. Research indicates that the etiology of asthma is multifactorial, encompassing genetic predisposition, environmental influences (such as air pollution), and perinatal factors, including low birth weight [10–12].
AD, a prevalent inflammatory skin condition during childhood, is marked by chronic, pruritic lesions in a characteristic distribution. The general prevalence of AD ranges from approximately 10 to 20%, affecting a significant portion (14.7%) of school-aged children in Korea, the number that has notably increased over the last 2–3 decades [13, 14]. Factors contributing to AD include skin barrier dysfunction, a feature prominently observed in preterm infants. Existing literature on the relationship between preterm birth and AD yielded conflicting results. Some European studies report that preterm birth is associated with a decreased risk of AD [15–18]. Conversely, research by Kvenshagen et al. [19] involving 512 children monitored from birth to 2 years found no significant association between AD and preterm birth.
As preterm birth is increasing, managing their quality of life is important. Asthma and AD become important factors in the health-related quality of life of such patients. Therefore, we aimed to determine whether premature infants and their accompanying medical conditions influence the incidence and severity of asthma and AD. Various past studies have analyzed the correlation between premature infants and both diseases. However, controversial results were shown regarding atopic dermatitis. To our knowledge, comprehensive studies examining the impact of preterm birth on asthma and AD while utilizing nationwide data are scarce in Korea. Furthermore, the incidence of these diseases segmented by gestational age (GA) remains unexplored. Therefore, we aimed to confirm a more accurate correlation through large-scale analysis using national data, and also investigate the difference in incidence rates according to GA. Our study seeks to the overall incidence of asthma and AD in preschool-aged children in Korea, utilizing medical claims data for newborns born between 2008 and 2014. We would classify them by GA to determine differences in incidence and severity depending on GA. Additionally, we aim to identify neonatal risk factors for asthma and AD.
Materials and methods
Data source
The data for this study were obtained from the Korean National Health Insurance Service (NHIS), the entity responsible for administering the health insurance coverage mandated by the Korean government. In Korea, each individual is assigned a unique identification number, ensuring that over 98% of the population is covered by a single-payer healthcare system. Medical claims data were collected from all people who visited the hospital (all 1st, 2nd, and 3rd medical institutions).
The NHIS compiles medical claims data, demographic details, and outcomes from the National Health Screening Program for Infants and Children (NHSPIC). The NHSPIC provides annual health screening tests for children of preschool age. Collected medical claims data were cataloged using the ICD-10 codes. This study was performed according to the principles of the Declaration of Helsinki and received an exemption from review by the Institutional Review Board of Hanyang University Guri Hospital (approval no. GURI 2022–04-017). Additionally, this study was reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Online Resource 1).
Study population
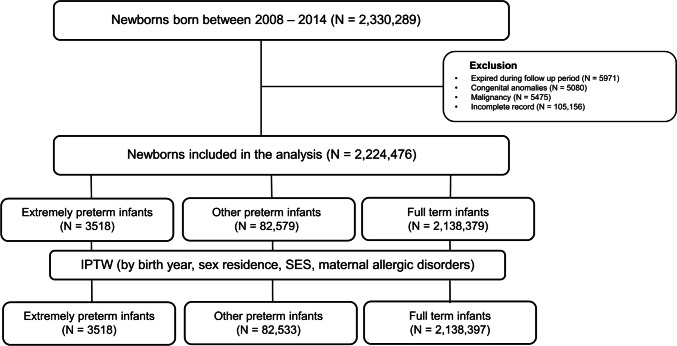
Figure 1 depicts the flow diagram of the study population. We identified newborns born between 2008 and 2014 and classified them according to the ICD-10 codes for preterm birth (P07) and full-term birth (Z38), finding a total of 2,330,289 individuals. These individuals were monitored up to the age of 6 years. The cohort was divided into three groups based on gestational age (GA): (1) extremely preterm (EP) infants born at < 28 weeks of GA; (2) other preterm (OP) infants born between 28 and 36 weeks of GA; and (3) full-term (FT) infants born at ≥ 37 weeks of GA. Given the strong correlation between birth weight (BW) and GA, both indicate the level of prematurity in preterm infants.
Fig. 1.
Flow diagram of the study population. Abbreviations: IPTW, inverse probability of treatment-weighted; SES, socioeconomic status
Individuals who (1) died during the follow-up after birth (n = 5971), (2) were diagnosed with chromosomal anomalies (n = 5080), (3) had any malignancies (n = 5475), or (4) possessed incomplete medical records (n = 106,056) were excluded. Finally, a total of 2,224,476 children were included in the final analysis.
Definition of asthma and atopic dermatitis
Asthma and AD were the primary outcomes of interest. Asthma was defined as having > 3 outpatient or inpatient visits coded as J45 or J46 according to the ICD-10 codes within a year, with at least 6 months separating the first and last visits. Early-onset asthma was categorized as a clinical diagnosis made before the child reached 2 years of age. Severe asthma was determined by at least 1 hospital visit of status asthmaticus (coded as J46 in ICD-10) or > 1 emergency department visit primarily for asthma.
AD was characterized by > 3 hospital visits for AD coded as J20.8 and J20.9 within a year, using the ICD-10 codes. An early AD diagnosis was defined as that made before the child reached 2 years of age. Severe AD was identified in patients who, upon diagnosis, were prescribed high-potency topical steroids, methotrexate, or cyclosporine.
Covariates
Demographic data, including the child’s sex, birth year, residence, and socioeconomic status (SES), were sourced from the NHIS database. Residence was classified into metropolitan or rural, with metropolitan areas including Seoul and the six major Korean cities, exceeding a population of 1 million. SES was inferred from health insurance premium quartiles, which are indicative of household income levels.
Maternal and perinatal conditions, such as delivery type, maternal gestational diabetes mellitus (GDM), and pregnancy-induced hypertension (PIH), were linked to each child through maternal data in the NHIS database. Neonatal comorbidities, including respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), pulmonary hypertension, and allergic proctocolitis, were considered potential confounders and included in the analysis. However, given their prevalence in preterm infants, these comorbidities were excluded from the inverse probability of treatment-weighted (IPTW) analysis.
Furthermore, neonatal comorbidities, such as RDS, BPD, patent ductus arteriosus (PDA), pulmonary hypertension, necrotizing enterocolitis (NEC), intraventricular hemorrhage (IVH), hypothyroidism, neonatal sepsis, neonatal jaundice, and retinopathy of prematurity (ROP), were investigated as risk factors for asthma and AD. Online Resource 2 provides comprehensive details of variables.
Statistical analysis
We employed the IPTW based on the propensity score (PS) to mitigate selection bias for assessing the incidence of asthma and AD among the three population subgroups. This approach involved creating a pseudo-dataset, in which each participant was weighted according to the IPTW. Then, the dataset was subjected to regression analysis. The PS for each subgroup was determined using a logistic regression model incorporating baseline demographic information (sex, birth year, residence, and SES) and maternal factors (maternal asthma, maternal AD, GDM, cesarean section, PIH, and intrauterine growth restriction (IUGR)).
Baseline demographic characteristics and comorbidities across groups were compared using a one-way analysis of variance for continuous variables and the chi-squared test for categorical variables. We calculated the maximum absolute standardized difference (ASD) for each variable to assess the balance of characteristics among the groups, considering the maximum ASD of < 0.1 indicative of well-balanced variables between the groups.
Adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs) for the risk of developing asthma and AD were derived from Cox proportional hazards models. A multivariate Cox regression analysis was conducted to adjust for potential confounders specific to each disease. For asthma, variables such as RDS, BPD, and pulmonary hypertension were adjusted. Regarding AD, adjustments were made for allergic proctocolitis. The assumption of proportional hazards was verified using log-minus-log plots.
The incidence of asthma and AD by age was depicted using Kaplan–Meier survival curves, with differences among subgroups assessed using the log-rank test. Additionally, logistic regression analysis was employed to identify major risk factors for diseases. A p-value < 0.01 indicated statistical significance. Statistical analyses were conducted using the SAS Enterprise Guide (version 7.1, SAS Institute Inc., Cary, NC, USA).
Sensitivity analysis
An additional sensitivity analysis was conducted to validate our statistical analysis. We divided the study population into three groups according to BW: extremely low birth weight (ELBW) including those with BW < 1 kg, low birth weight (LBW) including those with BW < 2.5 kg, and normal birth weight (NBW). We selected children who were assigned birth codes regarding BW. The IPTW with covariates, such as sex, birth year, residence, SES, maternal allergic disorders, GDM, cesarean section, PIH, and IUGR, was included in the analysis. Then, the association between BW and diseases (asthma and AD) was validated and expressed as HR with 95% CI using the Cox proportional hazards model.
Results
Participants’ characteristics
Among the study cohort of 2,224,476 individuals, 3518 (0.2%) were categorized as EP, 82,579 (3.7%) were categorized as OP, and 2,138,379 (96.1%) were categorized as FT (Table 1). Maternal asthma prevalence was lowest in the FT group compared to the EP and OP groups (5.1% in EP, 5.1% in OP, and 4.4% in FT; p < 0.001). Similarly, maternal AD was the commonest in the EP group, followed by the OP and FT groups (2.3% in EP, 2.2% in OP, and 2.1% in FT; p < 0.001). The frequencies of cesarean section, PIH, and IUGR were significantly higher among preterm infant groups than in the FT group. Before applying the IPTW, the maximum ASD for GDM, cesarean section, PIH, and IUGR exceeded 0.1. However, after IPTW adjustment, all baseline demographic characteristics were well balanced with a maximum ASD of < 0.1 except for IUGR.
Table 1.
Baseline characteristics of the pediatric population before and after inverse probability of treatment weighting (IPTW)
| Pre-IPTW | Post-IPTW | |||||||
|---|---|---|---|---|---|---|---|---|
| EP group n = 3518 (0.2) |
OP group n = 82,579 (3.7) |
FT group n = 2,138,379 (96.1) |
Maximum ASD | EP group n = 3549 (0.2) |
OP group n = 82,938 (3.7) |
FT group n = 2,138,358 (96.1) |
Maximum ASD |
|
| Variables included in IPTW | ||||||||
| Sex | 0.07 | 0.03 | ||||||
| Boys | 1796 (51.0) | 45,097 (54.6) | 1,093,814 (51.1) | 1877 (52.9) | 43,303 (52.2) | 1,096,585 (51.2) | ||
| Girls | 1722 (49.0) | 37,482 (45.4) | 1,044,565 (48.9) | 1672 (47.1) | 39,635 (47.8) | 1,041,773 (48.7) | ||
| Birth year | 0.08 | 0.05 | ||||||
| 2008–2010 | 1235 (35.1) | 32,362 (39.2) | 821,608 (38.4) | 1337 (37.7) | 29,248 (35.3) | 821,966 (38.4) | ||
| 2011–2014 | 2283 (64.9) | 50,217 (60.8) | 1,316,771 (61.6) | 2212 (62.3) | 53,690 (64.7) | 1,316,393 (61.6) | ||
| Residence | 0.03 | 0.03 | ||||||
| Metropolitan | 1608 (45.7) | 37,968 (46.0) | 952,951 (44.6) | 1563 (44.0) | 37,532 (45.3) | 954,128 (44.6) | ||
| Rural | 1910 (54.3) | 44,611 (54.0) | 1,185,428 (55.4) | 1986 (56.0) | 45,406 (54.7) | 1,184,230 (55.4) | ||
| SES | 0.05 | 0.07 | ||||||
| Q1 | 410 (11.7) | 9368 (11.3) | 226,689 (10.6) | 366 (10.3) | 8809 (10.6) | 227,311 (10.6) | ||
| Q2 | 795 (22.6) | 18,420 (22.3) | 495,053 (23.2) | 822 (23.2) | 19,200 (23.2) | 494,359 (23.1) | ||
| Q3 | 1429 (40.6) | 32,164 (39.0) | 856,067 (40.0) | 1470 (41.4) | 33,207 (40.0) | 855,219 (40.0) | ||
| Q4 | 884 (25.1) | 22,627 (27.4) | 560,570 (26.2) | 891 (25.1) | 21,722 (23.2) | 561,469 (26.3) | ||
| Maternal asthma | 181 (5.1) | 4172 (5.1) | 94,938 (4.4) | 0.03 | 173 (4.9) | 3733 (4.5) | 95,450 (4.5) | 0.02 |
| Maternal AD | 82 (2.3) | 1843 (2.2) | 44,967 (2.1) | 0.01 | 72 (2.0) | 1758 (2.1) | 45,077 (2.1) | < 0.01 |
| Variables not included in IPTW | ||||||||
| GDM | 371 (10.6) | 21,187 (25.7) | 476,637 (22.3) | 0.40 | 848 (23.9) | 19,315 (23.3) | 478,865 (22.4) | 0.04 |
| Cesarean section | 2136 (60.7) | 45,903 (55.6) | 731,008 (24.2) | 0.79 | 1221 (34.4) | 28,649 (34.5) | 748,858 (35.0) | 0.23 |
| PIH | 120 (3.4) | 4904 (5.9) | 61,824 (2.9) | 0.15 | 110 (3.1) | 2375 (2.9) | 64,242 (3.0) | 0.01 |
| IUGR | 105 (3.0) | 2899 (3.5) | 6000 (0.3) | 0.23 | 95 (2.7) | 2621 (3.2) | 6008 (0.3) | 0.22 |
| RDS | 3017 (85.8) | 18,020 (21.8) | 11,485 (0.5) | 3.39 | 2951 (83.2) | 16,838 (20.3) | 11,519 (0.5) | 3.07 |
| BPD | 2620 (74.5) | 4964 (6.0) | 202 (< 0.1) | 2.41 | 2511 (70.8) | 4612 (5.6) | 204 (< 0.1) | 2.20 |
| Pulmonary hypertension | 258 (7.3) | 745 (0.9) | 708 (< 0.1) | 0.39 | 241 (6.8) | 708 (0.9) | 715 (< 0.1) | 0.38 |
| Allergic proctocolitis | 56 (1.6) | 1044 (1.3) | 25,830 (1.2) | 0.03 | 74 (2.1) | 1003 (1.2) | 25,855 (1.2) | 0.07 |
| Birth weight, kg | 1.1 (0.9, 2.0) | 2.3 (1.9, 2.6) | 3.2 (3.0, 3.5) | 8.23 | 1.1 (0.9, 2.1) | 2.3 (1.9, 2.6) | 3.2 (3.0, 3.5) | 8.10 |
Categorical variables are presented as number (%). Abbreviations: IPTW, inverse probability of treatment weighting; EP, extremely preterm; OP, other preterm; FT, full term; SES, socioeconomic status; Q, quartile; AD, atopic dermatitis; ASD, absolute standardized difference; GDM, gestational diabetes mellitus; PIH, pregnancy-induced hypertension; IUGR, intrauterine growth restriction; RDS, respiratory distress syndrome; BPD, bronchopulmonary dysplasia. Categorical variables are presented as number (%) and continuous variables are presented as median (Q1, Q3)
Median BW was 3.2 kg (Q1, Q3: 3.0, 3.5) for the FT group, 2.3 kg (Q1, Q3: 1.9, 2.6) for the OP group, and 1.1 kg (Q1, Q3: 0.9, 2.1) for the EP group. The incidence of neonatal morbidities, including RDS, BPD, pulmonary hypertension, and allergic proctocolitis, was significantly higher in the EP group compared to the other groups (p < 0.001).
Incidence and risk of pediatric asthma and AD
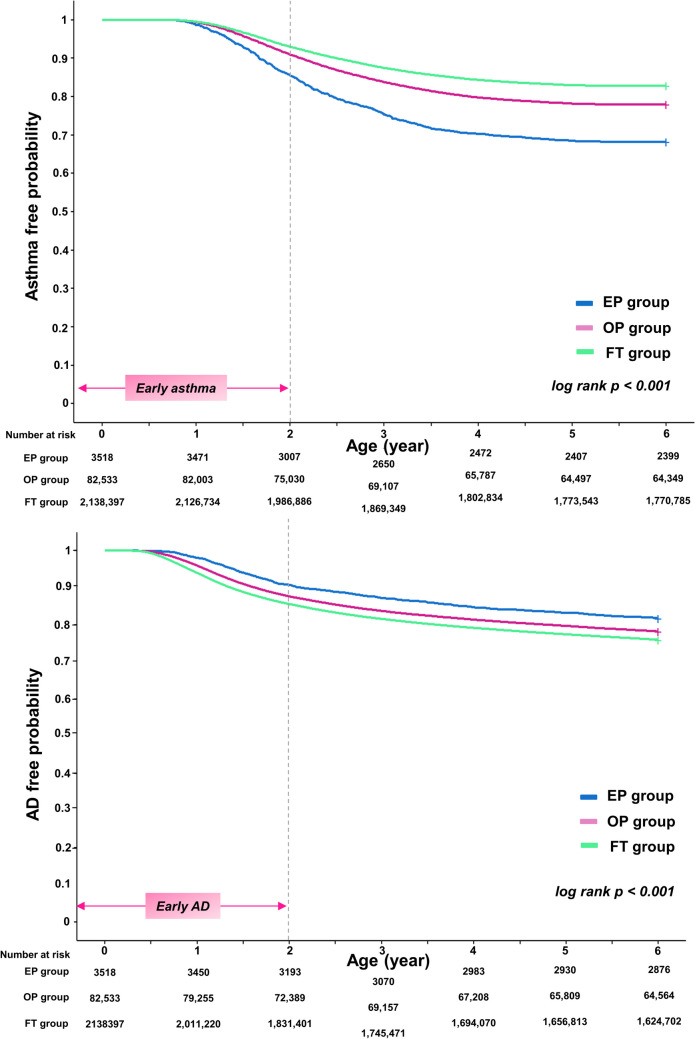
Table 2 presents the incidence of diseases and the results of regression analysis. The incidence of asthma was the highest in the EP group, followed by the OP group, and then the FT group (32.0% vs. 21.8% vs. 17.2%, p < 0.001). This pattern was consistent for both early-onset and severe asthma, with the highest incidence in the EP group, followed by the OP and FT groups. Conversely, the incidence of AD was the highest in the FT group, followed by the OP and EP groups (24.0% vs. 21.6% vs. 18.5%, p < 0.001). The Kaplan–Meier curves illustrated the incidence of asthma and AD across the three groups over the observation period from ages 0 to 6 years (Fig. 2). Asthma was the most prevalent in the EP group, while AD was the most prevalent in the FT group (log-rank p < 0.001).
Table 2.
Generalized linear model presenting the association between preterm birth and pediatric chronic disease (asthma and AD)
| N (%) | Crude model | Adjusted modeld | |
|---|---|---|---|
| Asthmaa,b,c | |||
| FT group | 367,673 (17.2) | Reference | Reference |
| OP group | 18,044 (21.8) | 2.05 (1.93–2.17) | 1.51 (1.41–1.63) |
| EP group | 1133 (32.0) | 1.30 (1.28–1.32) | 1.24 (1.22–1.26) |
| Early asthmaa,b,c | |||
| FT group | 66,588 (3.1) | Reference | Reference |
| OP group | 3050 (3.7) | 1.19 (1.15–1.24) | 1.10 (1.06–1.15) |
| EP group | 190 (5.3) | 1.78 (1.55–2.06) | 1.15 (0.97–1.36) |
| Severe asthmaa,b,c | |||
| FT group | 20,437 (1.0) | Reference | Reference |
| OP group | 1230 (1.5) | 1.59 (1.50–1.69) | 1.40 (1.32–1.50) |
| EP group | 107 (3.0) | 3.47 (2.87–4.20) | 1.62 (1.28–2.05) |
| ADa,b,c | |||
| FT group | 513,769 (24.0) | Reference | Reference |
| OP group | 17,949 (21.6) | 0.73 (0.68–0.79) | 0.73 (0.67–0.79) |
| EP group | 656 (18.5) | 0.88 (0.87–0.89) | 0.88 (0.87–0.89) |
| Early ADa,b,c | |||
| FT group | 307,050 (14.4) | Reference | Reference |
| OP group | 10,088 (12.2) | 0.83 (0.81–0.85) | 0.83 (0.81–0.85) |
| EP group | 336 (9.5) | 0.64 (0.57–0.71) | 0.63 (0.57–0.71) |
| Severe ADa,b,c | |||
| FT group | 137,784 (6.4) | Reference | Reference |
| OP group | 4565 (5.5) | 0.83 (0.81–0.86) | 0.83 (0.81–0.86) |
| EP group | 150 (4.2) | 0.63 (0.54–0.74) | 0.63 (0.53–0.73) |
Abbreviations: EP, extremely preterm; OP, other preterm; FT, full term; AD, atopic dermatitis
Numbers are expressed with an adjusted hazard ratio (95% confidence interval)
ap < 0.001 in post hoc analysis (EP group vs. OP group), bp < 0.001 in post hoc analysis (EP group vs. FT group), cp < 0.001 in post hoc analysis (OP group vs. FT group)
dAdjusted for RDS, BPD, and pulmonary hypertension for asthma; adjusted for allergic proctocolitis for AD
Fig. 2.
The Kaplan–Meier curve presenting the risk of asthma and AD according to the degree of prematurity. a Risk of asthma. b Risk of AD. The x-axis represents the age of the population, and the y-axis represents the disease-free probability. The number of children at risk of each study group is included. Abbreviations: EP, extremely preterm infants; OP, other preterm infants; FT, full-term infants; AD, atopic dermatitis
Regression analysis indicated that both preterm groups had a significantly higher risk of asthma (aHR for the EP group 1.24, 95% CI 1.22–1.26; aHR for the OP group 1.51, 95% CI 1.41–1.63). Notably, the OP group exhibited a significant risk for both early-onset and severe asthma during preschool ages (aHR 1.10, 95% CI 1.06–1.15 for early-onset asthma; aHR 1.40, 95% CI 1.32–1.50 for severe asthma). Regarding AD, preterm infants demonstrated a significantly lower risk (aHR for the OP group 0.73, 95% CI 0.67–0.79; aHR for the EP group 0.88, 95% CI 0.87–0.89). This association remained significant for both early-onset and severe AD.
Risk factors for pediatric asthma and AD
Analysis revealed several neonatal comorbidities associated with the later development of asthma and AD (Table 3). Cardiorespiratory conditions, such as RDS, BPD, PDA, and pulmonary hypertension, significantly increased the risk of asthma. Specifically, BPD emerged as a significant risk factor for both severe and early-onset asthma (odds ratio (OR) 1.36, 95% CI 1.21–1.37 for severe asthma; OR 1.55, 95% CI 1.30–1.85 for early asthma), while it was associated with a decreased risk of AD (OR 0.86, 95% CI 0.80–0.92). Neonatal sepsis, jaundice, and ROP were also identified as significant risk factors for later asthma. Online Resource 3 provides association between pediatric asthma and atopic dermatitis according to baseline characteristics of the pediatric population and neonatal comorbidities.
Table 3.
Logistic regression analysis presenting the association between neonatal comorbidities and pediatric chronic disease (asthma and AD)
| Comorbidity | Asthma | Early asthma | Severe asthma | AD | Early AD | Severe AD |
|---|---|---|---|---|---|---|
| RDS | 1.09 (1.05–1.14) | 1.16 (1.06–1.26) | 1.11 (0.98–1.27) | 0.91 (0.88–0.95) | 0.89 (0.84–0.93) | 0.93 (0.87–1.00) |
| BPD | 1.29 (1.21–1.37) | 1.36 (1.20–1.55) | 1.55 (1.30–1.85) | 0.86 (0.80–0.92) | 0.81 (0.74–0.89) | 0.77 (0.67–0.88) |
| PDA | 1.12 (1.06–1.18) | 1.01 (0.90–1.13) | 1.36 (1.17–1.59) | 0.95 (0.90–1.00) | 0.93 (0.87–1.00) | 0.88 (0.79–0.98) |
| Pulmonary hypertension | 1.16 (1.01–1.33) | 1.29 (0.98–1.70) | 1.25 (0.87–1.85) | 1.10 (0.94–1.28) | 1.10 (0.91–1.35) | 1.01 (0.74–1.36) |
| NEC | 0.93 (0.83–1.04) | 0.90 (0.71–1.15) | 0.68 (0.48–0.97) | 0.87 (0.77–0.99) | 0.97 (0.82–1.14) | 0.73 (0.56–0.94) |
| IVH | 1.02 (0.96–1.08) | 0.98 (0.86–1.11) | 0.88 (0.73–1.06) | 1.00 (0.94–1.06) | 0.98 (0.91–1.06) | 1.04 (0.93–1.16) |
| Hypothyroidism | 1.15 (1.08–1.23) | 1.02 (0.88–1.18) | 1.14 (0.93–1.38) | 1.08 (1.01–1.16) | 1.04 (0.95–1.13) | 1.11 (0.98–1.26) |
| Neonatal sepsis | 1.07 (1.03–1.13) | 0.99 (0.90–1.10) | 1.30 (1.13–1.48) | 1.04 (0.99–1.09) | 1.06 (0.99–1.12) | 1.07 (0.98–1.16) |
| Neonatal jaundice | 1.04 (1.00–1.08) | 1.07 (0.98–1.17) | 1.04 (0.91–1.19) | 1.04 (0.99–1.08) | 1.07 (1.01–1.12) | 1.08 (1.00–1.16) |
| ROP | 1.09 (1.05–1.13) | 1.06 (0.97–1.14) | 1.20 (1.06–1.35) | 1.04 (1.00–1.07) | 1.03 (0.98–1.08) | 1.00 (0.94–1.07) |
Adjusted for sex, residence, socioeconomic status, maternal asthma, maternal AD, maternal GDM, cesarean section, PIH
Numbers are expressed as odds ratios (95% confidence interval). Abbreviations: AD, atopic dermatitis; RDS, respiratory distress syndrome; BPD, bronchopulmonary dysplasia; NEC, necrotizing enterocolitis; IVH, intraventricular hemorrhage; PDA, patent ductus arteriosus; ROP, retinopathy of prematurity
Sensitivity analyses
The analysis included participants with ELBW (0.2%, n = 4025) and LBW (2.5%, n = 54,867) (Online Resource 4). Children born small had a significantly elevated risk of asthma and a decreased risk of AD, aligning with the main results (Table 4).
Table 4.
Sensitivity analysis of the association between population subgroup and pediatric chronic disease (asthma and AD) according to birth weight
| Adjusted modela | |
|---|---|
| Asthma | |
| NBW group | Reference |
| LBW group | 1.17 (1.14–1.20) |
| ELBW group | 1.43 (1.32–1.55) |
| Early asthma | |
| NBW group | Reference |
| LBW group | 1.06 (0.99–1.12) |
| ELBW group | 0.99 (0.80–1.24) |
| Severe asthma | |
| NBW group | Reference |
| LBW group | 1.39 (1.26–1.52) |
| ELBW group | 2.28 (1.74–3.00) |
| AD | |
| NBW group | Reference |
| LBW group | 0.89 (0.87–0.91) |
| ELBW group | 0.77 (0.71–0.84) |
| Early AD | |
| NBW group | Reference |
| LBW group | 0.84 (0.82–0.87) |
| ELBW group | 0.76 (0.68–0.85) |
| Severe AD | |
| NBW group | Reference |
| LBW group | 0.87 (0.83–0.90) |
| ELBW group | 0.52 (0.43–0.64) |
Abbreviations: FT, full term; LBW, low birth weight; ELBW, extremely low birth weight; NBW, normal birth weight; AD, atopic dermatitis
aAdjusted for RDS, BPD, and pulmonary hypertension for asthma and adjusted for allergic proctocolitis for AD
Discussion
In our study, we explored the risk of asthma and AD in preterm infants, leveraging nationwide medical claims data from Korea. Our analysis not only assessed the overall risk associated with asthma and AD but also examined their onset and severity across different degrees of prematurity according to GA. We found that preterm infants exhibit a significantly higher risk of developing asthma and a relatively lower risk of AD compared to the FT control group. Moreover, our findings indicate an increased vulnerability among preterm children to both early-onset and severe forms of asthma. In our study, the prevalence of asthma and the incidence of early-onset and severe asthma were higher in the EP group than in the OP group. Thus, the lower the GA, the higher the prevalence of asthma, early-onset asthma, and severe asthma. Additionally, the presence of cardiorespiratory comorbidities, such as RDS, BPD, PDA, and pulmonary hypertension, in the neonatal period was identified as a significant risk factor for asthma in preschool-aged children.
Asthma is coded as J45 when it is confirmed through a pulmonary function test, but pulmonary function tests are difficult in children. Additionally, diagnosing asthma is challenging because wheezing is frequent in children aged < 6 years. For coding asthma in children under 6 years, we use the “asthma prediction index” [20, 21]. Asthma was diagnosed and coded as J45 in patients with ≥ 2 wheezing episodes (clinically or coded R06.2) or bronchiolitis diagnosis if parents have asthma or high IgE. According to the Korean pediatric asthma treatment guidelines, treatment adjustment and step-down are performed at least in 2-month intervals after diagnosis [22, 23]. We suggest that patients visiting the hospital 2–3 times in at least 3-month intervals can be accurately diagnosed with asthma; thus, we set this criteria. Although these strict standards may have decreased the number of enrollees, it is believed that there was no major error given that the prevalence rate was similar to the results of other cohort studies. Our results align with those of Sol et al. [9], who reported asthma prevalence of 26.5% in children aged < 2 years and 25.8% in children aged < 6 years, corroborating our findings.
Regarding AD, our incidence was higher than that reported by a previous study: 17.2% in patients aged < 2 years and 7.3% in those aged < 5 years [24]. J20 code is used when simple eczematous skin lesions persist for > 6 weeks and are accompanied by itching [25]. Only patients who consistently visited the hospital according to the guidelines received treatment for AD and were coded as AD each time they were selected as study subjects to rule out the possibility that simple eczematous lesions or other differential diseases were misdiagnosed as AD [26, 27].
The main symptom of asthma is airway hyperresponsiveness, with still unclarified underlying mechanisms. Several studies showed that bronchial hyperresponsiveness can occur in premature infants due to various reasons. Clemm et al. [28] showed that exposure to tracheal intubation and oxygen therapy could induce structural deformations possibly resulting in airway hyperresponsiveness. Another potential cause of asthma may be that preterm infants with GA < 32 weeks are born with a structurally immature respiratory system. Preterm children lack sufficient time for alveolar maturation, mainly occurring after 32 weeks of GA, and receiving large quantities of maternal transplacental antibodies that could be protective against frequent respiratory infections [29–32]. This increases the risk of respiratory infections and can lead to bronchial hyperresponsiveness. Additionally, the inflammatory response caused by proinflammatory cytokines, such as TNF-α and IL-6, occurring in RDS also causes bronchial hyperresponsiveness. Moreover, the high incidence of RDS in premature infants might contribute to the high incidence of asthma [33]. Delaying treatment for asthma can lead to impaired lung function in adulthood. Thus, early diagnosis and management of asthma are critical to estimate the prognosis for lung function [34].
Unlike asthma, we found that the risk of AD was higher in the FT group. Moreover, the EP group had the lowest incidence and aHRs for early and severe AD. Some previous studies showed conflicting results as to whether preterm birth increases the risk of AD [35, 36]. The significance between AD and the degree of prematurity has not yet been clarified. There are many studies showing that the skin barrier and immune response affect the development of AD [19].
Compared with other studies, our study was based on the most recent births (over 2 million) in a nationwide population representing the general pediatric population (a large-scale national study). In our study, the risk of AD was higher in the FT group. We suggest that still developing immune response might contribute to atopic sensitization. As GA increases, the fetal immune response to specific antigens strengthens, and the intestinal non-immune defense system matures [37, 38]. Consistent with the findings of Mitselou et al. [39], preterm birth is associated with a reduced risk of sensitization to food or inhalant allergens compared to FT births. This diminished risk of allergen sensitization could be attributed to early microbial exposure, fostering an enhanced Th-1-type adaptive immune response [40]. Therefore, we speculate that the earlier exposure to allergens in FT infants might account for their increased risk of both early-onset and severe AD.
Individuals with AD may suffer from diverse issues, including chronic fatigue from insomnia, psychological distress, and a diminished quality of life [41]. While AD may improve with age, severe cases are less likely to resolve spontaneously and persistent AD increases may precede the development of other diseases, such as allergic rhinitis and asthma [42, 43]. It is called as “allergic march.” Therefore, effectively managing AD is crucial to preventing its progression.
Our study also explored risk factors for asthma and AD among neonatal comorbidities, finding that cardiopulmonary complications, such as RDS, PDA, BPD, and pulmonary hypertension, significantly predict the later development of asthma. Although the precise mechanisms underlying asthma development remain unclear, early lung injury — including exposure to repetitive oxygen therapy and tracheal intubation — might play a contributory role.
This study has several limitations. First, the reliance on medical claims data, collected primarily for reimbursement purposes, might influence the accuracy of the diagnoses coded by physicians. Second, the lack of data on allergic history or environmental factors (e.g., exposure to house dust mites or sanitation status) limits our analysis. Third, our observation period extended only until the preschool age, precluding the identification of asthma and AD that may emerge during pre-adolescence. Additionally, detection bias is a concern. Since preterm birth is likely associated with increased medical treatment, children born preterm may be more likely to be diagnosed with asthma or AD, thereby biasing results.
Despite these limitations, this study analyzed a large nationwide population to estimate the risk of asthma and AD in preterm infants in Korea, covering 6 years including both infancy and toddlerhood. We differentiated the population by GA and categorized asthma and AD into early-onset and severe forms. Preterm birth was significantly associated with asthma and inversely associated with AD. Further studies regarding its clinical mechanism are required, as well as studies for targeted respiratory and dermal management and prevention of asthma and AD for this high-risk population.
Conclusion
Preterm children exhibit a significantly higher risk of asthma but a lower risk of AD compared to their FT counterparts. Emphasizing long-term respiratory care for preterm infants, especially those with cardiopulmonary comorbidities, is crucial for asthma prevention. Conversely, to prevent the development of AD, more attention should be paid to FT children. Continued and larger research for prevention is needed.
Role of funder/sponsor
The funding organization had no role in the design and conduct of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study used the National Health Insurance Service database (No. NHIS-2022-1-673). The interpretations and conclusions reported herein do not represent those of the National Health Insurance Service.
Abbreviations
- AD
Atopic dermatitis
- ASD
Absolute standardized difference
- BPD
Bronchopulmonary dysplasia
- CI
Confidence interval
- EP
Extremely preterm infants
- FT
Full-term infants
- GA
Gestational age
- GDM
Gestational diabetes mellitus
- HR
Hazard ratio
- ICD-10
International Classification of Diseases-Tenth Revision
- IUGR
Intrauterine growth restriction
- IPTW
Inverse probability of treatment-weighted
- NHSPIC
The National Health Screening Program for Infants and Children
- NHIS
The Korean National Health Insurance Service
- OP
Other preterm infants
- OR
Odds ratio
- PIH
Pregnancy-induced hypertension
- PS
Propensity score
- RDS
Respiratory distress syndrome
- SES
Socioeconomic status
Authors’ Contributions
Conceptualization: Jong Ho Cha, Jae-Kyoon Hwang, Young-Jin Choi. Data curation: Soorack Ryu, Young-Jin Choi. Formal analysis: Jong Ho Cha, Soorak Ryu, and Jae Yoon Na. Funding acquisition: Young-Jin Choi. Methodology: Jong Ho Cha, Jae-Kyoon Hwang, Soorack Ryu, and Young-Jin Choi. Project administration: Jae-Won Oh, Young-Jin Choi. Visualization: Jong Ho Cha, Young-Jin Choi. Writing – original draft: Jong Ho Cha, Jae-Kyoon Hwang. Writing – review and editing: Jae Yoon Na, Jae-Won Oh, and, Young-Jin Choi.
Funding
This research was supported by a grant from Hanyang University (HY-202300000001168).
Data availability
The data presented in this study are available on request from the corresponding author.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jong Ho Cha and Jae Kyoon Hwang contributed equally to this work.
References
- 1.Chawanpaiboon S, Vogel JP, Moller A-B, Lumbiganon P, Petzold M, Hogan D, Landoulsi S, Jampathong N, Kongwattanakul K, Laopaiboon M (2019) Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 7:e37–e46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R (2008) Epidemiology and causes of preterm birth. The lancet 371:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santhakumaran S, Statnikov Y, Gray D, Battersby C, Ashby D, Modi N (2018) Survival of very preterm infants admitted to neonatal care in England 2008–2014: time trends and regional variation. Arch Dis Child Fetal Neonatal Ed 103:F208–F215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lai C, Beasley R, Crane J, Foliaki S, Shah J, Weiland S (2009) International Study of Asthma and Allergies in Childhood Phase Three Study Group. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 64:476–483 [DOI] [PubMed] [Google Scholar]
- 5.Trepka MJ, Martin P, Mavunda K, Rodriguez D, Zhang G, Brown C (2009) A pilot asthma incidence surveillance system and case definition: lessons learned. Public Health Rep 124:267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin JY, Sohn KH, Shin JE, Park M, Lim J, Lee JY, Yang MS (2018) Changing patterns of adult asthma incidence: results from the National Health Insurance Service-National Sample Cohort (NHIS-NSC) database in Korea. Sci Rep 8:15052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park SY, Kim JH, Kim HJ, Seo B, Kwon OY, Chang HS, Kwon HS, Kim TB, Kim H, Park CS et al (2018) High prevalence of asthma in elderly women: findings from a Korean national health database and adult asthma cohort. Allergy, Asthma Immunol Res 10:387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J-H, Lee H, Park S-Y, Kim J-Y, Choi SH, Kwon H-S, Song W-J, Kim S-H, Yu J, Song DJ (2023) Epidemiology of patients with asthma in Korea: analysis of the NHISS database 2006–2015. World Allergy Organ J 16:1007689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sol IS, Kim YH, Kim SY, Choi SH, Kim JD, Kim BO, Moon JE, Kim KW, Sohn MH (2019) Prescription patterns and burden of pediatric asthma in Korea. Allergy, Asthma Immunol Res 11:280–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz J, Gold D, Dockery DW, Weiss ST, Speizer FE (1990) Predictors of asthma and persistent wheeze in a national sample of children in the United States. Am Rev Respir Dis 142:555–562 [DOI] [PubMed] [Google Scholar]
- 11.Windham GC, Hopkins B, Fenster L, Swan SH (2000) Prenatal active or passive tobacco smoke exposure and the risk of preterm delivery or low birth weight. Epidemiol 11(4):427–433 [DOI] [PubMed] [Google Scholar]
- 12.Kelly Y, Brabin B, Milligan P, Heaf D, Reid J, Pearson M (1995) Maternal asthma, premature birth, and the risk of respiratory morbidity in schoolchildren in Merseyside. Thorax 50:525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson WF, Leung DY, Beck LA, Berin CM, Boguniewicz M, Busse WW, Chatila TA, Geha RS, Gern JE, Guttman-Yassky E (2019) Report from the National Institute of Allergy and Infectious Diseases workshop on “Atopic dermatitis and the atopic march: mechanisms and interventions.” J Allergy Clin Immunol 143:894–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Czarnowicki T, Krueger JG, Guttman-Yassky E (2017) Novel concepts of prevention and treatment of atopic dermatitis through barrier and immune manipulations with implications for the atopic march. J Allergy Clin Immunol 139:1723–1734 [DOI] [PubMed] [Google Scholar]
- 15.Trønnes H, Wilcox AJ, Lie RT, Markestad T, Moster D (2013) The association of preterm birth with severe asthma and atopic dermatitis: a national cohort study. Pediatr Allergy Immunol 24:782–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pulakka A, Risnes K, Metsälä J, Alenius S, Heikkilä K, Nilsen SM, Näsänen-Gilmore P, Haaramo P, Gissler M, Opdahl S (2023) Preterm birth and asthma and COPD in adulthood: a nationwide register study from two Nordic countries. European Respiratory Journal 61 [DOI] [PMC free article] [PubMed]
- 17.Egeberg A, Andersen YM, Gislason G, Skov L, Thyssen JP (2016) Neonatal risk factors of atopic dermatitis in Denmark–results from a nationwide register-based study. Pediatr Allergy Immunol 27:368–374 [DOI] [PubMed] [Google Scholar]
- 18.Goedicke-Fritz S, Härtel C, Krasteva-Christ G, Kopp MV, Meyer S, Zemlin M (2017) Preterm birth affects the risk of developing immune-mediated diseases. Front Immunol 8:1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvenshagen B, Jacobsen M, Halvorsen R (2009) Atopic dermatitis in premature and term children. Arch Dis Child 94:202–205 [DOI] [PubMed] [Google Scholar]
- 20.Castro-Rodriguez JA, Cifuentes L, Martinez FD (2010) The Asthma Predictive Index: a very useful tool for predicting asthma in young children. J Allergy Clin Immunol 126:212–216 [DOI] [PubMed] [Google Scholar]
- 21.Castro-Rodriguez JA, Cifuentes L, Martinez FD (2019) Predicting asthma using clinical indexes. Front Pediatr 7:320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kom WK (2018) Studies and proposals of childhood asthma in Korea. Allergy, Asthma Respir Dis 6:S52–S57 [Google Scholar]
- 23.Tesse R, Borrelli G, Mongelli G, Mastrorilli V, Cardinale F (2018) Treating pediatric asthma according guidelines. Front Pediatr 6:234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JY, Yang H-k, Kim M, Kim J, Ahn K (2017) Is the prevalence of atopic dermatitis in Korean children decreasing?: National Database 2009–2014. Asian Pac J Allergy Immunol 35:144–149 [DOI] [PubMed] [Google Scholar]
- 25.Sidbury R, Kodama S (2018) Atopic dermatitis guidelines: diagnosis, systemic therapy, and adjunctive care. Clin Dermatol 36:648–652 [DOI] [PubMed] [Google Scholar]
- 26.Kim JE, Kim HJ, Lew BL, Lee KH, Hong SP, Jang YH, Park KY, Seo DJ, Bae JM, Choi EH et al (2015) Consensus guidelines for the treatment of atopic dermatitis in Korea (part II): systemic treatment. Ann Dermatol 27:578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saeki H, Furue M, Furukawa F, Hide M, Ohtsuki M, Katayama I, Sasaki R, Suto H, Takehara K et al (2009) Guidelines for management of atopic dermatitis. J Dermatol 36:563–577 [DOI] [PubMed] [Google Scholar]
- 28.Clemm HH, Engeseth M, Vollsæter M, Kotecha S, Halvorsen T (2018) Bronchial hyper-responsiveness after preterm birth. Paediatr Respir Rev 26:34–40 [DOI] [PubMed] [Google Scholar]
- 29.Haataja P, Korhonen P, Ojala R, Hirvonen M, Korppi M, Gissler M, Luukkaala T, Tammela O (2018) Hospital admissions for lower respiratory tract infections in children born moderately/late preterm. Pediatr Pulmonol 53:209–217 [DOI] [PubMed] [Google Scholar]
- 30.Stephens AS, Lain SJ, Roberts CL, Bowen JR, Nassar N (2016) Survival, hospitalization, and acute-care costs of very and moderate preterm infants in the first 6 years of life: a population-based study. J Pediatr 169(61–68):e63 [DOI] [PubMed] [Google Scholar]
- 31.Townsi N, Laing IA, Hall GL, Simpson SJ (2018) The impact of respiratory viruses on lung health after preterm birth. European Clinical Respir J 5:1487214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shin JS, Kim YB, Lee YH, Shim GH, Chey MJ (2016) Comparisons of clinical characteristics affecting readmission between late preterm infants and moderate preterm infants or full-term infants. Neonatal Med 23(4):211–217 [Google Scholar]
- 33.Varvarigou AA, Thomas I, Rodi M, Economou I, Mantagos S, Mouzaki A (2012) Respiratory distress syndrome (RDS) in premature infants is underscored by the magnitude of Th1 cytokine polarization. Cytokine 58:355–360 [DOI] [PubMed] [Google Scholar]
- 34.Pascual RM, Peters SP (2005) Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J Allergy Clin Immunol 116:477–486 [DOI] [PubMed] [Google Scholar]
- 35.Steffensen FH, Sørensen HT, Gillman MW, Rothman KJ, Sabroe S, Fischer P, Olsen J (2000) Low birth weight and preterm delivery as risk factors for asthma and atopic dermatitis in young adult males. Epidemiol 11(2):185–188 [DOI] [PubMed] [Google Scholar]
- 36.Olesen AB, Ellingsen AR, Olesen H, Juul S, Thestrup-Pedersen K (1997) Atopic dermatitis and birth factors: historical follow up by record linkage. BMJ 314:1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones A, Miles E, Warner J, Colwell B, Bryant T, Warner J (1996) Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr Allergy Immunol 7:109–116 [DOI] [PubMed] [Google Scholar]
- 38.Kuitunen OO, Savilahti E, Sarnesto A (1994) Human alpha-lactalbumin and bovine beta-lactoglobulin absorption in premature infants. Pediatr Res 35:344–347 [DOI] [PubMed] [Google Scholar]
- 39.Mitselou N, Andersson N, Bergström A, Kull I, Georgelis A, van Hage M, Hedman AM, Almqvist C, Ludvigsson JF, Melén E (2022) Preterm birth reduces the risk of IgE sensitization up to early adulthood: a population-based birth cohort study. Allergy 77:1570–1582 [DOI] [PubMed] [Google Scholar]
- 40.Anderson J, Do LAH, Wurzel D, Licciardi PV (2023) Understanding the increased susceptibility to asthma development in preterm infants. Allergy 78:928–939 [DOI] [PubMed] [Google Scholar]
- 41.Lewis-Jones M, Finlay A (1995) The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. Br J Dermatol 132:942–949 [DOI] [PubMed] [Google Scholar]
- 42.Illi S, von Mutius E, Lau S, Nickel R, Grüber C, Niggemann B, Wahn U, Group MAS (2004) The natural course of atopic dermatitis from birth to age 7 years and the association with asthma. Journal of Allergy and Clinical Immunology 113:925-931 [DOI] [PubMed]
- 43.Silverberg JI, Simpson EL (2013) Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol 24:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.