Abstract
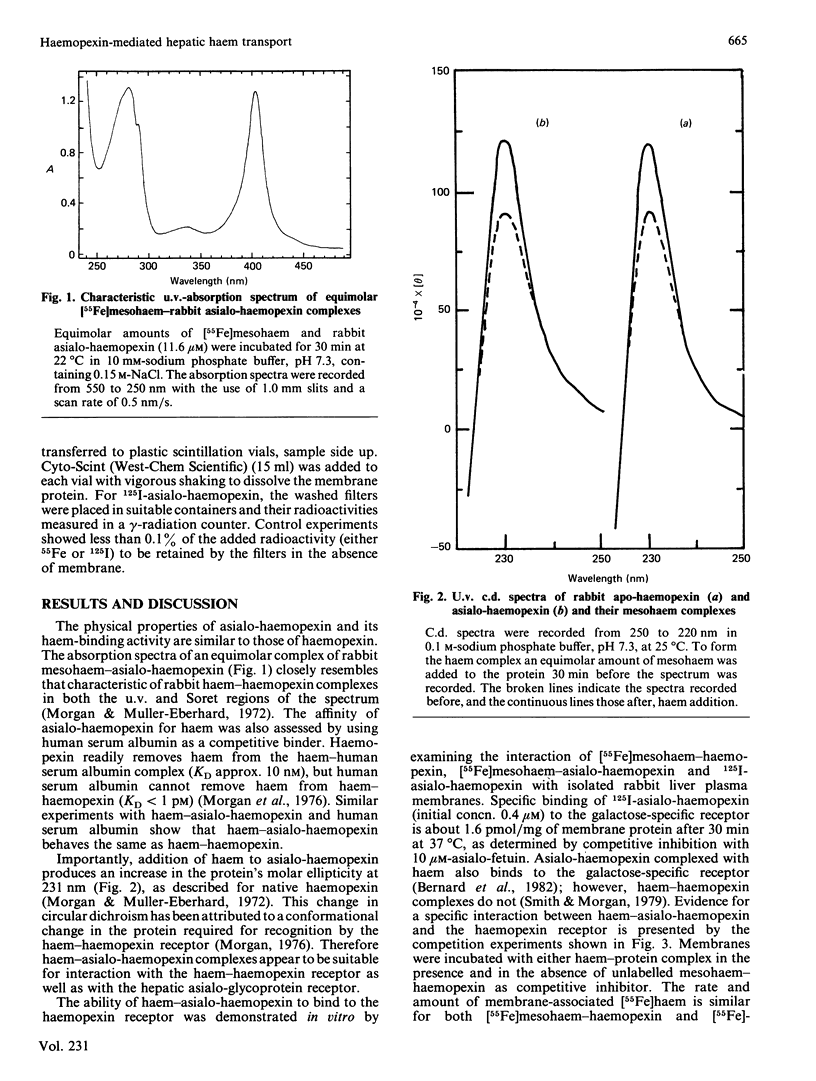
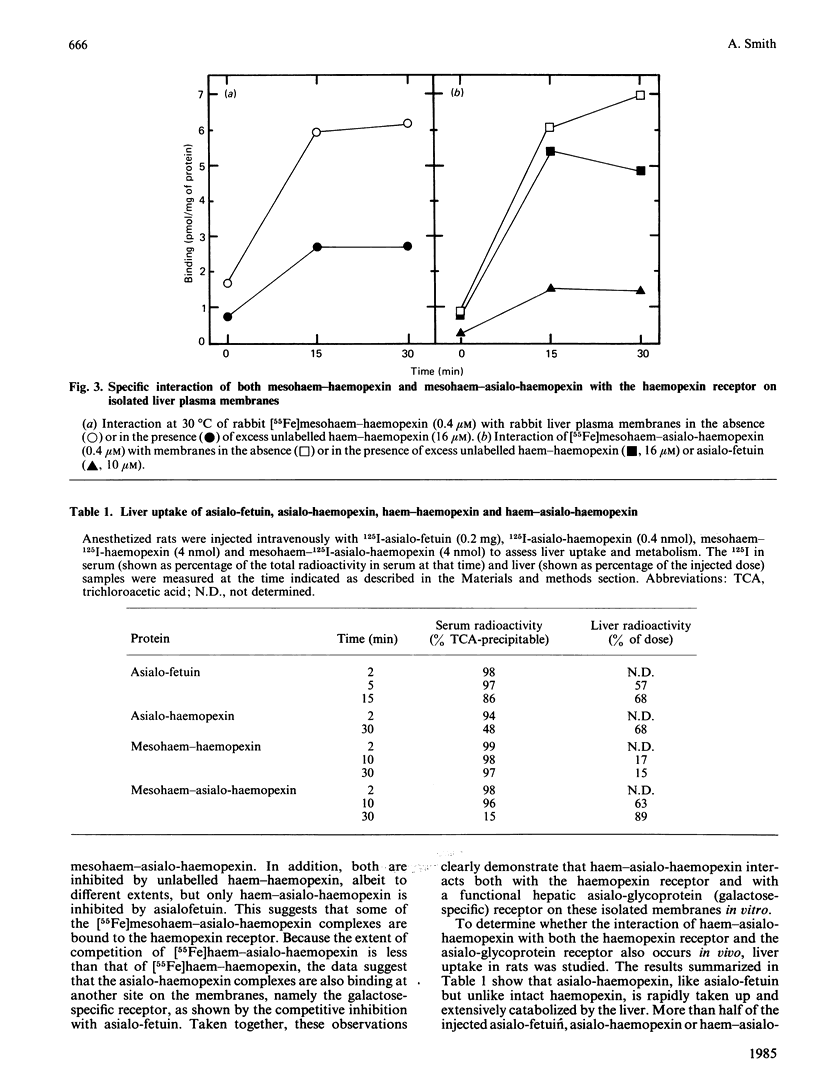
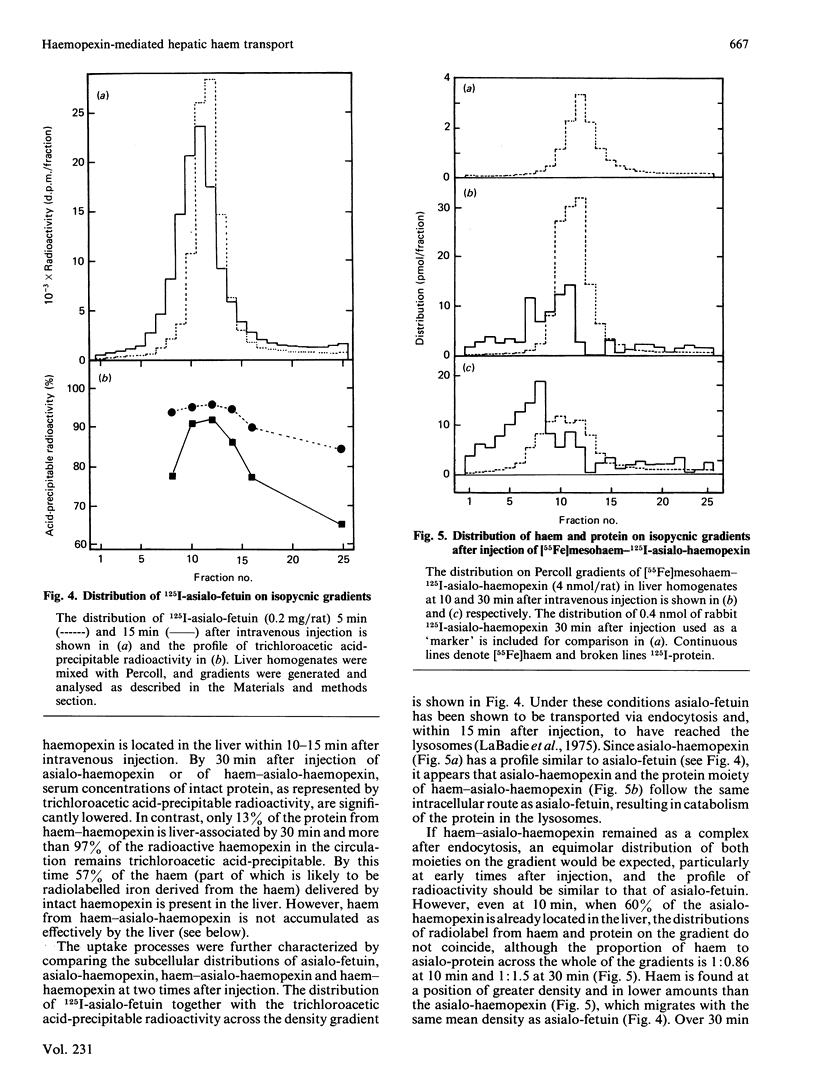
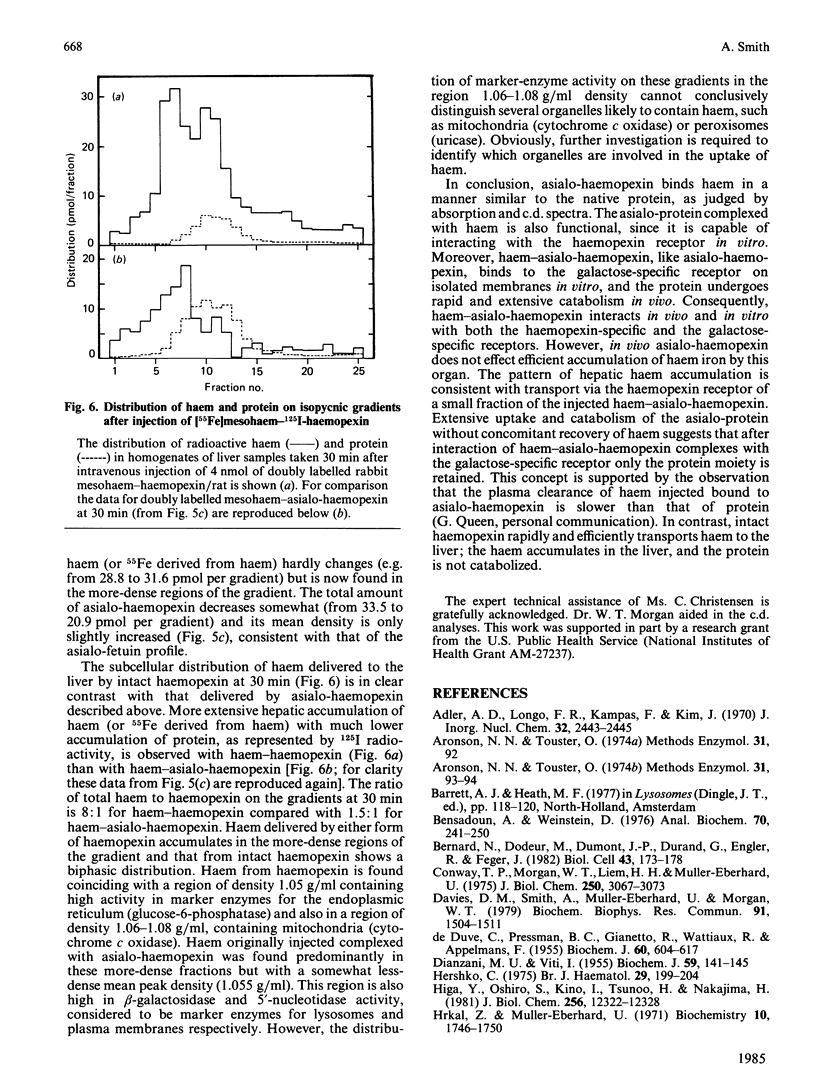
How the interaction of haemopexin with two different receptors affects its subsequent metabolism and 'intracellular' haem transport was examined by using mesohaem-haemopexin and mesohaem-asialo-haemopexin. The physical properties of the two haem proteins, including their absorption and c.d. spectra, are similar. Binding studies in vitro showed that haem-asialo-haemopexin interacts with both the haemopexin-specific and galactose-specific receptors on liver plasma membranes, but that haem-haemopexin interacts only with the haemopexin receptor. In vivo haem-asialo-haemopexin rapidly interacts with the liver via the galactose-specific receptor, since the protein is extensively catabolized and uptake is blocked by asialofetuin. Haem iron from haem-asialo-haemopexin is not accumulated in the liver to the same extent as from intact haem-haemopexin, and the native sialylated protein is not proteolysed. Moreover, after fractionation of homogenized liver by using colloidal-silica gradients, liver-associated haem-haemopexin and haem-asialo-haemopexin produced distinctly different patterns for both protein and ligand, consistent with their uptake by two distinct receptors. These results demonstrate that the interaction of haemopexin with different receptors influences its subsequent metabolic fate and that haem iron from haem-haemopexin is efficiently conserved only if it enters the liver cell via the specific haemopexin receptor.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Conway T. P., Morgan W. T., Liem H. H., Muller-Eberhard U. Catabolism of photo-oxidized and desialylated hemopexin in the rabbit. J Biol Chem. 1975 Apr 25;250(8):3067–3073. [PubMed] [Google Scholar]
- DE DUVE C., PRESSMAN B. C., GIANETTO R., WATTIAUX R., APPELMANS F. Tissue fractionation studies. 6. Intracellular distribution patterns of enzymes in rat-liver tissue. Biochem J. 1955 Aug;60(4):604–617. doi: 10.1042/bj0600604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIANZANI M. U., VITI I. The content and distribution of cytochrome c in the fatty liver of rats. Biochem J. 1955 Jan;59(1):141–145. doi: 10.1042/bj0590141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies D. M., Smith A., Muller-Eberhard U., Morgan W. T. Hepatic subcellular metabolism of heme from heme-hemopexin: incorporation of iron into ferritin. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1504–1511. doi: 10.1016/0006-291x(79)91235-x. [DOI] [PubMed] [Google Scholar]
- Hershko C. The fate of circulating haemoglobin. Br J Haematol. 1975 Feb;29(2):199–204. doi: 10.1111/j.1365-2141.1975.tb01814.x. [DOI] [PubMed] [Google Scholar]
- Higa Y., Oshiro S., Kino K., Tsunoo H., Nakajima H. Catabolism of globin-haptoglobin in liver cells after intravenous administration of hemoglobin-haptoglobin to rats. J Biol Chem. 1981 Dec 10;256(23):12322–12328. [PubMed] [Google Scholar]
- Hrkal Z., Muller-Eberhard U. Partial characterization of the heme-binding serum glycoproteins rabbit and human hemopexin. Biochemistry. 1971 May 11;10(10):1746–1750. doi: 10.1021/bi00786a002. [DOI] [PubMed] [Google Scholar]
- Hrkal Z., Vodrázka Z., Kalousek I. Transfer of heme from ferrihemoglobin and ferrihemoglobin isolated chains to hemopexin. Eur J Biochem. 1974 Mar 15;43(1):73–78. doi: 10.1111/j.1432-1033.1974.tb03386.x. [DOI] [PubMed] [Google Scholar]
- Kino K., Tsunoo H., Higa Y., Takami M., Hamaguchi H., Nakajima H. Hemoglobin-haptoglobin receptor in rat liver plasma membrane. J Biol Chem. 1980 Oct 25;255(20):9616–9620. [PubMed] [Google Scholar]
- LaBadie J. H., Chapman K. P., Aronson N. N., Jr Glycoprotein catabolism in rat liver: Lysosomal digestion of iodinated asialo-fetuin. Biochem J. 1975 Nov;152(2):271–279. doi: 10.1042/bj1520271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Morell A. G., Scheinberg I. H. Solubilization of hepatic binding sites for asialo-glycoproteins. Biochem Biophys Res Commun. 1972 Aug 21;48(4):808–815. doi: 10.1016/0006-291x(72)90679-1. [DOI] [PubMed] [Google Scholar]
- Morgan W. T., Liem H. H., Sutor R. P., Muller-Ebergard U. Transfer of heme from heme-albumin to hemopexin. Biochim Biophys Acta. 1976 Sep 24;444(2):435–445. doi: 10.1016/0304-4165(76)90387-1. [DOI] [PubMed] [Google Scholar]
- Morgan W. T., Muller-Eberhard U. Interactions of porphyrins with rabbit hemopexin. J Biol Chem. 1972 Nov 25;247(22):7181–7187. [PubMed] [Google Scholar]
- Seery V. L., Hathaway G., Eberhard U. M. Hemopexin of human and rabbit: molecular weight and extinction coefficient. Arch Biochem Biophys. 1972 May;150(1):269–272. doi: 10.1016/0003-9861(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Skoza L., Mohos S. Stable thiobarbituric acid chromophore with dimethyl sulphoxide. Application to sialic acid assay in analytical de-O-acetylation. Biochem J. 1976 Dec 1;159(3):457–462. doi: 10.1042/bj1590457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Haem transport to the liver by haemopexin. Receptor-mediated uptake with recycling of the protein. Biochem J. 1979 Jul 15;182(1):47–54. doi: 10.1042/bj1820047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated heme uptake by liver. Characterization of the interaction of heme-hemopexin with isolated rabbit liver plasma membranes. J Biol Chem. 1984 Oct 10;259(19):12049–12053. [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated transport of heme into isolated rat hepatocytes. J Biol Chem. 1981 Nov 10;256(21):10902–10909. [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Transport of heme by hemopexin to the liver: evidence for receptor-mediated uptake. Biochem Biophys Res Commun. 1978 Sep 14;84(1):151–157. doi: 10.1016/0006-291x(78)90276-0. [DOI] [PubMed] [Google Scholar]
- Tsunoo H., Sussman H. H. Characterization of transferrin binding and specificity of the placental transferrin receptor. Arch Biochem Biophys. 1983 Aug;225(1):42–54. doi: 10.1016/0003-9861(83)90005-x. [DOI] [PubMed] [Google Scholar]


